Abstract
Dendritic cells (DCs) possess numerous dendrites that may be of great advantage to interaction with T cells. However, it has been poorly understood how the dendritic morphology of a DC is controlled. In the present study, using a murine spleen-derived DC line, we analyzed effects of CCR7 ligands, CCL19 and CCL21, on dendritic morphology. Mature DCs, but not immature DCs, showed vigorous migration to either CCL19 or CCL21. CCL19 also rapidly (within 30 minutes) induced marked extension of dendrites of mature DCs that was maintained at least for 24 hours. On the other hand, CCL21 failed to induce rapid dendritic extension, even though a modest dendritic extension of mature DCs, compared to that by CCL19, was induced 8 or 24 hours after treatment with CCL21. In addition, pretreatment with a high concentration of CCL21 significantly inhibited the rapid dendritic extension induced by CCL19. Thus, it is suggested that CCL19 and CCL21 exert agonistic and antagonistic influences on the initiation of dendritic extension of mature DCs. The CCL19-induced morphologic changes were completely blocked by Clostridium difficiletoxin B that inhibits Rho guanosine triphosphatase proteins such as Rho, Rac, and Cdc42, but not by Y-27632, a specific inhibitor for Rho-associated kinase. These findings suggest that Rac or Cdc42 (or both), but not Rho, are involved in the CCL19-induced dendritic extension of mature DCs.
Introduction
Dendritic cells (DCs) are potent antigen-presenting cells (APCs) and play major roles in the regulation of immune responses to various antigens.1-3 DCs exhibit a unique ability to activate naive T cells. The antigen-presenting capability of DCs depends on their maturational stage. Immature DCs are present in almost all tissues and internalize antigens from the environment with high efficiency, but exhibit no considerable APC activity to naive T cells. On encountering foreign antigens, these immature DCs are rapidly activated and mature by complex processes.2 3 The mature DCs highly express major histocompatibility (MHC) and costimulatory molecules including CD80, CD86, and CD40 on the surface, which are associated with the capability of potent antigen presentation.
During the maturational processes, DCs also change surface expressions of chemokine receptors to migrate from each peripheral tissue to the regional lymph nodes (LNs). Chemokines are small proteins, with molecular weight around 10 kDa, that regulate the migration and the subsequent distribution of leukocytes.4-8 Chemokines are divided into at least 4 subfamilies (CXC, CC, C, and CX3C) depending on the number of cysteines and the space between the first 2 cysteines. These chemokines bind G protein-coupled receptors with 7-transmembrane domains.9-11 On mature DCs only high densities of CCR7, which is a chemokine receptor for CCL19 (Epstein-Barr virus–induced receptor ligand chemokine [ELC]) and CCL21 (secondary lymphoid tissue chemokine [SLC]), are expressed.12-14 Both CCL19 and CCL21 are constitutively and highly expressed in LNs and spleen.15,16 Recently, it has been reported that mature DCs fail to migrate into the regional LNs and, thereby, adaptive immune responses are abolished in CCR7-knockout mice.17 Thus, interaction between CCR7 and its ligands, CCL19 and CCL21, is an essential process for migration of mature DCs into the regional LNs. However, it has been totally unclear whether CCL19 and CCL21 play some roles in the regulation of the morphologic, phenotypical, and functional properties of mature DCs.
Rho guanosine triphosphatase (GTPase) proteins such as Rho, Rac, and Cdc42 belong to Ras superfamily and act as molecular switches to control cellular processes by cycling between active (GTP-bound) and inactive (GDP-bound) states.18 Rho GTPase proteins control the organization of the actin cytoskeleton. Indeed, Rho, Rac, and Cdc42 have been characterized well as morphologic regulators in fibroblasts.19-23 Rho is involved in the bundling of actin filaments into stress fibers and the clustering of integrins and associated proteins into focal adhesion complexes. Rac promotes actin polymerization at the cell periphery to form lamellipodial extension and membrane ruffles. Cdc42 triggers actin polymerization to form filopodia or microspikes. Thus, it seems that Rho GTPase proteins are involved in various morphologic changes in cells.
Mature DCs possess numerous dendrites, which may be of great advantage in interactions with T cells. However, it has been poorly understood how the dendritic morphology of DCs is controlled. Stable DC lines are needed to elucidate the molecular mechanisms underlying the regulation of dendritic morphology during DC activation. Winzler and colleagues24 established a growth factor-dependent immature DC line from splenocytes of adult C57BL/6 mice. Although this DC line showed an immature phenotype, various activating signals such as living bacteria, lipopolysaccharide (LPS), or cytokines including tumor necrosis factor-α (TNF-α) and interleukin 1 promoted maturation of the precursor line. Using a similar in vitro differentiation system of DCs, a number of important findings have been reported and verified.25-30 We also previously established a homogeneous immature DC line (BC1) from BALB/c splenocytes.31 In the present study, we analyzed the effect of chemokines CCL19 and CCL21 on dendritic morphology using the BC1 line. We demonstrate that CCL19, but not CCL21, induces rapid dendritic extension of mature DCs. It has been suggested that Rac and Cdc42, but not Rho, are involved in the CCL19-induced dendritic extension of mature DCs.
Materials and methods
Reagents
Recombinant murine granulocyte-macrophage colony-stimulating factor (GM-CSF) was purchased from Peprotech (London, United Kingdom). Recombinant murine CCL19 and CCL21 were purchased from Genzyme (Cambridge, MA) and R & D Systems (Minneapolis, MN), respectively (both chemokines were produced using Escherichia coli). Pertussis toxin (PTX) and LPS were purchased from Sigma Chemical (St Louis, MO).Clostridium difficile toxin B and Y-27632 were purchased from Calbiochem (La Jolla, CA).
