Abstract
In epithelial cells β-catenin plays a critical role as a component of the cell-cell adhesion apparatus and as a coactivator of the TCF/LEF (T-cell transcription factor/lymphoid enhancer binding factor) family of transcription factors. Deregulation of β-catenin has been implicated in the malignant transformation of cells of epithelial origin. However, a function for β-catenin in hematologic malignancies has not been reported. β-Catenin is not detectable in normal peripheral blood T cells but is expressed in T–acute lymphoblastic leukemia cells and other tumor lines of hematopoietic origin and in primary lymphoid and myeloid leukemia cells. β-Catenin function was examined in Jurkat T–acute lymphoblastic leukemia cells. Overexpression of dominant-negative β-catenin or dominant-negative TCF reduced β-catenin nuclear signaling and inhibited Jurkat proliferation and clonogenicity. Similarly, these constructs inhibited proliferation of K562 and HUT-102 cells. Reduction of β-catenin expression with β-catenin antisense down-regulated adhesion of Jurkat cells in response to phytohemagglutinin. Incubation of Jurkat cells with anti-Fas induced caspase-dependent limited proteolysis of β-catenin N- and C-terminal regions and rapid redistribution of β-catenin to the detergent-insoluble cytoskeleton, concomitant with a marked decline in nuclear β-catenin signaling. Fas-mediated apoptosis was potentiated by inhibition of β-catenin nuclear signaling. The data suggest that β-catenin can play a significant role in promoting leukemic cell proliferation, adhesion, and survival.
Introduction
β-Catenin is a multifunctional protein that acts as a component of the homotypic cell-cell adhesion apparatus and as a coactivator of the transcription of T-cell transcription factor/lymphoid enhancer binding factor (TCF/LEF) target genes. In epithelial cells β-catenin is localized in the cytoplasm and in the plasma cell membrane, where it functions as part of the adherens junction, a specialized cytoskeletal complex that regulates cell-cell adhesion.1
β-Catenin levels are regulated posttranslationally by the canonical Wnt signaling pathway. In the absence of secreted Wnts, the modular protein axin provides a scaffold for the binding of glycogen synthase kinase-3β (GSK-3β), adenomatous polyposis coli (APC) protein, and β-catenin. This facilitates the phosphorylation of β-catenin by GSK-3β and subsequent rapid degradation of β-catenin by a proteasome-dependent process.2,3 Binding of Wnts to their cell surface receptors, consisting of a seven-pass transmembrane molecule of the Frizzled family4 and either LRP5 or LRP6/Arrow,5-7 results in disruption of the axin/GSK-3β/APC/β-catenin complex. This involves the action of cytoplasmic proteins Dishevelled and Frat,8-12dephosphorylation of axin,13,14 and recruitment of axin to LRP5, which is associated with axin destabilization.15 As a result, β-catenin phosphorylation and degradation are inhibited, and the protein accumulates in the cytoplasm and nucleus, where it interacts with members of the TCF/LEF family to regulate gene expression. Mutations in APC, β-catenin, or axin that increase the steady state level of soluble β-catenin have been observed in various tumors of epithelial origin.1,16 In addition, constitutive expression of Wnt protein has been documented in animal models of breast cancer.17-19
Overexpression of wild-type β-catenin can transform normal epithelial cells by promoting G1/S transition and inhibiting apoptosis.20 β-Catenin is a target of apoptosis-associated proteolysis in various adherent cell types, including NIH 3T3 fibroblasts, Madine-Darby canine kidney cells, human umbilical vein endothelial cells, the human breast epithelial cell line H184A1, the hepatoma cell line McA-RH7777, and the colon carcinoma cell lines WiDr and DLD-1.21-24 Furthermore, in Alzheimer disease destabilization of β-catenin potentiates neuronal apoptosis.25 Thus, β-catenin has been shown to be a substrate for caspase-induced proteolysis in apoptotic adherent cells, and β-catenin is thought to act as an antiapoptotic factor in these cell populations.
β-Catenin has been reported to be expressed in Jurkat cells and several other leukemic cell lines.26 However, the function of β-catenin in hematologic malignancies has not been reported. We therefore examined the role of β-catenin in Jurkat cells, which express a high level of this protein. Recently, β-catenin was shown to activate transcription of growth-promoting genes, including c-myc27 and cyclin D1.28 29We hypothesized that β-catenin may constitutively activate transcription of genes associated with leukemogenesis and leukemic cell survival. To begin to investigate this possibility, we expressed proteins that interfere with nuclear β-catenin signaling and tested their effect on Jurkat cell proliferation, clonogenicity, and Fas-related apoptosis. Inhibition of β-catenin nuclear signaling inhibited proliferation and clonogenicity and potentiated anti-Fas–induced apoptosis in Jurkat cells. During Fas-mediated apoptosis, β-catenin was subject to caspase-dependent proteolysis at both the C- and N-terminus. The caspase-dependent cleavage of β-catenin during Jurkat apoptosis is consistent with a role for β-catenin in Jurkat cell survival. We also observed a redistribution of β-catenin to the detergent-insoluble cytoskeleton during apoptosis, contemporaneously with a loss of nuclear β-catenin signaling. Furthermore, β-catenin antisense down-regulated cellular β-catenin protein levels and markedly inhibited Jurkat cell homotypic adhesion. The data show that β-catenin plays important roles in leukemic cells analogous to its multifunctional activity in adherent cells.
Materials and methods
Cell lines and patient samples
The human T–acute lymphoblastic leukemia (T-ALL) cell lines Jurkat, Molt-4, CCRF-CEM, and HSB; the human T-cell lymphoma/leukemia virus-1 (HTLV-I)–associated T-cell line HUT102 derived from a patient with cutaneous T-cell lymphoma; the AML-M2 cell line HL-60; and the CML blast crisis cell line K-562 were acquired from the American Type Culture Collection (Manassas, VA). CA46 Burkitt lymphoma cells were a gift from Dr Ian Magrath, the International Network for Cancer Treatment and Research. The diffuse large B-cell lymphomas SUDHL4 and SUDHL5 30 were a gift from Dr Mark Raffeld of the National Cancer Institute. Cells were cultured in RPMI 1640 medium containing 2 mM l-glutamine, supplemented with 10% fetal bovine serum, 10 mM HEPES (pH 7.4), 100 U/mL penicillin, and 100 mg/mL streptomycin (Biofluids, Rockville, MD).
For patient samples bone marrow aspirates were obtained from 11 patients with acute or chronic leukemias. Seven patients (6 acute myelocytic leukemia [AML], 1 chronic myelocytic leukemia [CML]) were newly diagnosed, 2 had refractory AML, 1 had CML in blast crisis, and 1 had relapsed T-cell ALL characterized by aberrant expression of the myeloid antigen CD13. Mononuclear cells were isolated from heparinized aspirates by Ficoll-Hypaque sedimentation and washed in RPMI 1640 medium with 10% normal human serum. Marrow cells were frozen viably by controlled-rate freezing in 20% normal human serum plus 10% dimethyl sulfoxide and stored in liquid nitrogen until being thawed prior to preparation for Western blot analysis. All patients provided written informed consent according to the University of Maryland, Baltimore Institutional Review Board prior to bone marrow aspirates being obtained for study.
