Introduction
At diagnosis, approximately 80% of patients with follicular lymphoma (FL) have disseminated disease with one or more extranodal localizations (Ann Arbor stage IV), mostly corresponding to bone marrow (BM) involvement. Conventional chemotherapy is not curative nor does it substantially modify the natural course of the disease. Most patients with disseminated FL ultimately die from the disease, with a median survival time of 8 to 10 years.1 Innovative therapies have to be proposed to these patients. Some form of intensive therapy—for example, high-dose chemotherapy (HDT) or chemoradiotherapy followed by autologous or allogeneic hematopoietic stem cell transplantation—is an option. Recently, the autologous stem cell transplantation (ASCT) procedure has become easier and cheaper, and it has a mortality rate of below 5% and manageable morbidity. It could therefore be considered for patients with FL if it could be shown to improve survival. Allogeneic stem cell transplantation is another innovative approach, adding a graft-versus-lymphoma (GvL) effect. However, the high mortality rate of the procedure limits its indications. Nonmyeloablative allogeneic transplantation could increase the indications by decreasing the transplantation-related mortality (TRM).
Autologous stem cell transplantation
Clinical results
Progressing or relapsing patients.
Johnson et al2 have clearly shown that, after each subsequent relapse of FL, the response rate to treatment decreases and the progression-free survival (PFS) time shortens (Table1). This explains why most intensive therapies have been performed in patients with disseminated disease who have either not responded to initial conventional treatment or relapsed after one or more regimens of chemotherapy. The data from several retrospective series of ASCT in progressing/relapsing FL patients have been published. The main reports are summarized in Table2.3-17 They all indicate encouraging results, with a response rate higher than 90%, low (5%-10%) TRM, and long median relapse-free survival (RFS) and overall survival (OS) times. Nevertheless, most patients still relapse after ASCT, and cure has not been demonstrated. Besides, conclusions are difficult to draw on the basis of these reports because of the presence of one or more skewing factors, including different classifications of low-grade lymphomas, patients with and without histological transformation, the selection of patients who were suitable for ASCT, differences in conditioning regimens, the use of BM or peripheral blood stem cells, reinjection of purged or unpurged stem cells, and short follow-up periods.
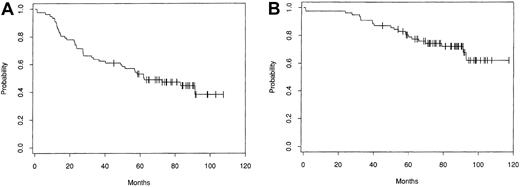
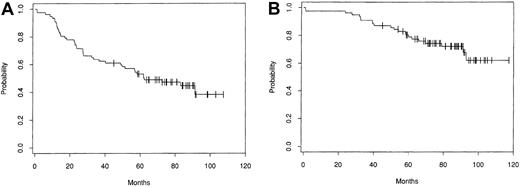
Disease-free and OS patterns from the largest single center series, which included 153 patients treated with intensive therapy followed by autologous bone marrow transplantation (ABMT) at the Dana Farber Cancer Institute (DFCI, Boston, MA), are shown in Figure1.6 Several groups have compared progression-free survival (PFS) and OS in patients treated with intensive therapy to that in matched historical controls treated with conventional chemotherapy. Patients treated with ASCT had a significantly better rate of freedom from second relapse5,8and, in some series8 but not in others,5better OS. None of these comparisons with historical control groups are case-controlled studies, and they suffer from a major skewing factor in that only those patients who were suitable candidates for intensive therapy, especially with respect to age, performance status, chemosensitivity, marrow infiltration, and response to chemotherapy, were selected.
Kaplan-Meier estimates of probability of disease-free survival (A) and overall survival (B) for 153 patients treated with ABMT for progressive/relapsing indolent follicular lymphoma at the DFCI (from Freedman et al6 with the authorization of the author and editor).
Kaplan-Meier estimates of probability of disease-free survival (A) and overall survival (B) for 153 patients treated with ABMT for progressive/relapsing indolent follicular lymphoma at the DFCI (from Freedman et al6 with the authorization of the author and editor).