Preparation of DCs
The DC line (BC1) from BALB/c splenocytes was generated as described in our previous report.31 BC1 cells were cultured and expanded in Iscove modified Dulbecco medium (IMDM) containing 10% fetal calf serum (FCS), 30% NIH/3T3 supernatant, and 10 ng/mL mouse recombinant GM-CSF (henceforth referred as R1 medium).24,31 BC1 cells are CD11c+, major histocompatibility complex (MHC) class I+, MHC class II+, CD80+, and CD86+.31 Although BC1 cells show an immature phenotype, various activating signals such as TNF-α and LPS promote maturation of this precursor line. The maturated BC1 cells highly express MHC and costimulatory molecules and have potent allostimulatory capability compared with immature ones.31 In the present study, BC1 cells (2 × 105 cells/mL) treated with 5 μg/mL LPS for 24 hours were used as mature BC1 cells.
Spleen-derived DCs (SPDCs) were generated from C57BL/6 (B6) splenocytes. B6 female mice were purchased from Japan SLC (Hamamatsu, Shizuoka, Japan). Spleens were removed from B6 mice (6-9 weeks of age) and single-cell suspensions were prepared by passing through a stainless mesh. Erythrocytes were lysed by treatment with ammonium chloride. Remaining unfractionated cell populations were plated at a density of 1 × 106 cells/mL in R1 medium. Cultures were fed with fresh R1 medium every 3 to 4 days. On day 14 of the culture, suspended and weakly adherent cells (DC-enriched fraction) were collected by treatment with 3 mM EDTA (ethylenediaminetetraacetic acid). DCs were then positively selected using anti-CD11c (N418) MicroBeads and MACS column (Miltenyi Biotec, Auburn, CA). The purified cells were routinely more than 90% CD11c+ and used as SPDCs. SPDCs expressed moderate levels of MHC class II and CD86, and these expressions were markedly enhanced by treatment with LPS (data not shown). SPDCs (2 × 105 cells/mL) treated with 5 μg/mL LPS for 24 hours were used as mature SPDCs.
Chemotaxis assay
Chemotaxis assay was conducted as described.32,33In brief, 1.5 × 105 BC1 cells were added to upper wells of 5-μm pore, polycarbonate 24-well tissue culture inserts (Costar, Cambridge, MA) in 100 μL, with 600 μL chemokine or medium in the bottom wells. All migration assays were conducted in IMDM with 2% FCS in 5% CO2 for 90 minutes at 37°C. Migrated cells recovered from each lower well were counted using comparison to a known number of beads as an internal standard (Flow-Count, Coulter, Miami, FL) as described.27 28 The percent migration was determined from numbers of the starting cells and the migrated cells.
Confocal microscopy
A space of 0.25 mm thickness was made between a slide glass and a cover glass, and BC1 cells (1 × 106 cells/mL) were infused into the space in the presence or absence of CCL19 or CCL21. The cells were incubated for 15 to 120 minutes at 37°C. In the case of pretreatment with CCL21, the cells were pretreated with CCL21 (1 μg/mL) for 15 minutes on ice; then the cells were cultured on the slide glass with CCL19 (20 ng/mL) and CCL21 (1 μg/mL) for 60 minutes at 37°C. The cell morphology was analyzed using a confocal microscopy (BX61-2CHAr-HeNeG-SP, FV500, Ver3.1, Olympus, Tokyo, Japan). The number of spread cells with long dendrites was counted by 2 observers in a blind fashion and the percentage was calculated. Approximately 100 cells observed in the field of 0.407 mm2were counted based on their morphology at each point. The data are presented as the mean percentage ± SE of 4 independent experiments.
Microscopy
The BC1 cells (6 × 104 cells) were cultured using 48-well culture plates in the presence or absence of CCL19 or CCL21 for 2 to 24 hours at 37°C. In some experiments, cells were pretreated with PTX (100 ng/mL) for 2 hours at 37°C. Pretreatment of cells with toxin B or Y-27632 was performed for 5 hours at 37°C. The cell morphology was analyzed by invert microscopy (IX70, Olympus). The number of spread cells with long dendrites was counted by 2 observers in a blind fashion and the percentage was calculated. One hundred to 200 cells were counted based on their morphology at each point. The data are presented as the mean percentage ± SE of 4 independent experiments.
Mature SPDCs were preincubated in the absence of chemokines for 4 hours at 37°C to decrease the constitutive extension of dendrites. The cells were then harvested and cultured at a density of 6 × 104 cells/300μL/well using 48-well culture plates in the presence or absence of chemokines for 90 minutes at 37°C. The cell morphology was analyzed by invert microscopy (IX70, Olympus).
Statistical analyses
In chemotaxis assays, statistical differences compared with the control were calculated by the Dunnett test. In other experiments, the Student t test was used to analyze data for significant differences. Differences between groups were considered significant atP < .05.