Cell death induction
Antihuman Fas monoclonal antibody clone CH-11 was purchased from Upstate Biotechnology (Lake Placid, NY) and was used at a concentration of 100 ng/mL. Staurosporine and etoposide (VP-16) were obtained from Sigma Chemical (St Louis, MO) and used at a concentration of 1 μM and 100 μM, respectively. Soluble recombinant TRAIL (tumor necrosis factor-α–related apoptosis-inducing ligand) (Apo-2L) was acquired from Biomol Research Laboratories (Plymouth Meeting, PA) and used at a concentration of 100 ng/mL. To induce apoptosis, 4.0 × 105 cells per milliliter were treated as indicated and incubated at 37°C for the indicated times.
Antibodies
Monoclonal antibody to β-catenin amino acid residues 56 to 75 was purchased from Alexis (San Diego, CA). Monoclonal antibody to β-catenin amino acid residues 571 to 781 was purchased from Transduction Laboratories (Lexington, KY). Polyclonal antibody to β-catenin amino acid residues 768 to 781 was purchased from Sigma Chemical. Polyclonal antiactin antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Peroxidase-labeled sheep anti–mouse immunoglobulin and peroxidase-labeled donkey anti–rabbit immunoglobulin were obtained from Amersham Life Sciences (Cleveland, OH). Protein A–Sepharose beads were acquired from Santa Cruz Biotechnology.
Chemical reagents
N-acetyl-leucyl-leucyl-norleucinal (calpain inhibitor I, ALLnL) was purchased from Sigma Chemical. The caspase inhibitor N-acetyl-Tyr-Val- Ala-Asp-chloromethylketone (YVAD-CMK) was acquired from Bachem (Torrance, CA). The peptide caspase inhibitors CBZ-Val-Ala-Asp-fluoromethylketone (ZVAD-FMK), CBZ-Asp-Glu-Val-Asp-fluoromethylketone (ZDEVD-FMK), and Boc-Asp-fluoromethylketone (BD-FMK) and the cathepsin inhibitor CBZ-Phe-Ala-fluoromethylketone (ZFA-FMK) were purchased from Enzyme Systems Products (Dublin, CA).
Isolation of normal peripheral blood T cells
Buffy coats were separated on a Ficoll-Paque Plus (Amersham, Buckinghamshire, United Kingdom) gradient; the mononuclear cell interface was incubated on plastic dishes for 2 hours at 37°C to remove the majority of monocytes; and the B-cell and natural killer cell populations were depleted with beads (Dynal, Lake Success, NY) coated with anti-CD19 and anti-CD16 antibodies (Pharmingen, San Diego, CA), respectively. The resulting population was 85% to 90% T cells as determined by flow cytometric analysis (data not shown). Buffy coats were provided anonymously as a byproduct of whole blood donations from paid healthy volunteer donors through a National Institutes of Health Institutional Review Board–approved protocol.
Western blot analysis
Cells were rinsed with phosphate-buffered saline (PBS) and lysed in CSK buffer (100 mM NaCl, 300 mM sucrose, 10 mM PIPES [pH 6.8], 3 mM MgCl2, 0.5% Triton X-100, 0.1 mM sodium orthovanadate, 2 μg/mL aprotinin, 2 μg/mL leupeptin, and 100 μg/mL phenylmethylsulfonyl fluoride) for 10 minutes at 4°C. Samples were spun at 9000g for 10 minutes to remove insoluble material followed by measurement of protein concentrations by the Bradford method (Bio-Rad, Hercules, CA). Samples containing equal amounts of protein were subjected to Western blot analysis as described.31 The blots were probed with primary antibody as indicated, and where specified the blot was stripped by washing the membrane at room temperature for 2 hours in stripping buffer (200 mM glycine, 500 mM NaCl, adjusted to pH 2.8 with HCl) and reprobed with the antibody indicated.
Immunoprecipitation
Jurkat cell lysates were prepared in CSK buffer, precleared for 1 hour at 4°C with protein A–Sepharose beads (Santa Cruz Biotechnology), and then incubated with 1 μg of primary antibody for 3 hours at 4°C. Protein A–Sepharose beads were added, and the mixture was rocked for 2 hours at 4°C. The beads were subsequently washed 5 times with lysis buffer and mixed with sodium dodecyl sulfate sample buffer. The samples were boiled to elute bound proteins, separated on 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels, and Western blot analysis was performed.
Immunocytochemistry
For a standard, noncytoskeletal extraction, cells that had been attached to glass slides by cytocentrifugation (Shandon, Pittsburgh, PA) were fixed with 3.7% formaldehyde in PBS for 10 minutes and extracted with 0.2% Triton X-100 for another 10 minutes at room temperature. For cytoskeletal preparations, the cells were permeabilized with 1% Triton X-100 in PHEM buffer (60 mM piperazine-N, N'-bis 2-ethane-sulfonic acid [PIPES], 25 mM HEPES, 10 mM ethyleneglycoltetraacetic acid, and 2 mM MgCl2, pH 6.9) for 2 minutes and fixed with 3.7% formaldehyde for 10 minutes at room temperature. The cells were stained with monoclonal anti–β-catenin antibody (Transduction Laboratories) and Cy3-conjugated goat antimouse immunoglobulin (Jackson Immunoresearch Laboratories, West Grove, PA), as described previously.32
Promoter-reporter assays
Jurkat cells, 2 × 107 in 200 μL, were transfected by electroporation (Electro Square Porator T820, BTX, San Diego, CA; 250 V, 65 msec) with 10 μg of the TCF/LEF-responsive reporter plasmid pTOPFLASH33 or the control reporter plasmid containing mutated TCF/LEF sites, pFOPFLASH.33Jurkat cells were cotransfected with expression vectors (10 μg each) encoding dominant-negative β-catenin, dominant-negative TCF,34 or E-cadherin.35 Following transfection the cells were incubated for 18 hours and further incubated for the time indicated in the presence or absence of anti-Fas. The cells were then lysed, and luciferase assays were performed as described previously.36 For normalization 3 μg of β-galactosidase reporter vector pCMV-β (Promega, Madison, WI) was cotransfected, and β-galactosidase activity was assayed using the β-galactosidase enzyme assay system (Promega).
Flow cytometric analysis of apoptosis
Jurkat cells were transfected by electroporation as described above with 10 μg expression vectors encoding either E-cadherin, dominant-negative TCF, dominant-negative β-catenin, or with pcDNA4 as a control vector. All transfection reactions included enhanced green fluorescent protein plasmid (5 μg pEGFP, Clontech, Palo Alto, CA) as a marker of transfected cells. Following transfection the cells were treated with anti-Fas antibody (100 ng/mL) for 6 hours. These cells were washed twice with cold PBS and resuspended in 0.5 mL PBS containing 50 μg/mL propidium iodide. After incubation for 10 minutes at room temperature, the cells were analyzed on a FACScan flow cytometer (Becton Dickinson, Bedford, MA). Analysis was confined to the GFP-positive live cell population as defined by green fluorescence and forward and side scatter profiles, and the percent of apoptotic propidium iodide–positive cells within this population was determined using the CellQuest program (Becton Dickinson).