Although these results clearly suggest that intensive therapy is probably superior to conventional chemotherapy in relapsing FL patients, only a randomized trial could confirm this hypothesis. Such a trial was launched in Europe for chemotherapy unpurged bone marrow or purged bone marrow (the CUP trial) but was stopped because of poor patient recruitment (89 randomized patients in 4 years). The trial showed a significant improvement in the progression/relapse rate in the ASCT group (44% in the chemotherapy arm as opposed to 32% in the intensive arm, P = .006) without any difference in OS.18
Several prognostic factors that significantly influenced PFS and/or OS after ASCT have been described: (1) age (with a threshold around 50 years)13; (2) B symptoms at diagnosis and/or on relapse6,19 and/or increased serum lactate dehydrogenase (LDH) levels20; (3) number of previous chemotherapy regimens11,13,21; (4) clinical status at the time of ASCT (patients who have resistant disease are less likely to benefit from ASCT than those with chemosensitive disease)4,11,13,20; (5) presence or absence of histological transformation (several groups have reported on small numbers of patients with histologically transformed FL treated with HDT).11,15,22-27 In the first report of high-risk patients with histologically transformed FL, poor performance status, and bulky or chemoresistant disease, the results were poor with only 1 patient of 10 still surviving one year after HDT.22 More recent series have reported better results (Table3).11,15,23,24,27 The European Bone Marrow Transplantation (EBMT) registry reported on 50 patients treated with HDT for histologically transformed FL.25 Median PFS after HDT was 13 months, and OS was 64% and 51% at 2 and 5 years, respectively. Results of HDT performed after histological transformation were not different from those of 200 matched, untransformed FL and 200 de novo cases of aggressive non-Hodgkin lymphoma (NHL) treated with HDT (P = .67 and .44).25 These results can be considered as better than those reported for patients treated with conventional chemotherapy.28 However, results of HDT in histologically transformed FL are too limited to allow any firm conclusions to be drawn.); (6) histological BM involvement at the time of stem cell harvest6; (7) the bcl-2-JH polymerase chain reaction (PCR) graft status after the purge procedure, if any was performed. The influence of a negative PCR bcl-2-JH rearrangement has been clearly demonstrated by retrospective analysis of patients who were treated with ABMT at the DFCI. PCR detected residual disease at the time of marrow stem cell harvest in 113 of 153 patients. After immunologically purged ABMT, BM PCR analysis showed no detectable disease in 48 patients (42%), whereas 65 (58%) remained PCR positive. Patients whose BM was negative for the bcl-2-JH rearrangement after purged ABMT experienced longer freedom from relapse (FFR) than those whose BM remained positive [6 of 48 relapses versus 49 of 65, and 8-year FFRs of 83% vs 19%, respectively,P < .001].)6; and, finally, (8) residual disease after ASCT. In the DFCI study, continued bcl-2-JH PCR negativity in blood or BM during follow-up was also strongly predictive of prolonged complete remission (CR) after ABMT (5 relapses among the 47 patients with persistently negative samples during follow-up compared to 36 of 39 relapses in patients with positive PCR in all BM samples after ABMT). PCR positivity after purging and in BM samples after transplantation were the only parameters associated with a poorer FFR after ABMT in a univariate analysis (P < .0001).6 Some studies have confirmed the prognostic influence of a negative bcl-2-JH PCR result after ASCT,29-31 but others showed a similar relapse rate whatever the PCR status.32 Real-time quantitative PCR evaluations that provide an accurate and reproducible estimate of the number of gene copies with a low carry-over contamination risk could account for these discrepancies.33 Using this technique as well as limiting dilution assays combined with 2-step PCR, relapses among 15 patients were associated with increasing numbers of circulating t(14;18)-positive cells and continuous CR with stable cell counts.34 For patients without any detectable chromosomal rearrangement, amplification of the third complementary determining region (CDR III) can be useful as a clonal marker for minimal residual disease.35 No relapse occurred in any of the 10 patients in whom HDT eradicated PCR-detectable lymphoma cells with this technique, whereas all 8 patients with PCR-detectable lymphoma cells after ABMT relapsed (P = .0002).35
In conclusion, intensive therapy with ASCT should be considered for patients younger than 60 to 65 years with chemosensitive relapsing FL, especially if they have poor prognosis factors at relapse.8 36 This conclusion holds true even for patients with histological transformation, which is, per se, an adverse prognostic factor.
ASCT as first-line therapy.
