The type I hexokinase (HK-I) deficiency is a rare red cell enzymopathy associated with hereditary nonspherocytic hemolytic anemia. To date, only 16 affected families have been reported.1 We report here prenatal diagnosis and molecular analysis of a Japanese family of HK deficiency. The proband's mother was hospitalized at 29 weeks of gestation due to intrauterine growth retardation (IUGR) of the fetus. To investigate the cause of IUGR, fetal blood was sampled at 30 weeks of gestation, showing that the fetus had severe hemolytic anemia: hemoglobin level, 37g/L; reticulocyte percentage, 42.1%; and indirect bilirubin level, 99.2 μM. There was no evidence of fetomaternal blood type incompatibility. The fetus had a normal karyotype: 46XX.
Red blood cells (RBCs) and reticulocytes were purified by using alpha- and microcrystalline cellulose as described,2 and RBC enzymes were measured by standardized protocols.3 RBC enzyme activity was compared to those in cord blood of the extremely low–birth weight infants, whose weights were below 1000 g.4 The HK activity of the proband (0.40 IU/gHb) was decreased to about 12% of the control mean value (3.28 IU/gHb), whereas activities of other enzymes such as glucose phosphate isomerase (GPI), pyruvate kinase (PK), and glucose-6-phosphate dehydrogenase (G6PD) were 95.2, 30.4, and 18.3 IU/gHb, respectively, almost comparable to the controls. RBC HK activity of the parents was also decreased to 65% and 73% of the normal mean value (1.01 IU/gHb), suggesting that they are heterozygous for HK-I deficiency.
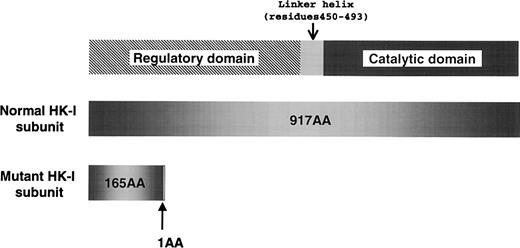
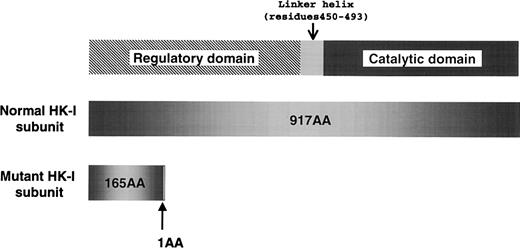
To elucidate the molecular lesion responsible for the HK deficiency, we analyzed the reticulocyte RNA of the proband, and identified aberrant HK-I mRNA, which lacks exons 5 through 8. The skipping of those exons consequently caused a frameshift mutation; as a result, the mutant HK-I polypeptide lacked 82% of the C-terminal amino acids due to premature termination of translation (Figure 1). Sequencing of the polymerase chain reaction (PCR) products containing the break point showed that the proband was homozygous for a 9490-bp intragenic deletion flanking exons 5 through 8 of the HK-Igene. The deleted sequence corresponds to nucleotide number 12937-22425 of the human chromosome 10 clone RP11-404C6 (GenBank accession AC016821). The break points are located 482-bp upstream of the 3′ splice site of intervening sequence 4 (Ivs 4) and 454-bp downstream of the 5′ splice site of Ivs 8. Between the sequences derived from Ivs 4 and 8, an additional 10 nucleotides of unknown origin (tgggcaacat) were found. Although the parents have no apparent consanguinity, they were found to be heterozygous with an identical deletion confirmed by sequencing of the break points.
The domain structure of type 1 hexokinase (HK-I) and a premature translational termination caused by the intragenic gene deletion that was identified in the Japanese HK-I variant.
The domain structure of type 1 hexokinase (HK-I) and a premature translational termination caused by the intragenic gene deletion that was identified in the Japanese HK-I variant.
These findings suggest that null expression of the HK-Igene has deleterious effects on red blood cell metabolism, resulting in fetal death due to severe hemolytic anemia. Magnetic resonance image of the head of the stillborn infant showed injury of cerebral white matter, which was represented as periventricular leucomalacia (PVL). The lesion occurred after prolonged exposure of the brain to either hypoxia or ischemia.5 Whether PVL observed in this case was caused by either a direct effect of HK-I deficiency in white matter or a secondary phenomenon of severe anemia remains unclear.
We thank J. Oka and A. Sakuma for their excellent technical assistance.