Growth curve
Jurkat, HL-60, HUT-102, SUDHL4, and K562 cells were transiently transfected by electroporation with vectors encoding pEGFP (5 μg) and β-catenin, dominant-negative β-catenin, or dominant-negative TCF (10 μg each). The cells were analyzed in a Becton Dickinson flow cytometer, and the transfected GFP-positive cells were separated from GFP-negative cells by sorting under sterile conditions. Cells were seeded in 96-well plates at 3000 cells per well and incubated at 37°C. The number of cells per well was determined daily by removing cells from triplicate wells, pelleting by centrifugation, resuspension in a small volume of medium, and counting of viable cells using trypan blue in a hemocytometer.
Clonogenic assay
Jurkat and HL-60 cells were transiently transfected with vectors encoding pEGFP (5 μg) and β-catenin, dominant-negative β-catenin, or dominant-negative TCF (10 μg each). GFP-positive cells were sorted by flow cytometry. Clonogenicity was measured as described.37 Briefly, 1 × 103 GFP-positive sorted cells were transferred to semisolid medium containing 0.2% agar and plated into 24-well plates (0.3 mL per well). Colony formation, defined as clusters of 20 or more cells, was scored on an inverted microscope after 7 days. At least 4 wells per condition were used.
Cell aggregation assay
Jurkat cells were transiently transfected with vectors encoding pEGFP (5 μg) and wild-type β-catenin, full-length β-catenin antisense, or full-length antisense to the interferon-inducible guanosine triphosphatase MxA (10 μg each), used as a control for antisense transfection. GFP-positive cells were sorted by flow cytometry. Homotypic cell aggregation was induced as described previously.38 Briefly, 2.5 × 106GFP-positive cells were suspended in 5 mL complete medium containing 2 μg/mL phytohemagglutinin (PHA), and then 100 μL of this cell suspension was added per well to round-bottomed 96-well microtiter plates. After 30 minutes of incubation at 37°C, aggregation was analyzed by evaluating the number of individual cells, aggregates of 2 to 10 cells, and aggregates of more than 10 cells using an inverted microscope and a hemocytometer. At least 4 wells per condition were analyzed.
Results
Western blot analysis of normal peripheral blood T cells, leukemia/lymphoma cell lines, and primary leukemia cells
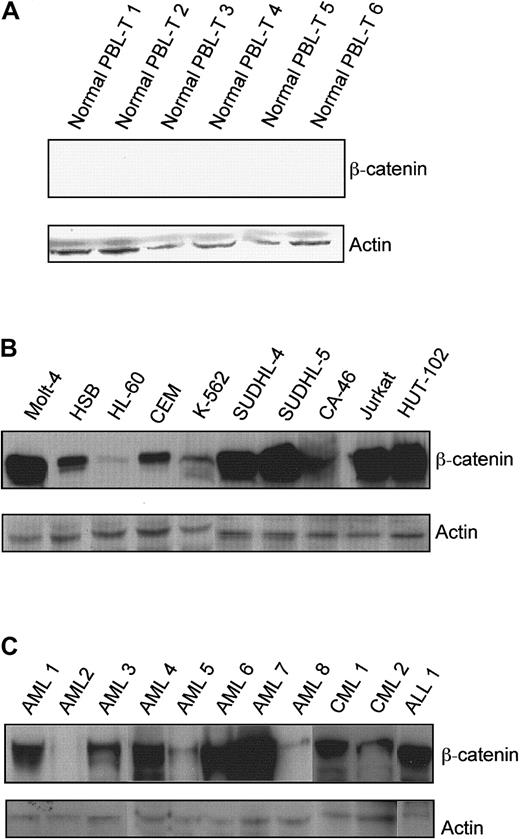
Resting T cells were isolated from peripheral blood obtained from healthy donors, and β-catenin expression was determined by Western blot analysis. There was no detectable β-catenin protein in the normal peripheral blood T-cell samples (Figure1A). The blot was reprobed for actin, and signal was detectable in all lanes. In contrast, all of the leukemia and lymphoma cell lines expressed β-catenin protein (Figure 1B). However, there was marked heterogeneity in the level of β-catenin among the cell lines tested. The highest signals were seen in Jurkat, Molt-4, SUDHL4, and SUDHL5, while HL-60 expressed the lowest level of β-catenin. These results are consistent with a report that showed β-catenin protein in leukemic cell lines and not in normal leukocytes.26
β-Catenin protein levels in normal peripheral blood T cells, leukemia/lymphoma cell lines, and primary leukemia cells.
(A) Peripheral blood obtained from healthy volunteers was separated on Ficoll gradients, and the mononuclear cells were depleted of monocytes by brief incubation on plastic. T cells were enriched by removal of natural killer cells and B cells using Dynal beads coated with anti-CD16 and anti-CD19 antibodies, respectively. β-Catenin and actin protein levels in the resulting populations were measured by Western blot analysis. (B) β-Catenin and actin in total cell lysates from leukemia/lymphoma cell lines were determined by Western blot analysis with chemiluminescence detection. (C) β-Catenin and actin in total cell lysates from primary leukemia cells were determined. (Lane 1, AML-M4, newly diagnosed; lane 2, AML-M2, newly diagnosed; lane 3, AML-M5, newly diagnosed; lane 4, AML-M4, refractory; lane 5, AML-M5, refractory; lane 6, AML-M5, newly diagnosed; lane 7, AML-M0, newly diagnosed; lane 8, AML-M3, newly diagnosed; lane 9, CML blast crisis; lane 10, CML, chronic phase; lane 11, relapsed T-ALL).
β-Catenin protein levels in normal peripheral blood T cells, leukemia/lymphoma cell lines, and primary leukemia cells.
(A) Peripheral blood obtained from healthy volunteers was separated on Ficoll gradients, and the mononuclear cells were depleted of monocytes by brief incubation on plastic. T cells were enriched by removal of natural killer cells and B cells using Dynal beads coated with anti-CD16 and anti-CD19 antibodies, respectively. β-Catenin and actin protein levels in the resulting populations were measured by Western blot analysis. (B) β-Catenin and actin in total cell lysates from leukemia/lymphoma cell lines were determined by Western blot analysis with chemiluminescence detection. (C) β-Catenin and actin in total cell lysates from primary leukemia cells were determined. (Lane 1, AML-M4, newly diagnosed; lane 2, AML-M2, newly diagnosed; lane 3, AML-M5, newly diagnosed; lane 4, AML-M4, refractory; lane 5, AML-M5, refractory; lane 6, AML-M5, newly diagnosed; lane 7, AML-M0, newly diagnosed; lane 8, AML-M3, newly diagnosed; lane 9, CML blast crisis; lane 10, CML, chronic phase; lane 11, relapsed T-ALL).