Results
Migratory responses of immature and mature DCs to CCL19 and CCL21
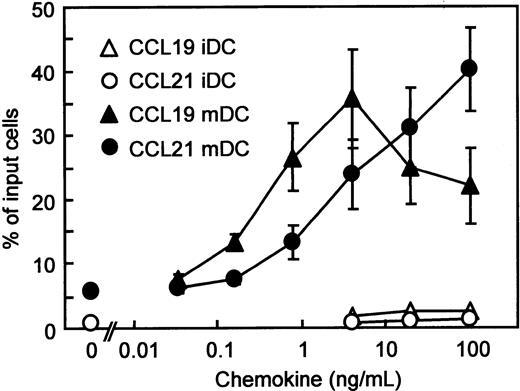
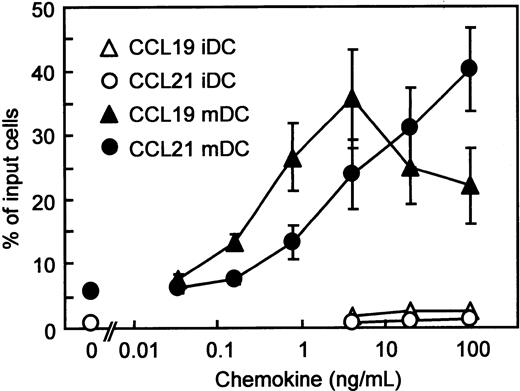
Using BC1 cells, we first compared the migratory responses of immature and mature DC to CCR7 ligands, CCL19 and CCL21. We have reported that unstimulated BC1 cells are phenotypically and functionally immature DCs.31 BC1 cells treated with LPS for 24 hours were used as mature DCs. It has been reported that LPS promotes DC maturation and the mature DCs express CCR7 to migrate into the regional LNs.12-14 Immature DCs showed negligible migration to CCL19 or CCL21 (Figure 1). In contrast, mature DCs vigorously migrated to either CCL19 or CCL21. The considerable migration of mature DCs to CCL19 was observed at concentrations of 0.16 to 100 ng/mL (0.017-10.6 nM) with a peak at 4 ng/mL (0.426 nM) and a subsequent decrease at 20 and 100 ng/mL (2.13-10.6 nM). CCL19-induced migration was statistically greater than that of control (without chemokine) at 0.8 ng/mL (0.085 nM;P < .05) and 4 ng/mL (0.426 nM; P < .01). On the other hand, the migration of mature DCs to CCL21 increased at concentrations of 0.8 to 100 ng/mL (0.067-8.33 nM) in a dose-dependent manner. The CCL21-induced migration was statistically greater than that of control at 4 ng/mL (0.333 nM; P < .05), 20 ng/mL (1.67 nM; P < .01), and 100 ng/mL (8.33 nM;P < .01).
Chemotactic preferences of CCL19 and CCL21 for murine DC line (BC1 cells).
BC1 cells were activated by LPS and used as mature DCs (mDC). Unstimulated BC1 cells were used as immature DCs (iDC). Numbers of migrated cells into the lower well are expressed as the percentage of input cells in the upper well at the start time of chemotaxis assay. Each symbol represents the mean ± SE of 4 or 5 independent experiments.
Chemotactic preferences of CCL19 and CCL21 for murine DC line (BC1 cells).
BC1 cells were activated by LPS and used as mature DCs (mDC). Unstimulated BC1 cells were used as immature DCs (iDC). Numbers of migrated cells into the lower well are expressed as the percentage of input cells in the upper well at the start time of chemotaxis assay. Each symbol represents the mean ± SE of 4 or 5 independent experiments.
Dendritic extension induced by CCL19 and CCL21
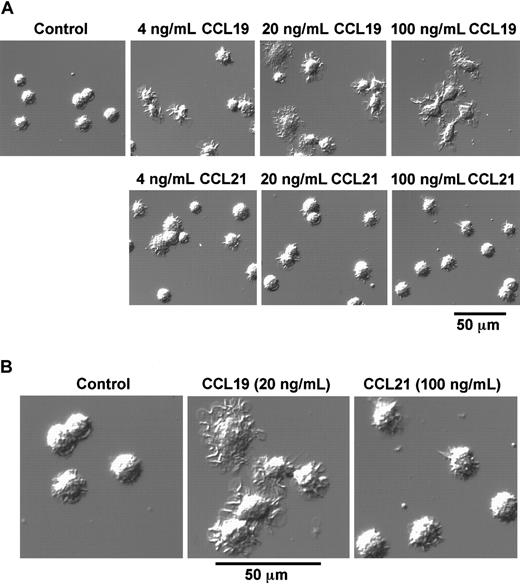
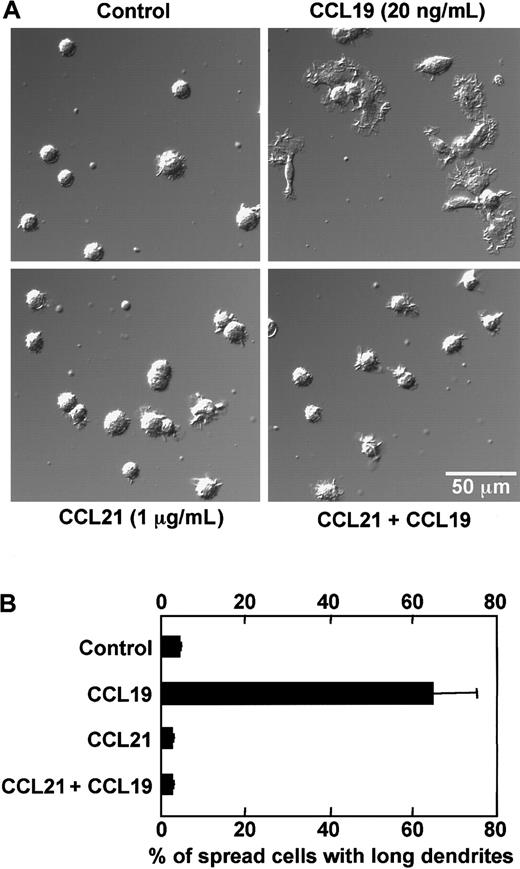
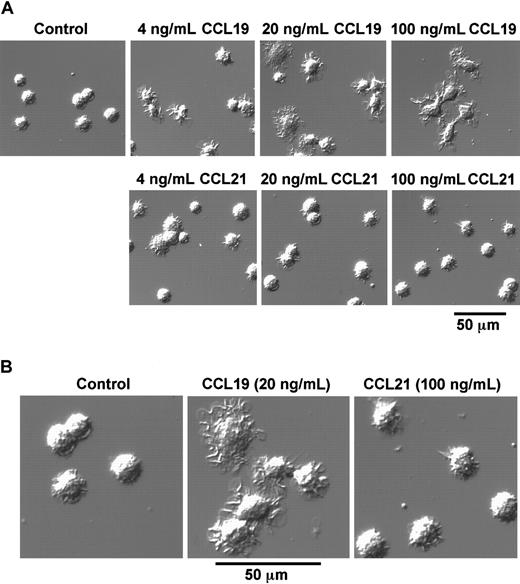