A few Phase II trials of intensive therapy after maximum response to conventional chemotherapy have been reported in FL patients.4,16,37-44 The results are summarized in Table4. In a nonrandomized comparison of 26 patients treated with a high-dose CHOP-like regimen (cyclophosphamide, vincristine, prednisone, and doxorubicin) followed by ABMT, and 14 patients treated with the same regimen without ABMT, there was a significant improvement in DFS in the ABMT group (a DFS of 54% at 36 months in the group treated with ABMT versus 26% in the group treated with conventional chemotherapy) without any difference in OS.38 Horning et al have compared the survival of 37 patients treated with ABMT after a maximum response to conventional chemotherapy and 61 matched historical controls in CR or unconfirmed CR after conventional chemotherapy. With a median follow-up of 6.5 years, the estimated 10-year survival rate was 86% in the transplantation group and 59% in the conventional chemotherapy group (P = .074).39 Recently, Sanz-Rodriguez et al45 has reported on a retrospective comparison of a group of patients initially treated with ASCT and a group of matched controls treated with a conventional treatment (cyclophosphamide, vincristine, and prednisone [CVP] chemotherapy associated with interferon alpha). With a median follow-up of 87 months and 112 months, respectively, there was no difference in PFS (49.5% and 48%, respectively) or OS (76% and 71%).45 In another nonrandomized comparison, when survival was calculated from the date of diagnosis, there was no difference in event-free survival and OS between a group of 33 patients treated with ASCT at the outset and a group of 22 patients treated with ASCT only on relapse.16
Several large Phase III trials are under way to test the potential benefits of initial ASCT in FL patients with adverse prognostic factors. The German Lymphoma Study Group is comparing conventional treatment (mitoxantrone-chlorambucil-prednisone followed by interferon alpha maintenance) and HDT (4 to 6 cycles of CHOP followed by DexaBEAM chemotherapy, then ASCT with a cyclophosphamide-TBI conditioning regimen). As of May 1999, 288 patients have been randomized. With a very short median follow-up of 17 months, there has been a significant improvement in DFS for patients treated with HDT, with a 32% risk reduction (P = .001) without any difference in OS.46 Two other trials conducted by the GELA group (Groupe d'Etude des Lymphomes de l'Adulte) and the GOELAMS group (Groupe Ouest-Est pour l'Etude des Leucémies Aigues et des Maladies du Sang) are comparing a conventional chemoimmunotherapy program (a CHOP-like regimen concomitantly associated with interferon alpha) and intensive therapy with the transplantation of either unselected (GELA trial) or purged (GOELAMS trial) peripheral stem cells. So far, 400 patients have been included in the GELA trial. The first interim analysis of the GOELAMS trial on 136 patients has been recently reported. The 4-year PFS was 61% in the HDT group and 27% in the conventional group (P = .027), without significant difference in 4-year OS (83% vs 80%).47
In conclusion, because of the poor outcomes associated with conventional chemotherapy in FL patients with disseminated disease and adverse prognostic factors, intensive therapy with ASCT is a potential improvement and can be considered for patients under 60 years of age.48 Nevertheless, because its efficacy has not yet been proven, this treatment should be considered only in the context of a clinical trial.
Optimal procedure
Collection and purging of stem cells.
Autologous peripheral blood stem cell transplantation (APBSCT) has progressively replaced ABMT since retrospective analyses49,50 and at least 3 randomized studies51-53 demonstrated that the time taken for neutrophils or platelet recovery, the duration of the hospital stay, the incidence of infectious complications, and the overall cost are less after APBSCT than after ABMT. Besides, the rate of contamination by lymphoma cells is similar with both harvest procedures,54,55 and posttransplantation PFS and OS rates are similar.50 Finally, immune reconstitution is faster after APBSCT than after ABMT.56
Leukapheresis for peripheral blood stem cell collection may be performed after either conventional chemotherapy followed by granulocyte colony-stimulating factor (G-CSF) treatment57 or G-CSF treatment alone.58,59 In a nonrandomized comparison between these 2 procedures, there were no differences in transplantation outcome (stem-cell engraftment, risk of relapse).60,61 A combination of stem cell factor (SCF) and G-CSF resulted in the collection of a higher number of stem cells62,63 and has to be more extensively evaluated in patients in whom the harvest is insufficient after chemotherapy plus G-CSF or G-CSF alone.64 65
Several factors influence the number of stem cells that can be collected, including BM involvement,66 the number of previous chemotherapy regimens,66-71 the time interval between collection and the last cycle of chemotherapy,68,72 previous treatments with radiation and/or cytotoxic drugs (especially the total cumulative dose of alkylating agents66), and fludarabine treatment in some series.73-75
In general, stem cell collections are poorer and the rate of failure to collect a sufficient number of CD34+ stem cells to perform ASCT is greater in low-grade lymphomas than in aggressive lymphomas. The reasons for this are not understood.68
Lymphoma cells often contaminate BM69,76,77 and peripheral blood54 stem cell collections and may contribute to relapse after ASCT.78 79 Thus, purging collected stem cells ought to help decrease contamination and lower the risk of relapse. Several procedures have been proposed to purge BM and/or peripheral blood stem cells.