Eight freshly isolated AML samples, 2 freshly isolated CML samples, and 1 freshly isolated ALL were also examined. β-Catenin protein was expressed in 7 of 8 AML, 2 of 2 CML, and 1 of 1 ALL specimens. A range of β-catenin expression was observed, consistent with the data from cultured cell lines (Figure 1C).
Attenuation of β-catenin signaling inhibits cell proliferation and clonogenicity
To examine the role of β-catenin in leukemic cell growth, Jurkat cells were transiently transfected with expression vectors encoding proteins that interfere with β-catenin–activated transcription. Two constructs were used, dominant-negative TCF, which has been reported to block β-catenin signaling, and truncated β-catenin, lacking the N- and C-terminal domains required for coactivation of transcription by β-catenin.24 Jurkat cells, which express high levels of β-catenin, were compared with HL-60 cells, which express a low level of β-catenin. In addition, the effect of overexpression of wild-type β-catenin was determined.
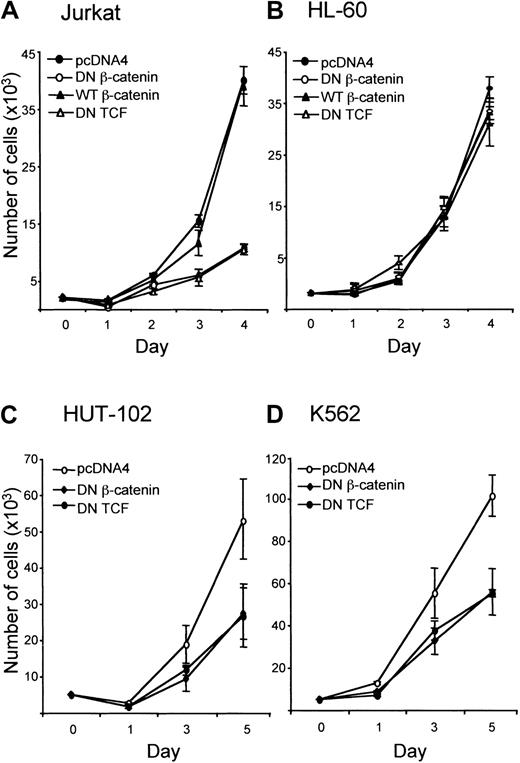
In Jurkat cells, growth was inhibited by overexpression of dominant-negative β-catenin and dominant-negative TCF (Figure2A). In contrast, in HL-60 cells, growth was not affected by expression of dominant-negative β-catenin or dominant-negative TCF. Expression of wild-type β-catenin also did not affect the growth rate of HL-60 cells (Figure 2B). In Jurkat cells overexpression of wild-type β-catenin did not increase the growth rate (Figure 2A). To confirm the role of β-catenin in leukemic cell growth, 2 other cell lines, HUT-102 and K562, were transfected with control vector, dominant-negative β-catenin, or dominant-negative TCF. Growth was inhibited by overexpression of constructs inhibiting β-catenin signaling (Figure 2C,D).
Effect of attenuation of β-catenin signaling on proliferation of leukemic cell lines.
(A) Jurkat, (B) HL-60, (C) HUT-102, and (D) K562 cells were transfected with pEGFP (5 μg) and other constructs (10 μg each) as indicated, and GFP-positive cells were isolated by flow cytometry as described in “Materials and methods.” Cells were seeded in 96-well plates at 3000 cells per well and incubated at 37°C. The number of viable cells per well was determined by counting trypan blue–excluding cells in a hemocytometer.
Effect of attenuation of β-catenin signaling on proliferation of leukemic cell lines.
(A) Jurkat, (B) HL-60, (C) HUT-102, and (D) K562 cells were transfected with pEGFP (5 μg) and other constructs (10 μg each) as indicated, and GFP-positive cells were isolated by flow cytometry as described in “Materials and methods.” Cells were seeded in 96-well plates at 3000 cells per well and incubated at 37°C. The number of viable cells per well was determined by counting trypan blue–excluding cells in a hemocytometer.
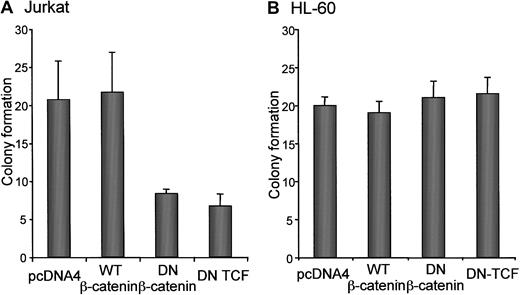
The effect of each construct was also tested in a clonogenicity assay in Jurkat and HL-60 cells. These results were similar to those observed in the cell growth assay. Jurkat cell clonogenicity was decreased by expression of dominant-negative β-catenin and dominant-negative TCF and not by wild-type β-catenin (Figure3A). However, clonogenicity of HL-60 cells was not affected by expression of dominant-negative β-catenin, dominant-negative TCF, or wild-type β-catenin (Figure 3B).
Effect of attenuated β-catenin signaling on clonogenicity.
(A) Jurkat and (B) HL-60 cells were transfected with pEGFP (5 μg) and other constructs (10 μg each) as indicated, and GFP-positive cells were isolated by flow cytometry as described in “Materials and methods.” A total of 1000 sorted cells were transferred to 5 mL semisolid medium containing 0.2% agar and plated into 24-well plates (0.3 mL per well). Colonies of 20 or more cells were scored on an inverted Leica microscope after 7 days. At least 4 wells per condition were counted in 3 independent experiments.
Effect of attenuated β-catenin signaling on clonogenicity.
(A) Jurkat and (B) HL-60 cells were transfected with pEGFP (5 μg) and other constructs (10 μg each) as indicated, and GFP-positive cells were isolated by flow cytometry as described in “Materials and methods.” A total of 1000 sorted cells were transferred to 5 mL semisolid medium containing 0.2% agar and plated into 24-well plates (0.3 mL per well). Colonies of 20 or more cells were scored on an inverted Leica microscope after 7 days. At least 4 wells per condition were counted in 3 independent experiments.
β-Catenin plays a role in PHA-induced homotypic cell aggregation
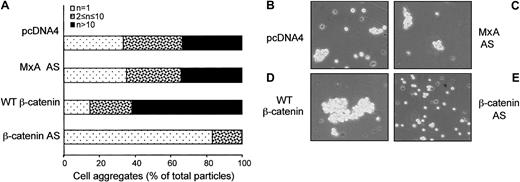
In adherent cells, β-catenin plays a critical role in homotypic cell-cell adhesion. Leukemic cells undergo homotypic adhesion in response to various stimuli both in vitro and in vivo.39,40 To analyze whether β-catenin is involved in homotypic aggregation after Jurkat cell activation, cells were transfected with control constructs or constructs encoding full-length β-catenin sense or antisense and cotransfected with a construct encoding GFP. After flow cytometric isolation of GFP-positive cells, the transfected cells were treated with PHA,38 and the effect of β-catenin antisense was determined. Incubation of Jurkat cells with 2 μg/mL PHA for 30 minutes strongly induced aggregation (Figure 4B). PHA-induced homotypic aggregation was markedly inhibited by full-length β-catenin antisense and not by a control antisense (Figure 4C,E). In contrast, PHA-induced aggregation was increased by wild-type β-catenin expression (Figure 4D).