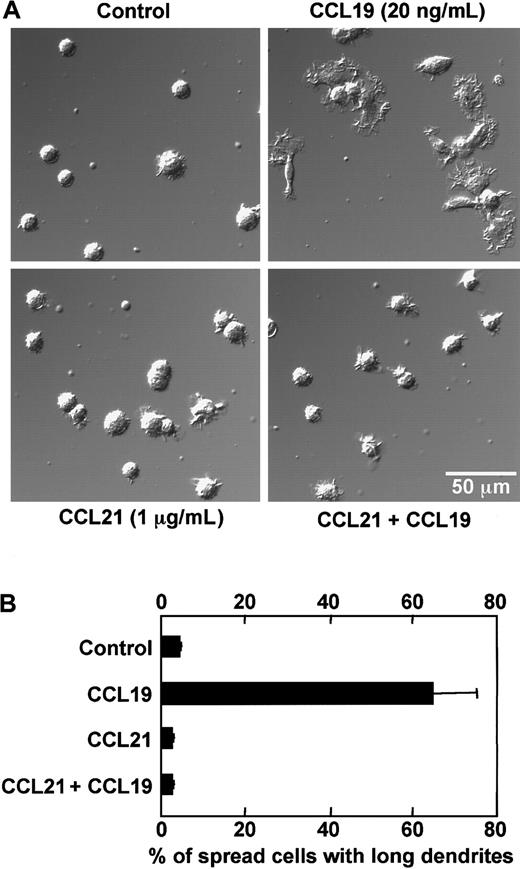
As described in the prior section, BC1 cells treated with LPS for 24 hours were used as mature DCs. These mature DCs were then treated with CCL19 or CCL21 in a space between a slide glass and a cover glass, and their morphologic changes were analyzed using confocal microscopy. Before treatment with chemokines, mature DCs had a round shape and possessed short dendrites (Figure 2A,B). After treatment with CCL19 at 20 or 100 ng/mL (2.13 or 10.6 nM) for 1 hour, the cells were spread and their dendrites were markedly extended; 4 ng/mL (0.426 nM) CCL19 also induced a similar but modest morphologic change compared with those at higher doses of CCL19 (Figure 2A). By contrast, no obvious change of dendritic morphology was detected after treatment of mature BC1 cells with CCL21 up to 100 ng/mL (8.33 nM) for 1 hour (Figure 2A,B).
CCL19-induced rapid dendritic extension of mature DCs.
BC1 cells were activated by LPS and used as mature DCs. The mature DCs were treated with CCL19 or CCL21 for 1 hour, and then the morphologic changes were analyzed by confocal microscopy (original magnification × 50 in panel A; pictures further magnified as indicated in panel B). The results are representative of at least 3 independent experiments.
CCL19-induced rapid dendritic extension of mature DCs.
BC1 cells were activated by LPS and used as mature DCs. The mature DCs were treated with CCL19 or CCL21 for 1 hour, and then the morphologic changes were analyzed by confocal microscopy (original magnification × 50 in panel A; pictures further magnified as indicated in panel B). The results are representative of at least 3 independent experiments.
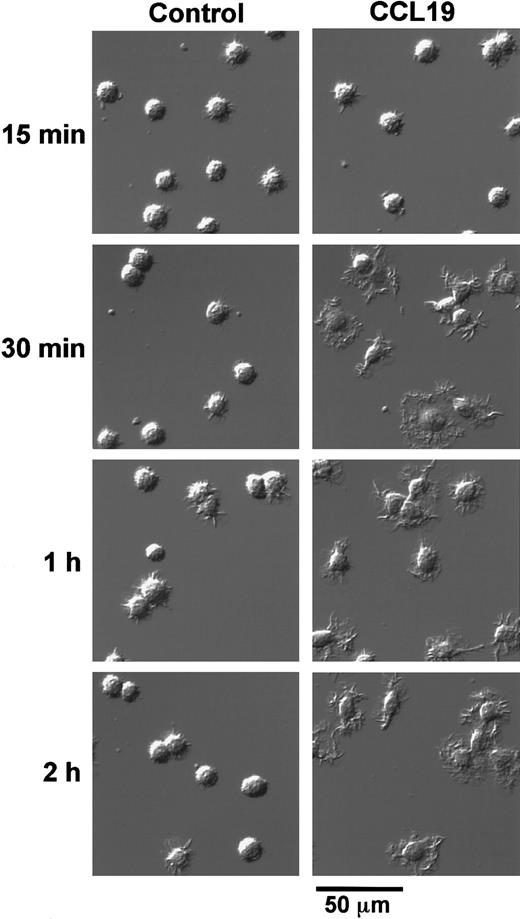
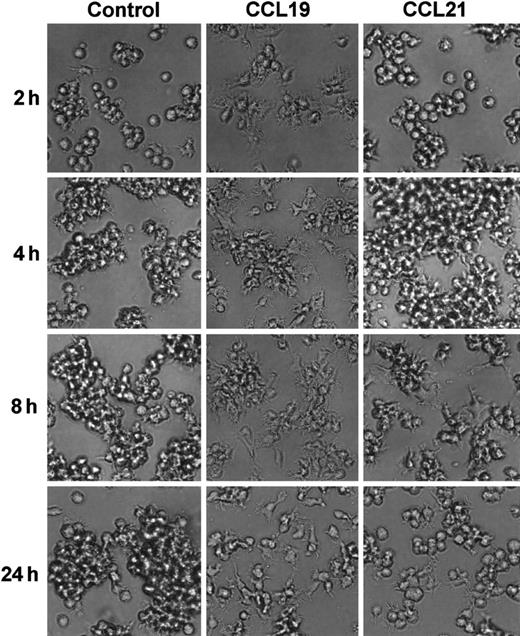
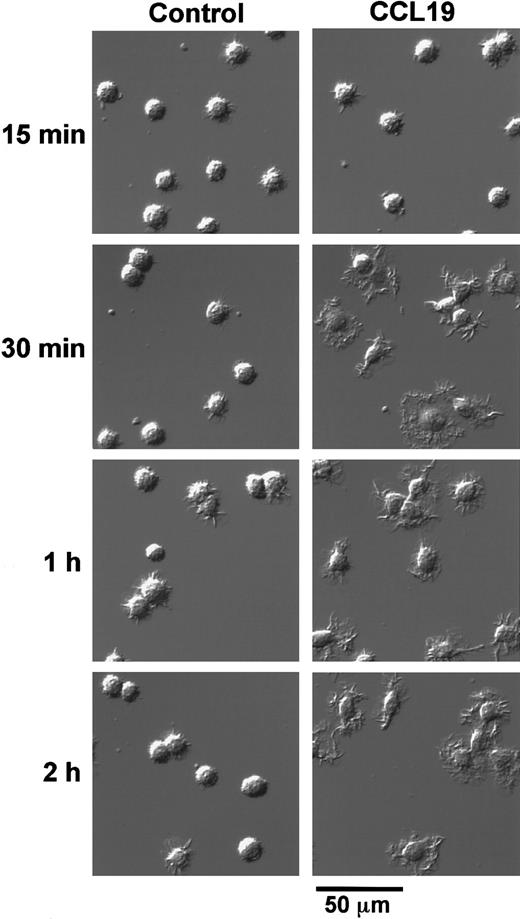
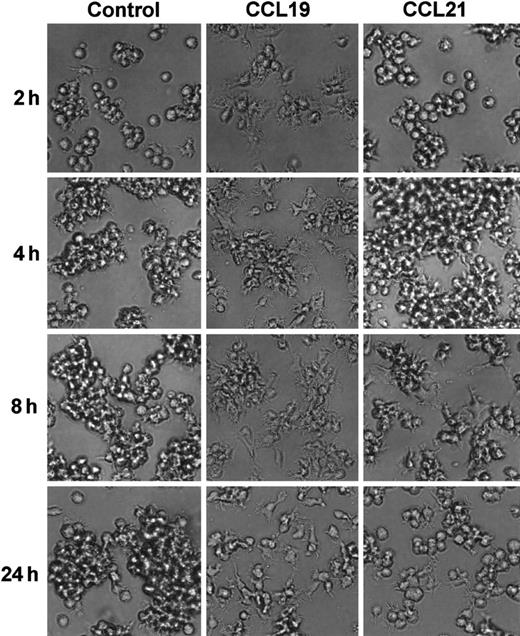
We next analyzed the time course of morphologic changes of mature DCs treated with 20 ng/mL (2.13 nM) CCL19 or 100 ng/mL (8.33 nM) CCL21. In these experiments, the cells were cultured in a space between a slide glass and a cover glass for 15 minutes to 2 hours or in 48-well culture plates for 2 to 4 hours, and the morphologic changes were analyzed using confocal microscopy or invert microscopy, respectively. Long-time culture more than 2 hours of BC1 cells on the slide glass resulted in the low cell viability (data not shown).