(1) In vitro treatment with cytotoxic agents (4-hydroperoxycyclophosphamide or mafosfamide) has deleterious effects on normal hematopoietic stem cells.19
(2) In vitro treatment with a cocktail of anti–B-cell monoclonal antibodies (anti-CD20, anti-CD10, and anti-CD19) and fresh rabbit complement,80 or passing the blood81 or BM stem cells82 over a column containing anti–B-cell antibodies bound to immunomagnetic beads. This procedure significantly decreases the number of lymphoma cells in a BM graft as assessed by PCR amplification of the bcl-2-JH rearrangement.83 In the DFCI study, 57 of 114 (50%) patients with PCR-positive BM became negative after purging.80 However, in another study, 18 of 21 (86%) patients remained positive after treatment with a cocktail of 4 monoclonal antibodies.84
(3) In vitro positive selection of CD34+ hematopoietic stem cells,85,86 which produces a 3- to 4-log reduction in tumor contamination.86,87 Although hematopoietic recovery is not delayed88 after ASCT with CD34-selected stem cells, slow T- and B-cell recovery may increase the risk of opportunistic infections.89,90 Nevertheless, CD34-selected grafts may remain contaminated with lymphoma cells.69 91
(4) Selection of the CD34+CD19− subpopulation that is less contaminated than the CD34+fraction.91 However, even a CD34+CD19−CD20− stem cell population may contain the bcl-2-JH rearrangement.92 93
(5) Ex vivo expansion of the hematopoietic stem cells using a combination of growth factors such as SCF, interleukin 3, interleukin 6, G-CSF, and Flt-3 ligand that accelerates hematological reconstitution after HDT.94,95 This ex vivo expansion of bcl-2-JH–negative CD34+ progenitor cells does not induce the expansion of lymphoma progenitor cells,93 96 but the data are scarce and need confirmation.
(6) More recently, an in vivo purging method has been proposed using a treatment with rituximab before stem cell collection.97,98The results of the first studies are encouraging. When rituximab is combined with chemotherapy, lymphoma cells became undetectable in peripheral blood stem cell collections in 75% (9 of 12),99 93% (14 of 15),97 or 100% (7 of 7)98 of informative cases, a significantly higher rate than in historical control (26%98 to 40%97). However, a single rituximab infusion 3 to 5 days before stem cell collection is insufficient since 10 of 11 stem cell grafts remained PCR-positive.100 Anti-CD20 monoclonal antibody in vivo purging does not have a deleterious effect on stem cell collection, engraftment time, or transplantation outcome.101 102 A trial conducted by the European Bone Marrow Transplantation group is currently assessing the role of rituximab in ASCT for relapsing FL in a 4-arm randomized trial, rituximab being (1) not given in a control group; (2) given before stem cell collection; (3) given after ASCT; and (4) given before collection and after ASCT.
The clinical benefit of the purge procedure remains to be established. After a reduction of lymphoma cell numbers to below a threshold level of 1 lymphoma cell of 104 to 105 mononucleated cells, the risk of relapse is decreased in some studies5,6,76 but is similar to the relapse rate after unpurged ASCT in other retrospective analyses32,103 or in a comparison of registry data.25 Only a randomized study could confirm the role of purging. One such trial has been initiated but was stopped because of poor patient recruitment (the CUP trial),18 and it is unfortunately unrealistic to undertake another trial comparing ASCT with or without purging of the stem cell graft. In vitro purging is an expensive and cumbersome technique. Treatment before stem cell collection with rituximab or another anti–B-cell antibody such as humanized anti-CD22 (hLL2)104is more promising, given that it is both an anti-lymphoma treatment and an in vivo purging procedure. Confirmatory data are needed.
Conditioning regimens.