β-Catenin contributes to homotypic cell aggregation of PHA-activated Jurkat cells.
Jurkat cells were transiently transfected with vectors encoding pEGFP (5 μg) and other constructs (10 μg each) as indicated, and GFP-positive cells were sorted by flow cytometry, suspended in complete medium containing 2 μg/mL PHA, and plated into a 96-well plate at 5 × 104/100 μL per well. After 30 minutes of incubation at 37°C, the cells were transferred to a hemocytometer using a wide-diameter pipette tip, and aggregation was determined using an inverted Leica microscope. Single cells, small clusters containing 2 to 10 cells, and clusters of more than 10 cells were counted by visual inspection of at least 4 wells per condition in 3 independent experiments.
β-Catenin contributes to homotypic cell aggregation of PHA-activated Jurkat cells.
Jurkat cells were transiently transfected with vectors encoding pEGFP (5 μg) and other constructs (10 μg each) as indicated, and GFP-positive cells were sorted by flow cytometry, suspended in complete medium containing 2 μg/mL PHA, and plated into a 96-well plate at 5 × 104/100 μL per well. After 30 minutes of incubation at 37°C, the cells were transferred to a hemocytometer using a wide-diameter pipette tip, and aggregation was determined using an inverted Leica microscope. Single cells, small clusters containing 2 to 10 cells, and clusters of more than 10 cells were counted by visual inspection of at least 4 wells per condition in 3 independent experiments.
Proteolysis of β-catenin in apoptotic Jurkat cells
The data presented thus far suggest that β-catenin can play a role in promoting Jurkat cell growth, adhesion, and survival. A number of proteins that regulate survival have been shown to act as substrates for caspase in response to apoptotic stimuli. In adherent cells undergoing apoptosis, β-catenin is degraded in a caspase-dependent manner. We examined the fate of β-catenin in Jurkat cells treated with multivalent anti-Fas antibody as a model for studying the fate of β-catenin in apoptotic hematopoietic cells.41 Anti-Fas antibody induced proteolysis of full-length β-catenin, which was evident within 2 hours of treatment and virtually complete by 6 hours (Figure 5). Anti-Fas induced sequential cleavage of β-catenin into two or three 64- to 70-kd fragments. The apparent half-life of intact β-catenin protein in apoptotic Jurkat cells was significantly less (t1/2 < 1.5 hours) than the apparent half-life in untreated cells (t1/2 > 3.5 hours) (data not shown).
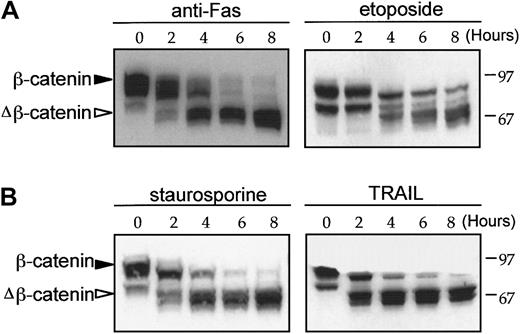
β-Catenin cleavage in response to apoptotic stimuli.
Time-dependent β-catenin cleavage in Jurkat cells in response to anti-Fas (100 ng/mL), staurosporine (1 μM), etoposide (100 μM), and TRAIL (100 ng/mL). After incubation for the time indicated, whole cell lysates were prepared using CSK buffer and β-catenin expression was determined by Western blot analysis. β-Catenin and degradative intermediates of β-catenin (closed and open triangles, respectively) are indicated.
β-Catenin cleavage in response to apoptotic stimuli.
Time-dependent β-catenin cleavage in Jurkat cells in response to anti-Fas (100 ng/mL), staurosporine (1 μM), etoposide (100 μM), and TRAIL (100 ng/mL). After incubation for the time indicated, whole cell lysates were prepared using CSK buffer and β-catenin expression was determined by Western blot analysis. β-Catenin and degradative intermediates of β-catenin (closed and open triangles, respectively) are indicated.
Jurkat cells were incubated with 3 other agents previously reported to induce apoptosis: staurosporine, which induces apoptosis through a protein kinase C–associated mechanism42; the topoisomerase II–reactive drug etoposide43; and soluble recombinant TRAIL, which induces apoptosis through activation of the plasma membrane TRAIL receptor.44 β-Catenin cleavage was induced in response to all of these reagents (Figure 5). The data demonstrate that β-catenin cleavage occurs during apoptosis induced by different apoptotic stimuli, which act through diverse signaling pathways.
Caspase-dependent proteolysis of β-catenin N- and C-terminal regions in response to anti-Fas
To determine whether caspases play a role in β-catenin proteolysis in Fas-treated Jurkat cells, we studied the effect of inhibitors of the caspase family. β-Catenin proteolysis was inhibited by YVAD-CMK, ZVAD-FMK, ZDEVD-FMK, and BD-FMK (Figure6A). These caspase inhibitors also blocked Fas-induced apoptosis (data not shown). As a control reagent for the FMK group we used the cathepsin inhibitor ZFA-FMK, which did not block β-catenin proteolysis (Figure 6A), showing that the caspase inhibitors did not work by blocking cathepsins.45 The peptide aldehyde ALLnL, which inhibits proteasome- and calpain-mediated proteolysis, also did not block proteolysis of full-length β-catenin (Figure 6A).
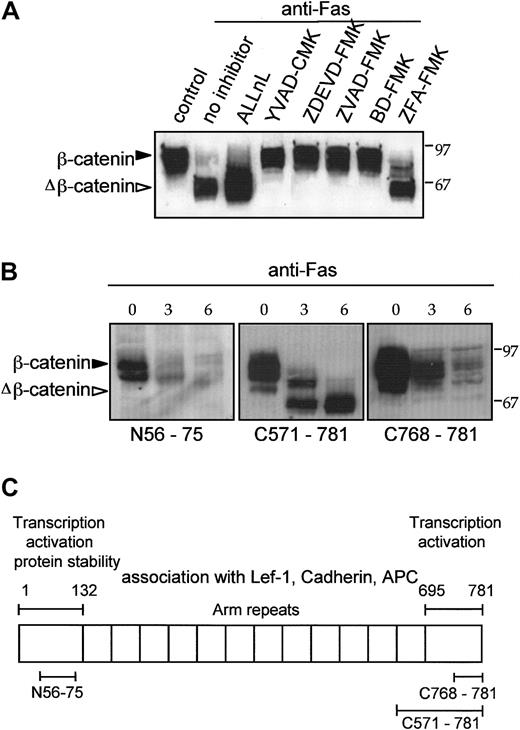
N- and C-terminal cleavage of β-catenin by a caspaselike protease in response to anti-Fas.