CCL19 rapidly (at 30 minutes) induced dendritic extension and the extension was maintained at least for 24 hours (Figures3 and 4). Aggregations of the mature DCs were observed in cultures with medium alone from 4 to 24 hours (Figure 4). No such marked aggregations were detected in cultures with either chemokine except that with CCL21 at 4 hours. It was also shown in Figures 2 and 4 that CCL21 induced no considerable morphologic change until 2 hours after the treatment. CCL21 increased the cell aggregations but not dendritic extension at 4 hours. CCL21-induced dendritic extension of BC1 cells could be detected at 8 or 24 hours, although the dendritic extension was not so impressive compared with that induced by CCL19 (Figure 4). Similar results were obtained with a higher concentration of CCL21 (1 μg/mL, 83.3 nM) (data not shown). Morphology of immature DCs (unstimulated BC1 cells) was unaffected by treatment with either CCL19 or CCL21 (data not shown).
Time-course change of CCL19-induced dendritic extension of mature DCs.
BC1 cells were activated by LPS and used as mature DCs. The mature DCs were treated with CCL19 (20 ng/mL) for 15 minutes to 2 hours, and then the morphologic changes were analyzed by confocal microscopy (original magnification × 50). The results are representative of 3 independent experiments.
Time-course change of CCL19-induced dendritic extension of mature DCs.
BC1 cells were activated by LPS and used as mature DCs. The mature DCs were treated with CCL19 (20 ng/mL) for 15 minutes to 2 hours, and then the morphologic changes were analyzed by confocal microscopy (original magnification × 50). The results are representative of 3 independent experiments.
Time course of mature DC morphology in the absence or presence of CCL19 or CCL21.
BC1 cells were activated by LPS and used as mature DCs. The matures DC were treated with CCL19 or CCL21 for 2 to 24 hours, and the morphologic changes were analyzed by invert microscopy (original magnification × 20). The results are representative of 3 independent experiments.
Time course of mature DC morphology in the absence or presence of CCL19 or CCL21.
BC1 cells were activated by LPS and used as mature DCs. The matures DC were treated with CCL19 or CCL21 for 2 to 24 hours, and the morphologic changes were analyzed by invert microscopy (original magnification × 20). The results are representative of 3 independent experiments.