Schematically there are 2 types of conditioning regimens for ASCT: (1) those based on chemotherapy alone, such as CBV (cyclophosphamide, carmustine, etoposide),105 BEAM (carmustine, etoposide, cytarabine, melphalan),13 or BEAC (carmustine, etoposide, cytarabine, cyclophosphamide)7; and (2) those using a chemoradiotherapy combination with total body irradiation (TBI).5 6
The efficacy of very low-dose TBI (1.0 to 2.0 Gy in 0.25 to 0.50 Gy fractions) was demonstrated in low-grade NHLs some 25 years ago.106 Usually, TBI as a conditioning regimen before ASCT is administered in 6 fractions of 2 Gy each (fractionated TBI) rather than as a single dose of 12 Gy, which is more toxic.107TBI is usually combined with high-dose cyclophosphamide5,6and, in some studies, VP16.3 108
There is no randomized study comparing conditioning regimens in FL. Several retrospective comparisons have been reported, but the results are conflicting. Some report better overall survival with chemotherapy alone,109 whereas the opposite was found in others with better OS when fractionated TBI was used.15 In some studies, no difference was observed.16 110
Treatment with a murine anti-CD20 monoclonal antibody coupled with a high dose of 131I may be an interesting alternative, given that it allows delivery of a significantly higher dose of radiation to the tumor without too much damage to normal organs. The results of a preliminary phase I-II trial where this high-dose radioimmunotherapy was combined with cyclophosphamide and VP16 were promising. Doses up to 75 Gy could be delivered to affected sites without exceeding doses of 20 Gy to the lungs or other radiosensitive organs.111However, such a conditioning regimen can be given only in a limited number of centers. Other Phase I-II trials using high-dose radioimmunotherapy with humanized anti-CD20 and anti-CD22 MoAbs are in progress.112
Treatments after ASCT
As previously mentioned, residual disease is often present after ASCT and may contribute to relapse.29,113Several post-ASCT adjuvant treatment modalities have been tested to eradicate residual disease and decrease the relapse rate.114 115
MoAbs conjugated to toxins such as the anti–B-cell immunotoxin anti–B4-blocked ricin have proven disappointing as an adjunctive therapeutic modality after ABMT.116 Rituximab used as an adjunctive immunotherapy after PBSCT is well tolerated but can induce transient neutropenia100,102 or delayed immune reconstitution, although without any increase in infectious complications.117 However, the ultimate determination of its efficacy will require the results of ongoing studies.
Immune-mediated antitumor therapy after ASCT has been recently reviewed in Blood.111
(1) Cytokines may be of therapeutic value as their endogenous production is decreased after ASCT,118,119 and their administration soon after ASCT120 can activate cytotoxic T cells directed against lymphoma cell antigens.121-123 A phase II trial of rIL-2 (3 to 6 IU/m2/d × 5 d/wk for 4 weeks) combined with interferon (IFN) alpha (3 × 106U/d × 5 d/wk) given after hematological recovery in relapsing lymphoma patients treated with ABMT has shown a significant improvement in survival when compared to historical controls without post-ABMT immunotherapy (4-year survival of 90% vs 46%) and resulted in a significant decrease in relapse rate (20% vs 46%).124However, the hematological and systemic toxicities of IL-2 restrict its usefulness after ASCT.
(2) Cyclosporine A (CyA), which prevents the thymic clonal deletion of autoreactive T lymphocytes, can increase the incidence of autologous graft-versus-host disease (GVHD),125 a syndrome similar to allogeneic cutaneous GVHD, which is observed in 5% to 10% of patients.126 Encouraging results have been reported in a phase II trial of CyA (in combination with αIFN) given after ABMT for NHL,127 but confirming data are lacking.
(3) An anti-idiotype immune reaction may be induced by infusions of specific idiotypes coupled with a potent adjuvant128-130such as granulocyte-macrophage colony-stimulating factor (GM-CSF),131 the C fragment of tetanus toxin,132 or ex vivo–pulsed dendritic cells that act as antigen-presenting cells133,134 and considerably increase the immune response to idiotype proteins. About 50% of patients develop a cellular and/or an antibody-mediated anti-idiotypic reaction, and in these patients the risk of relapse after ASCT is significantly decreased.130,135,136 Tumor vaccination can be performed either after ASCT with minimal residual disease, or before stem cell harvest, which prevents posttransplantation immunosuppression. Using a syngeneic murine bone marrow transplantation (BMT) model, the tumor-free survival rate was greater after vaccination with irradiated GM-CSF–producing autologous tumor cells in the post-BMT period than in the nontransplantation context.137 Evaluating the potential clinical benefits of these modalities requires further studies.
Complications after ASCT
Relapse.
Relapses after ASCT often occur in sites of previous disease (78% among 33 patients who relapsed after ABMT for FL).138 In some retrospective analyses, posttransplantion radiation therapy improved event-free survival when compared to historical controls.19
Relapses after ASCT often have the same pathological aspects as before intensive therapy,138 but patients may relapse either with histological transformation138 or, conversely, after ASCT for transformed FL with a low-grade FL (“down-staging”).24 Relapses after ASCT tend to respond to conventional chemotherapy138 and/or to monoclonal anti-CD 20 antibodies.139 140
Myelodysplastic syndromes/acute leukemias and other secondary tumors.