(A) Tetrapeptide caspase inhibitors block β-catenin cleavage induced by anti-Fas. Jurkat cells were preincubated for 30 minutes with various inhibitors as indicated and then treated with 100 ng/mL anti-Fas for an additional 6 hours at 37°C. Untreated control cells were compared with cells treated with anti-Fas or cells treated with anti-Fas and ALLnL (100 μM), YVAD-CMK, ZVAD-FMK, ZDEVD-FMK, or BD-FMK (20 μM each). The cathepsin inhibitor ZFA-FMK (20 μM) was used as a negative control reagent for peptide-FMK compounds. (B) Differential recognition of cleaved β-catenin by 3 anti–β-catenin antibodies. Untreated log phase Jurkat cells were compared with cells treated with anti-Fas (100 ng/mL) for the time indicated. Control and apoptotic Jurkat cell lysates were analyzed for β-catenin by Western blotting with anti–β-catenin antibodies that recognize amino acids 56 to 75, 571 to 781, and 768 to 781. (C) Schematic diagram indicates location of structure-function elements in β-catenin protein and regions recognized by anti–β-catenin antibodies.
N- and C-terminal cleavage of β-catenin by a caspaselike protease in response to anti-Fas.
(A) Tetrapeptide caspase inhibitors block β-catenin cleavage induced by anti-Fas. Jurkat cells were preincubated for 30 minutes with various inhibitors as indicated and then treated with 100 ng/mL anti-Fas for an additional 6 hours at 37°C. Untreated control cells were compared with cells treated with anti-Fas or cells treated with anti-Fas and ALLnL (100 μM), YVAD-CMK, ZVAD-FMK, ZDEVD-FMK, or BD-FMK (20 μM each). The cathepsin inhibitor ZFA-FMK (20 μM) was used as a negative control reagent for peptide-FMK compounds. (B) Differential recognition of cleaved β-catenin by 3 anti–β-catenin antibodies. Untreated log phase Jurkat cells were compared with cells treated with anti-Fas (100 ng/mL) for the time indicated. Control and apoptotic Jurkat cell lysates were analyzed for β-catenin by Western blotting with anti–β-catenin antibodies that recognize amino acids 56 to 75, 571 to 781, and 768 to 781. (C) Schematic diagram indicates location of structure-function elements in β-catenin protein and regions recognized by anti–β-catenin antibodies.
To analyze the sites of β-catenin cleavage, we used antipeptide antibodies against defined regions of β-catenin to probe Western blots of control and anti-Fas–treated Jurkat cells. β-Catenin contains 12 armadillo repeats flanked by N- and C-terminal transcription activation domains (Figure 6C). Anti–β-catenin antibody directed to a region near the N-terminus (amino acids 56-75) and another antibody directed to the C-terminal 14 amino acids (768-781) recognized intact β-catenin but did not recognize the large proteolytic fragments of β-catenin in apoptotic Jurkat cells (Figure6B). The proteolytic fragments were recognized by antibody directed against the amino acids 571 to 781. The loss of immunoreactivity with the anti–β-catenin N-terminal antibody maps the N-terminal Fas-induced cleavage site to within the first 75 amino acids of β-catenin. These data demonstrate that in response to Fas receptor activation, β-catenin undergoes 2 proteolytic processing steps, in which cleavage occurs within both the N- and C-terminal domains.
Attenuation of β-catenin nuclear signaling and redistribution of β-catenin to the detergent-insoluble cytoskeleton are early events in Fas-induced apoptosis
The data demonstrate that β-catenin undergoes proteolysis in response to anti-Fas. The loss of N- and C-terminal amino acids in the transcription activation domains suggested that β-catenin nuclear signaling might be attenuated as an early event in Fas-induced apoptosis. A promoter-reporter assay was performed using the β-catenin–responsive promoter pTOPFLASH and the mutant control pFOPFLASH. Luciferase activity of pTOPFLASH decreased rapidly in response to anti-Fas, while activity of the mutant control pFOPFLASH declined more slowly (Figure 7A). The rapidity of the loss of β-catenin nuclear signaling suggested a mechanism other than β-catenin proteolysis, which occurs more slowly (Figure 7B). In adherent cells it has been reported that overexpression of E-cadherin or α-catenin reduces β-catenin nuclear signaling by a mechanism involving enhanced association of β-catenin with the cytoskeleton and sequestering of free β-catenin.46-48 We asked whether the loss of β-catenin signaling was associated with a shift in the subcellular localization of β-catenin in apoptotic Jurkat cells. Cytochemical analysis of log phase control Jurkat cells showed no detectable association of β-catenin with the cytoskeleton (compare Figure 7C with 7E). However, within 30 minutes after anti-Fas treatment there was a striking increase in β-catenin associated with the detergent-insoluble cytoskeletal fraction in the apoptotic cells (compare Figure 7D with 7F).
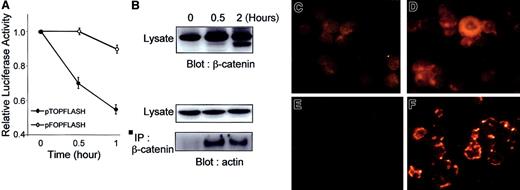
Anti-Fas induces down-regulation of β-catenin–associated transcription and redistribution of β-catenin to cytoskeleton.
(A) Jurkat cells were transfected with 10 μg pTOPFLASH or pFOPFLASH, incubated for 18 hours, and further incubated for the time indicated in the presence of anti-Fas antibody (100 ng/mL). Cells were then lysed, and luciferase activity was assayed as described in “Materials and methods.” (B) Immunoblot analysis. Jurkat cells were incubated with anti-Fas (100 ng/mL) for the indicated times and extracted in CSK buffer, and β-catenin was immunoprecipitated with anti–β-catenin antibody directed to amino acids 571 to 781. Immunoprecipitates and total cell lysates were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose, and probed either with antibody to β-catenin or actin. (C-F) Immunocytochemical analysis. β-Catenin distribution was analyzed in a standard preparation (C,D) and in a cytoskeletal preparation (E,F), in which soluble proteins were removed by incubation in 1% Triton X-100. Control (C,E) and 30-minute anti-Fas–treated (D,F) Jurkat cells were stained with anti–β-catenin antibody C571-781 followed by Cy3-conjugated goat antimouse antibody. (Magnification, ×630).
Anti-Fas induces down-regulation of β-catenin–associated transcription and redistribution of β-catenin to cytoskeleton.