Effect of CCL21 on rapid dendritic extension induced by CCL19
It has been reported that both CCL19 and CCL21 bind to CCR7 and attract the CCR7-expressing cells in the migratory responses.15,16,34 35 Thus, if rapid dendritic extension induced by CCL19 but not by CCL21 could be mediated via CCR7, CCL21 might compete with CCL19 in interaction with CCR7 and exert an inhibitory effect on the rapid morphologic change. To examine this possibility, we analyzed the effect of CCL21 on the dendritic extension at 1 hour after stimulation with CCL19. Mature BC1 cells were pretreated with a high concentration of CCL21 (1 μg/mL, 83.3 nM) for 15 minutes on ice, and then treated with CCL19 (20 ng/mL, 2.13 nM) in a space between a slide glass and a cover glass for 1 hour at 37°C. The morphologic properties of the cells were observed using confocal microscopy, and proportions of spread cells with long dendrites were calculated (Figure 5A,B). In the control culture without chemokines, the mean proportion of spread cells with long dendrites was less than 5%. CCL19 markedly increased the proportion of spread cells with long dendrites up to more than 60% (Figure 5B). Again no obvious change of the dendritic morphology was detected in mature BC1 cells 1 hour after treatment with the high concentration of CCL21 alone (Figure 5A,B). It should be noted in Figure 5, panels A and B, that pretreatment with a high concentration of CCL21 completely inhibited the CCL19-induced increase in the proportion of spread cells with long dendrites.
CCL21 inhibits CCL19-induced dendritic extension of mature DCs.
BC1 cells were activated by LPS and used as mature DCs. The mature DCs were pretreated with a high concentration of CCL21 (1 μg/mL) for 15 minutes and then incubated with CCL19 (20 ng/mL) for 1 hour. The morphologic changes were analyzed by confocal microscopy (original magnification × 50). The results are representative of 4 independent experiments (A). Each column represents the mean ± SE of 4 independent experiments (B).
CCL21 inhibits CCL19-induced dendritic extension of mature DCs.
BC1 cells were activated by LPS and used as mature DCs. The mature DCs were pretreated with a high concentration of CCL21 (1 μg/mL) for 15 minutes and then incubated with CCL19 (20 ng/mL) for 1 hour. The morphologic changes were analyzed by confocal microscopy (original magnification × 50). The results are representative of 4 independent experiments (A). Each column represents the mean ± SE of 4 independent experiments (B).
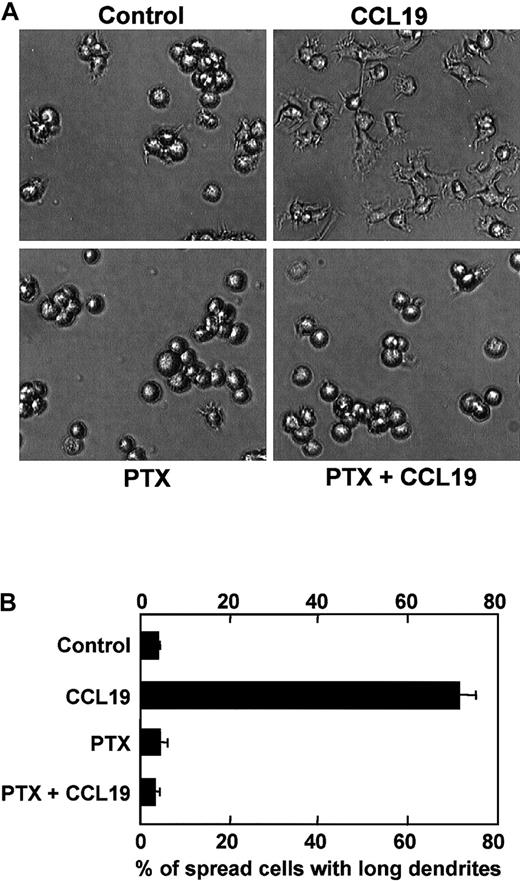
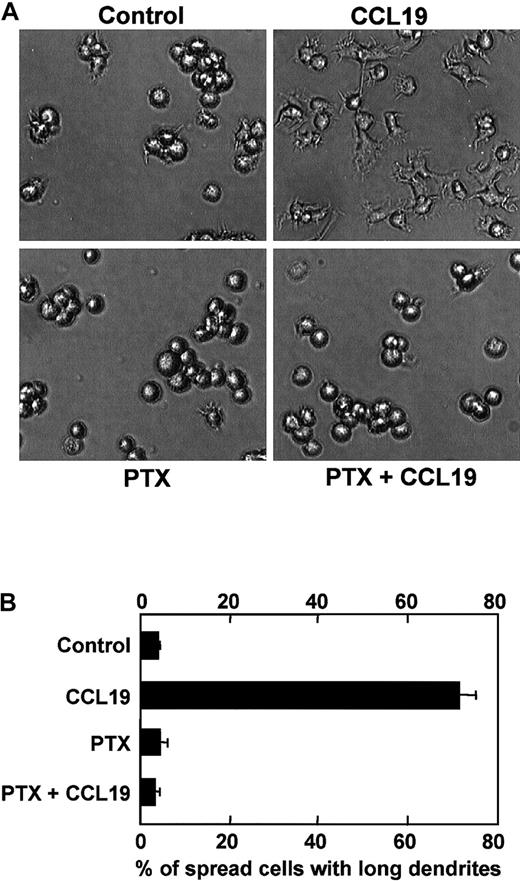
Effect of PTX on CCL19-induced dendritic extension
Pertussis toxin specifically inhibits Gi/Go proteins among other G-proteins such as Gq, Gs, or G12/G13 proteins, which results in inhibition of cell migration induced by chemokines. Indeed, it has been reported that CCL19-induced chemotaxis of mature DCs is inhibited by PTX at 100 ng/mL.14 Then, we examined whether PTX also inhibited CCL19-induced morphologic changes of mature DCs. Mature DCs were pretreated with PTX at 100 ng/mL for 2 hours, and then treated with CCL19 for 2 hours at 37°C. The morphologic properties of the cells were analyzed using invert microscopy, and the proportions of spread cells with long dendrites were calculated. CCL19 again markedly increased the proportion of spread cells with long dendrites up to approximately 70% (Figure 6A,B). It should be noted in Figure 6, panel B, that the CCL19-induced morphologic changes of mature BC1 cells are almost completely inhibited by PTX.