Myelodysplastic syndrome and acute leukemia (MDS/AL) are the major long-term complications of ASCT for FL.21,141-145 Their 5-year incidence for NHL following ASCT has been estimated to be 8% to 14%5,144-146 and may be higher in low-grade as opposed to aggressive NHL.147 The prognosis is very poor, with a median survival of 9.4 months.148
Some factors may increase the risk of MDS/AL, such as advanced age,142,144,149 prior fludarabine therapy,150the total cumulative dose of alkylating agents,141radiation therapy before ASCT,148,149,151 the use of TBI in the conditioning regimens142,144,149 or stem cell priming with high-dose VP-16,151 the use of peripheral blood stem cell (PBSC) rather than BM progenitors,144 or the median number of cells/kg reinfused.148 Cytogenetic BM analysis before ABMT may allow prediction of the risk of MDS/AL.149,152 The incidence of acquired karyotypic instability observed during serial assessment after ASCT may greatly exceed the clinical incidence of MDS/AL (13 instances of karyotypic instability versus 2 cases of MDS/AL in a total of 22 patients).153
Allogeneic stem cell transplantation
When the follow-up time is prolonged, the pattern of relapses after ASCT appears as continuous, without any plateau. Moreover, ASCT is not feasible when the marrow reserves are inadequate, and it is poorly effective for patients with refractory disease or extensive BM involvement. In such situations, the curative value of allogeneic SCT can now be assessed by analyzing a greater amount of information.
Clinical results
An analysis of 215 patients reported on in 8 different studies,9,10,154-160 including an observational study of 113 patients collected from 50 centers participating in the International Bone Marrow Transplant Registry (IBMTR),155is summarized in Table 5. From these results, it appears that standard allogeneic BMT was offered mainly to patients with advanced, chemotherapy-resistant disease with extensive BM involvement and after extensive prior therapy in most cases. In these conditions, a high TRM (around 40%) is counterbalanced by the very potent antitumoral effect of allogeneic BMT, allowing high lymphoma-free and OS rates (49% to 80%) due to a low recurrence rate (0% to 20%).10,154-157,159 Indeed, if the registry follow-up method is accurate, only 1 relapse occurred among 33 patients monitored for more than 2 years.155 Interestingly, and in contrast to the outcome with many other malignancies, a full response and long-term disease-free survival have been observed even in patients with refractory disease.10,154,158,161 These data indicate that patients with refractory or recurrent low-grade lymphoma have a better chance of cure when they are treated with allogeneic BMT than when they are treated with ASCT. However, in the IBMTR study, a higher survival rate was associated with good pretransplantation performance status, chemotherapy-sensitive disease, use of a TBI-containing conditioning regimen, and age younger than 40 years.155 Better patient selection and earlier transplantation could improve outcomes.159,162 In one study, patients with sensitive disease not heavily treated have a low TRM rate (14% vs 86% in patients with resistant disease).162 In contrast to ASCT, the long-term pattern of which does not reach a plateau, allogeneic SCT has a curative potential as witnessed by the fact that long-term remission (as determined by molecular analysis) was observed in 3 of 7 evaluable patients.163
Graft-versus-lymphoma effect
The lower relapse rate after allogeneic BMT compared with autologous BMT is likely due to the lack of BM contamination of the graft and to an immune-mediated graft-versus-lymphoma (GvL) effect. Evidence of a GvL effect come from 2 things.
It can come from the response to persistent disease following allogeneic BMT, which is observed at the same time as the development of active acute or chronic GVHD. In an EBMT retrospective study, a lower recurrence rate was found in patients with chronic GVHD compared with those without GVHD (0% vs 35%,P = .02),164 although this relationship is still controversial.155 However, the incidence of GVHD in FL seems to be remarkably high (Table 4) compared with that in leukemia patients, probably because FL recipients and donors tend to be older.156
Evidence of a GvL effect also comes from induction of remission after cessation of immunosuppression or donor-leukocyte infusion (DLI).156,165 Quantification of the disease activity by real-time PCR with an internal fluorogenic probe for the t(14;18) translocation has been used to investigate the GvL effect of donor leukocytes given for relapsed follicular NHL after allogeneic BMT.166 Cytoreduction after DLI may take several months to occur.154 159
So, the better outcome after allogeneic BMT in patients with FL compared to that in patients with high-grade subtypes may be explained not only by the more indolent course of the disease and the better condition of patients, but also by the GvL effect.