(A) Jurkat cells were transfected with 10 μg pTOPFLASH or pFOPFLASH, incubated for 18 hours, and further incubated for the time indicated in the presence of anti-Fas antibody (100 ng/mL). Cells were then lysed, and luciferase activity was assayed as described in “Materials and methods.” (B) Immunoblot analysis. Jurkat cells were incubated with anti-Fas (100 ng/mL) for the indicated times and extracted in CSK buffer, and β-catenin was immunoprecipitated with anti–β-catenin antibody directed to amino acids 571 to 781. Immunoprecipitates and total cell lysates were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose, and probed either with antibody to β-catenin or actin. (C-F) Immunocytochemical analysis. β-Catenin distribution was analyzed in a standard preparation (C,D) and in a cytoskeletal preparation (E,F), in which soluble proteins were removed by incubation in 1% Triton X-100. Control (C,E) and 30-minute anti-Fas–treated (D,F) Jurkat cells were stained with anti–β-catenin antibody C571-781 followed by Cy3-conjugated goat antimouse antibody. (Magnification, ×630).
In adherent cells, β-catenin associates with the actin cytoskeleton.49 Coimmunoprecipitation analysis was performed to ask if β-catenin associates with the actin cytoskeleton in log phase or anti-Fas–treated Jurkat cells. In control Jurkat cells β-catenin did not associate with actin as determined by coimmunoprecipitation analysis. In contrast, there was a marked rapid induction of β-catenin association with actin in anti-Fas–treated cells (Figure 7B).
Effect of interrupting β-catenin signaling on anti-Fas–induced apoptosis
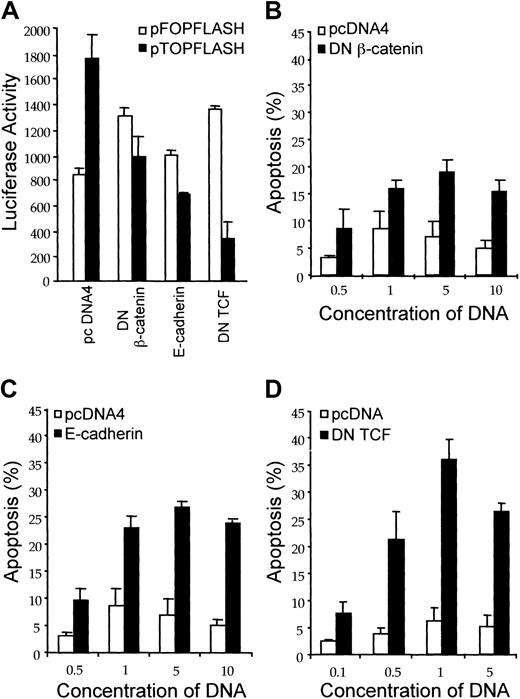
The data presented thus far, taken together with the role of β-catenin in other malignancies, suggested that β-catenin might function as a survival factor for leukemic cells and, consequently, loss of β-catenin signaling might facilitate apoptosis. To explore this possibility, we transfected Jurkat cells with 3 expression vectors encoding proteins that interfere with β-catenin–activated transcription: dominant-negative TCF, dominant-negative β-catenin, and E-cadherin. Cotransfection of each of these constructs with the pTOPFLASH reporter construct resulted in a decrease in the signal obtained from log phase Jurkat cells (Figure8A). In contrast, there was no inhibition of the signal when these constructs were cotransfected with the mutant pFOPFLASH reporter construct (Figure 8A). Thus, each of these constructs induced specific inhibition of the β-catenin–responsive promoter-reporter. To assess the effect of these constructs on anti-Fas–induced apoptosis, Jurkat cells were cotransfected with control vector, dominant-negative TCF, dominant-negative β-catenin, or E-cadherin together with a GFP expression vector. The cells were treated with anti-Fas, and the percent of apoptotic cells was determined by flow cytometric analysis of GFP-positive cells. Each of the constructs that attenuated β-catenin signaling, as detected by decreased pTOPFLASH activity, induced a concentration-dependent increase in apoptosis in response to anti-Fas (Figure 8B-D). Thus, the data indicated that loss of β-catenin signaling facilitates apoptosis.
Attenuation of β-catenin signaling increases susceptibility to anti-Fas–mediated apoptosis.
(A) Inhibition of β-catenin signaling by dominant-negative β-catenin, dominant-negative TCF, and E-cadherin. Jurkat cells (2 × 107) were transfected with either the empty pcDNA4 vector control, dominant-negative β-catenin, dominant-negative TCF, or E-cadherin and cotransfected with pTOPFLASH or pFOPFLASH (10 μg each). Following transfection, the cells were incubated for 18 hours and luciferase reporter activity was measured as described in “Materials and methods.” (B-D) Effect of attenuating β-catenin signaling on anti-Fas–induced apoptosis. Jurkat cells were transfected with either pcDNA4 vector control or with dominant-negative β-catenin (B), E-cadherin (C), or dominant-negative TCF (D) at the concentrations indicated. The pEGFP expression vector was cotransfected for identification of transfected cells. Quantitative analysis of apoptosis induced by anti-Fas in GFP-positive Jurkat cells was performed by flow cytometry. Values represent the mean ± SD of 3 separate experiments.
Attenuation of β-catenin signaling increases susceptibility to anti-Fas–mediated apoptosis.
(A) Inhibition of β-catenin signaling by dominant-negative β-catenin, dominant-negative TCF, and E-cadherin. Jurkat cells (2 × 107) were transfected with either the empty pcDNA4 vector control, dominant-negative β-catenin, dominant-negative TCF, or E-cadherin and cotransfected with pTOPFLASH or pFOPFLASH (10 μg each). Following transfection, the cells were incubated for 18 hours and luciferase reporter activity was measured as described in “Materials and methods.” (B-D) Effect of attenuating β-catenin signaling on anti-Fas–induced apoptosis. Jurkat cells were transfected with either pcDNA4 vector control or with dominant-negative β-catenin (B), E-cadherin (C), or dominant-negative TCF (D) at the concentrations indicated. The pEGFP expression vector was cotransfected for identification of transfected cells. Quantitative analysis of apoptosis induced by anti-Fas in GFP-positive Jurkat cells was performed by flow cytometry. Values represent the mean ± SD of 3 separate experiments.
Discussion
β-Catenin has several important and distinct roles in adherent cells, involving interactions at the plasma membrane and in the nucleus. Perhaps in part because of the absence of a classical adherens junction, a role for β-catenin in hematopoietic cells has not been evaluated extensively. It is now clear that β-catenin regulates adhesion, proliferation, and survival of solid tumor cells. The data presented here suggest that β-catenin has analogous functions in leukemic cells.