Effect of PTX on CCL19-induced dendritic extension of mature DCs.
BC1 cells were activated by LPS and used as mature DCs. The mature DCs were pretreated with PTX (100 ng/mL) for 2 hours and then incubated with CCL19 (20 ng/mL) for 2 hours. The morphologic changes were analyzed by invert microscopy (original magnification × 20). The results are representative of 4 independent experiments (A). Each column represents the mean ± SE of 4 independent experiments (B).
Effect of PTX on CCL19-induced dendritic extension of mature DCs.
BC1 cells were activated by LPS and used as mature DCs. The mature DCs were pretreated with PTX (100 ng/mL) for 2 hours and then incubated with CCL19 (20 ng/mL) for 2 hours. The morphologic changes were analyzed by invert microscopy (original magnification × 20). The results are representative of 4 independent experiments (A). Each column represents the mean ± SE of 4 independent experiments (B).
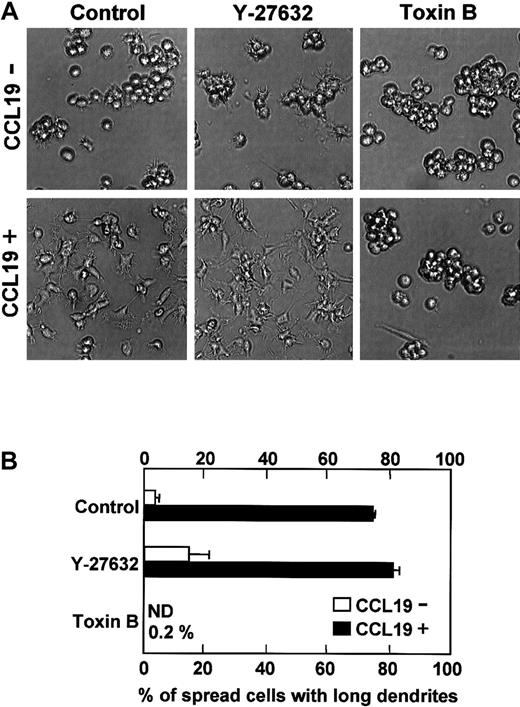
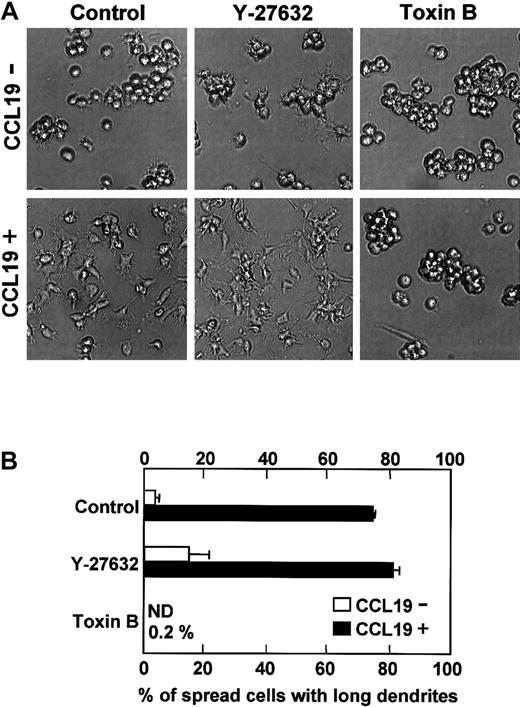
Effect of inhibitors for Rho GTPase-mediated pathways on CCL19-induced dendritic extension
Rho GTPase family including Rho, Rac, and Cdc42 is involved in morphologic change of fibroblasts.19-23 We then examined the effect of Y-27632 and C difficile toxin B on CCL19-induced dendritic extension. Y-27632 is a specific inhibitor for Rho-associated kinase that is a target molecule of Rho36and C difficile toxin B is an inhibitor for Rho GTPase proteins including Rho, Rac, and Cdc42.37 It has been reported that Y-27632 at 10 μM blocks neurite retraction in neuroblastoma,38 whereas toxin B at 1 to 100 ng/mL inhibits endocytosis of murine DCs and phagocytosis of rat basophil leukemia mast cells that need Rac1 and Cdc42 activations.39 40 Mature DCs were pretreated with Y-27632 (10 μM) or toxin B (4 ng/mL) for 5 hours, and then treated with medium alone or CCL19 (20 ng/mL, 2.13 nM) for 2 hours at 37°C. In the control culture without inhibitors and chemokines, the mean proportion of spread cells with long dendrites was 3.7% (Figure7B). CCL19 markedly increased the proportion of spread cells with long dendrites up to 74.4%. Pretreatment with Y-27632 induced moderate dendritic extension and increased the proportion of spread cells with long dendrites to 14.8% even in the absence of CCL19. In the presence of CCL19, pretreatment with Y-27632 enhanced the morphologic changes and significantly increased the proportion of spread cells with long dendrites to 81.3% (P < .05 versus CCL19 alone–treated culture). In contrast, no spread cells with long dendrites were observed in toxin B–pretreated cultures either in the absence or presence of CCL19 (Figure 7B).