Optimal procedure
Modalities to reduce the toxicity of the conditioning regimen and the risk of GVHD and to separate the latter from the beneficial GvL effect are required.
Conditioning regimens.
The high response rate after allogeneic or autologous BMT is due at least in part to the intensive conditioning regimen. Several attempts have been made to improve the efficiency and decrease the toxicity of such regimens, but no randomized data are available to assess their respective value. In the IBMTR survey, a higher survival rate was associated with the use of a TBI-containing conditioning regimen. However, only 18% of these patients received non-TBI regimens, and it is possible that unknown factors may have skewed the results.155 An increase in the intensity of the conditioning regimen in 15 patients was associated with an increase in severe acute GVHD despite T-cell depletion of the graft, probably due to supplementary impairment of the recipient's immune status.156
Source of allogeneic hematopoietic stem cells.
Apheresis products containing G-CSF–mobilized peripheral blood cells can be safely obtained from allogeneic donors. Seven recently reviewed167 randomized trials in various hematological neoplasias have demonstrated that neutrophil and platelet recovery and immune reconstitution were faster with peripheral blood cells than with marrow.168 The risk of acute GVHD is not increased, even though the number of T cells in the blood is 10-fold higher than that in the BM. Moreover, in 2 of these studies, the incidence of chronic GVHD was higher among patients who received peripheral blood cells. This procedure may offer advantages in terms of OS and DFS, especially in patients with high risk of relapse.
GVHD prophylaxis.
The incidence of acute and chronic GVHD following allogeneic BMT can be reduced by several modalities.
(1) T-cell depletion. T-cell depletion, performed in vitro by elutriation or monoclonal antibodies or in vivo by the addition of CAMPATH-1G, may decrease the high TRM rate (4.5% of 22 patients treated with alloBMT for relapsed indolent lymphoma at the DFCI).160,169 Conversely, it may increase graft failure (10%), delay engraftment, mixed chimerism, disease recurrence, and Epstein-Barr virus–associated lymphoproliferative disorder.10,156 Delayed engraftment secondary to T-cell depletion might be overcome by reinfusion of the fraction of small CD34+ cells that coseparate with the small lymphocytes.170 However, decreasing GVHD inhibited the GvL effect. Thus, after T-cell–depleted allogeneic BMT, 4 of 5 patients with clinical CR and informative PCR for the bcl2/IgH translocation remained PCR-positive after transplantation.171
(2) Delayed administration of small, graded doses of donor lymphocytes172 and the identification and reinjection of specific lymphocyte subtypes.169 For example, CD6+ T-cell–depleted allogeneic BMT resulted in 54% and 59% DFS and OS, respectively, in poor-risk relapsing FL over a median follow-up time of 30 months.169 T-cell–depleted allogeneic BMT followed by donor lymphocyte infusion (DLI) appears to be effective in poor-risk patients with relapsing FL.156Preliminary results of a randomized trial suggest that CD8 depletion of DLI reduces the incidence of GVHD associated with DLI without compromising antitumor activity, conversion to donor hematopoiesis, or immunologic recovery.173
(3) Transduction of the donor lymphocytes with a suicide gene, such as the herpesvirus thymidine kinase, that confers sensitivity to ganciclovir and allows the effector cells to be eradicated if GVHD occurs.174
(4) Other immunosuppressive agents such as mycophenolate mofetil, FK506, CAMPATH-1, or monoclonal IL-2 receptor are currently being evaluated.
Nonmyeloablative preparative regimen.