Normal resting peripheral blood mononuclear cells do not contain detectable levels of β-catenin protein. In contrast, β-catenin was easily observed in most leukemia cell lines26 (Figure 1) and primary leukemia cells (Figure 1C). β-Catenin protein was abundant in several T-ALL lines, the HTLV-I–associated T-cell line HUT-102, and the diffuse large B-cell lymphomas SUDHL4 and SUDHL5 (Figure 1). β-Catenin protein was less abundant in the Burkitt lymphoma line CA46 and in the CML blast crisis line K562 and was very weakly expressed in the AML cell line HL-60. Another AML cell line, U937, expressed abundant β-catenin protein (data not shown). The basis of the different levels of β-catenin expression remains to be determined. However, it is of interest that HL-60 cells are known to overexpress the β-catenin target gene c-myc.50Deregulation of a downstream target may obviate the need for overexpression of β-catenin itself to provide a selective advantage. Consistent with this hypothesis, manipulation of β-catenin signaling by dominant-negative β-catenin and dominant-negative TCF markedly down-regulated Jurkat growth and clonogenicity but had no effect on these parameters in HL-60 cells. The relatively low level of expression in the CA46 Burkitt lymphoma cells may also relate to c-myc deregulation.
The data raise the possibility that deregulation of at least one component of the β-catenin signaling pathway may be a characteristic feature of leukemic cells. Mutations might occur in β-catenin, the β-catenin degradation machinery (eg, APC, axin), or in a downstream gene target such as c-myc. The amino-terminal domain of β-catenin contains 4 consensus motifs for phosphorylation by GSK-3β, and mutations at these sites have been found in some tumors. These mutations stabilize β-catenin by blocking its ubiquitination and targeting to the proteasome. Although mutations of β-catenin have been observed thus far, they are relatively rare. With the striking exception of pilomatricomas, with a β-catenin mutation rate of more than 75%, other tumor types examined, including medulloblastomas, carcinoma of the colon, and hepatocellular, ovarian, prostatic, and endometrial carcinomas, have a mutation rate of less than 20%.51,52 An accumulation of nuclear β-catenin in the absence of β-catenin mutations has been observed in tumors of the uterine cervix, cholangiocarcinomas, gastrointestinal carcinoid tumor, and synovial sarcomas.53-56 Alternatively, deregulation might result from changes in an upstream component (ie, in a member of the Wnt family). Consistent with this idea, a Wnt protein was found to be overexpressed in pre–B ALL.57 In each of the leukemia/lymphoma cell lines studied here we sequenced the region of exon 3 encoding the N-terminal phosphorylation sites prone to mutation. No mutations were observed (data not shown).
Although inhibition of β-catenin nuclear signaling markedly reduced proliferation and clonogenicity in Jurkat cells, overexpression of β-catenin did not stimulate either activity (Figures 2 and 3). This is probably because Jurkat cells constitutively express very high levels of β-catenin and transfection with wild-type β-catenin only modestly increased the total β-catenin level (data not shown). Furthermore, while β-catenin promotes survival in various adherent cell types, overexpression of β-catenin also has been reported to induce apoptosis.58 These observations indicate that β-catenin effects can be context specific. Along with the many regulatory mechanisms that govern Wnt/β-catenin signaling, they also imply that the level of intracellular β-catenin is closely controlled and either too much or too little of the protein may be deleterious to the cell.
Because β-catenin plays a dual role in epithelial tumors, as a regulator not only of growth-promoting genes but also as a component of the homotypic adhesion apparatus, we investigated the possibility that β-catenin participates in the homotypic adhesion of leukemic cells. Adhesion of leukemic cells to each other occurs in vitro, both spontaneously and in response to mitogens and certain drugs, and in vivo, in certain pathological states.39,40 We triggered homotypic cell adhesion with PHA in Jurkat cells transiently transfected with expression vectors including wild-type β-catenin or full-length β-catenin antisense. The PHA-mediated homotypic adhesion of Jurkat cells, which occurs very rapidly and was analyzed after a 30-minute exposure, was increased by expression of wild-type β-catenin and, conversely, decreased by expression of full-length β-catenin antisense (Figure 4). Apparently, the adhesive properties of Jurkat cells were more sensitive to increased levels of β-catenin than the proliferative and clonogenic responses. We presume that cell-cell adhesion was mediated by interactions with cadherins, because Jurkat cells have been reported to express a cadherin59and the phenomenon was blocked by chelation of extracellular calcium (unpublished observation, February 2001). Our results demonstrate that, as in epithelial cells, β-catenin can regulate cell-cell adhesion of leukemic cells.
Proteolytic processing of cytosolic β-catenin is a key mechanism of its regulation. While proteasomal degradation is a well-established element in this process, caspase-dependent cleavage of β-catenin also has been described.21-24 In this study, we explored the fate of β-catenin in Jurkat cells following exposure to 4 different apoptotic stimuli (Figure 5). In each instance, large stable fragments of β-catenin were generated in a manner that was blocked by caspase-specific protease inhibitors. Both proteasomal and calpain inhibitors increased the concentration of these 64- to 70-kd fragments but did not increase the amount of intact β-catenin in Jurkat cells undergoing apoptosis. These results indicated that caspases were responsible for limited N- and C-terminal processing of β-catenin during apoptosis, while further degradation was mediated by the proteasome and calpains. Loss of N- and C-terminal portions presumably would disrupt transcriptional activity of β-catenin, but the persistence of large fragments with residual protein-protein binding sites implied that these derivatives might retain other functional activities.
The rapid loss of β-catenin transcriptional activity following treatment with anti-Fas antibody preceded evidence of apoptotic degradation of β-catenin protein but coincided with the redistribution of β-catenin to the actin cytoskeleton. This suggested that relocation to the cytoskeleton might have been responsible for the decline in reporter activity (Figure 7). The formation of a β-catenin/actin complex in apoptotic Jurkat cells might have another function, perhaps to facilitate shape changes associated with cell fragmentation.
In summary, the data in the present study suggest that β-catenin may have an important role in hematologic malignancies, similar to the one recently established for epithelial tumors. Thus, β-catenin signaling may present a new target for therapeutic intervention in leukemia and lymphoma.60 We documented that β-catenin, which is overexpressed in many hematopoietic cell lines, contributes to their proliferation, survival, and adhesive properties. Other recent studies indicate that β-catenin/TCF activity participates in differentiation, proliferation, and survival of normal developing lymphocytes.61 62 While these articles indicate a function for β-catenin in lymphocyte development, we have observed a striking increase in β-catenin transcript and protein following activation of normal lymphocytes (manuscript in preparation). Presumably, defects in regulation that result in constitutive β-catenin signaling would predispose to malignant transformation.
We thank Dr Stephen Byers for the E-cadherin expression vector, Dr Hans Clevers for pTOPFLASH and pFOPFLASH reporter plasmids, Dr Frank McCormick and Dr Osamu Tetsu for the dominant-negative TCF expression vector, and Dr Kenneth Kinzler and Dr Bert Vogelstein for the β-catenin expression vector. We are grateful to Dr Susan Leitman and the Department of Transfusion Medicine, Clinical Center, National Institutes of Health, for their help in obtaining and processing peripheral blood leukocytes from healthy donors. We thank Dr Giovanna Tosato and Dr Mark Udey for critical review of the manuscript.
E. J. C. and S.-G. H. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jane B. Trepel, Medical Oncology Clinical Research Unit and Developmental Therapeutics Program, NCI, Bldg 10, Room 12N230, NIH, Bethesda, MD 20892; e-mail: trepel@helix.nih.gov.