An alternative strategy to reduce TRM and improve the age cutoff is to use a lower dose, nonmyeloablative, preparative regimen designed not to eradicate the malignancy but to provide sufficient immunosuppression to achieve engraftment of an allogeneic graft, thus allowing the development of an immune GvL effect. Results from more than 600 patients with various hematological malignancies have been reported, including 18% with NHL.175-186
Nonmyeloablative preparative regimens contain fludarabine and 200 cGy TBI,175 cyclophosphamide,161 busulfan with181,182 or without176,185anti–T-lymphocyte globulin, melphalan with184 or without176,183,186 CAMPATH-1H or carmustine.177 Thiotepa-based regimens with fludarabine,180 cyclophosphamide,179 or both178 are also investigated. GVHD prophylaxis consisted in cyclosporine A and methotrexate,176-179tacrolimus,161,187 or mycophenolate mofetil and175,185 or T-cell depletion.186 If residual malignant cells or mixed chimerism are detected, donor lymphocytes are infused to potentiate the GvL effect. Graft rejection occurred in < 5%, whereas the frequency of mixed chimerism ranged from low175 to 40%184 of patients. TRM occurred in between 10% and 40% of cases. Median granulocyte and platelet nadirs are high, 0.3 and 60 G/L, respectively, in one study,175and platelet and red blood cell transfusions are significantly decreased.188 Outpatient allografting has even been performed without subsequent hospitalization.189 However, an increased incidence of early viral infection, especially cytomegalovirus (CMV),190 and late gram-negative infection was reported in nonneutropenic patients.191 GVHD remains the major drawback to this procedure and the main cause of death before day 100. The risk for grade III-IV GVHD in those patients whose median age was older than 50 years remains high (10% to 42%). The addition of in vivo CAMPATH-1H to melphalan and fludarabine significantly reduces TRM and the risk of GVHD with marrow transplants from both related and unrelated donors.184Nonmyeloablative allogenic transplantations are also performed with unrelated donors185,192,193 and in patients who relapsed after autologous BMT.177,178,192,194 195
FL should be a good indication for these nonmyeloablative regimens, as its long natural history gives time to exploit the potentially beneficial GvL effect. So far, few results of nonmyeloablative allogeneic BMT are available in FL161,187,195-200 (Table6). Khouri et al161 first reported on 6 cases of heavily pretreated FL and 9 similar cases of CLL. Eleven of the 15 patients had durable engraftment with 50% to 100% donor cells one month following transplantation, converting to 100% over the next 2 months either spontaneously or after DLI. Hematopoietic recovery was prompt, and 8 of the 11 patients with engraftment achieved CR.161 Maximal responses were slow to develop and gradually occurred over a period of several months to one year. Subsequently, nonmyeloablative allogeneic BMT has been reported in others cases of heavily pretreated, high-risk lymphoma. Evidence for GvL activity have been observed as residual posttransplantation active disease declined with the onset of chronic GVHD161,187,197,198 or after DLI infusion.187,196,197 High doses of rituximab (375 mg/m2 on day −6 and 1000 mg/m2 on days 1, 8, and 15 after transplantation) have been administered to enhance tumor control early after transplantation to allow time for the GvL effect to occur.187 This improves the antitumoral activity of the fludarabine-cyclophosphamide conditioning, as 8 of 8 patients in partial response before transplantation achieved CR, 2 of them concomitant with the withdrawal of tacrolimus and subsequent development of GVHD. At a median follow-up of 21 months, 84% of patients are still in CR despite advance recurrent disease. The low TRM allows to perform nonmyeloablative allogenic PSCT after ABMT conditioned by BEAM therapy.196 The high response rate (9 patients in partial remission after autologous grafting achieved CR after mini-allografting) in this group of heavily pretreated patients is promising. However, acute and chronic GVHD continue to be a problem,195-200 and controlled prospective trials are warranted to assess if nonmyeloablative alloBMT can indeed improve outcomes of patients with FL.
So far, given the encouraging results of allogeneic BMT in patients with advanced and refractory disease, it would be reasonable to consider allogeneic BMT in patients younger than 55 years with disease either refractory or relapsing early after conventional chemotherapy. Nonmyeloablative allogeneic transplantation might be beneficial to patients with FL who would otherwise have been excluded because of age, pre-existing organ dysfunction, or previous treatment.
Conclusion
There is not yet a clear demonstration that HDT followed by ASCT improves the OS of patients when used as first-line treatment. However, the results of several trials will soon allow determination of its role. For patients with chemoresistant disease, allogeneic SCT must be considered if patients have an HLA-identical sibling. Conventional allogeneic SCT is probably the best procedure for patients younger than 50 years. For older patients, nonmyeloablative treatment probably yields a similar cure rate with a lower TRM. For patients who relapse with disseminated disease after a complete or partial response, salvage chemotherapy followed by autologous SCT improves response rate, survival from second relapse, and probably overall survival compared with conventional therapy. Although there is no clear demonstration that stem cell harvest purging decreases the relapse rate and improves survival, in vivo treatment by anti–B monoclonal antibodies such as rituximab are an attractive option, because they have both an antilymphoma and a purging effect on blood and BM. These results must be confirmed by ongoing clinical trials. Despite these improvements, many patients still relapse, and additional therapies must be tested. Posttransplantation immunotherapy is a promising approach.
References
Author notes
Philippe Solal-Celigny, Centre Jean Bernard, 9 rue Beauverger 72 000 Le Mans, France; e-mail: p.solal-celigny@noos.fr.