Effects of Y-27632 or toxin B on CCL19-induced dendritic extension of mature DCs.
BC1 cells were activated by LPS and used as mature DCs. The mature DCs were pretreated with Y-27632 (10 μM), a Rho-associated kinase inhibitor, or toxin B (4 ng/mL), a Rho GTPase inhibitor, for 5 hours and then incubated with CCL19 (20 ng/mL) for 2 hours. The morphologic changes were analyzed by invert microscopy (original magnification × 20). The results are representative of 4 independent experiments (A). Each column represents the mean ± SE of 4 independent experiments (B).
Effects of Y-27632 or toxin B on CCL19-induced dendritic extension of mature DCs.
BC1 cells were activated by LPS and used as mature DCs. The mature DCs were pretreated with Y-27632 (10 μM), a Rho-associated kinase inhibitor, or toxin B (4 ng/mL), a Rho GTPase inhibitor, for 5 hours and then incubated with CCL19 (20 ng/mL) for 2 hours. The morphologic changes were analyzed by invert microscopy (original magnification × 20). The results are representative of 4 independent experiments (A). Each column represents the mean ± SE of 4 independent experiments (B).
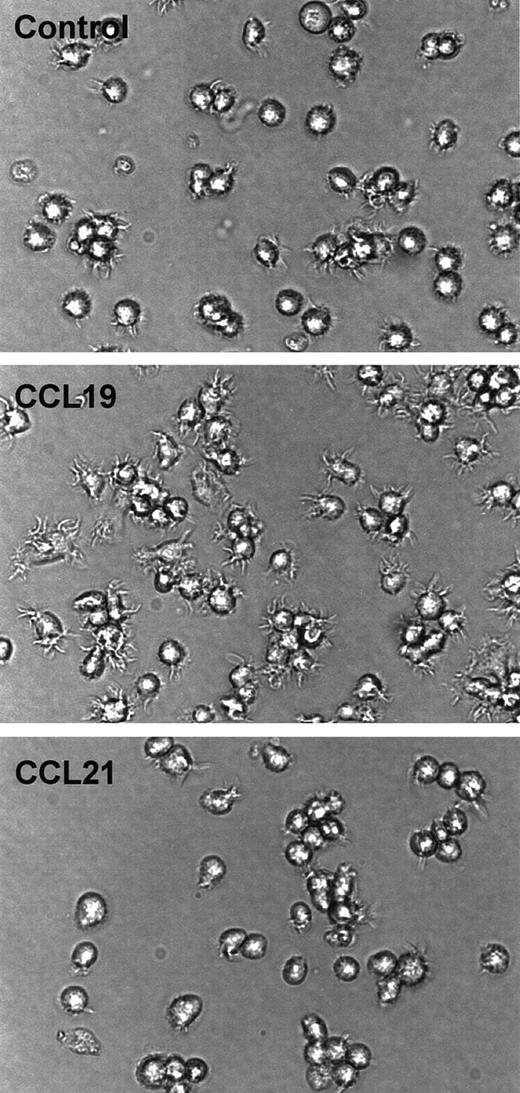
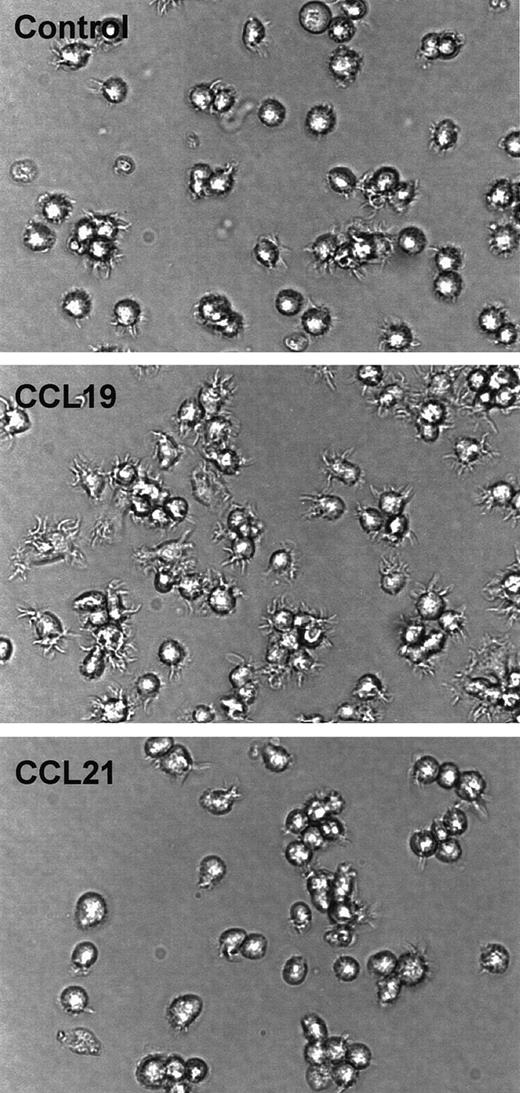
CCL19-induced dendritic extension of SPDCs
To examine whether CCL19-induced dendritic extension is observed in other DC systems, SPDCs were generated by culturing B6 splenocytes in the presence of GM-CSF and fibroblast supernatant for 14 days. SPDCs expressed moderate levels of MHC class II (I-Ab) and CD86, and these expressions were markedly enhanced by treatment with LPS (data not shown). Then, SPDCs treated with LPS for 24 hours were used as mature SPDCs, and these mature SPDCs were treated with CCL19 (20 ng/mL) or CCL21 (100 ng/mL) for 90 minutes. Figure8 shows that dendrites of mature SPDCs are markedly extended by treatment with CCL19 but not by CCL21. These findings are consistent with prior observations with the BC1 cell line.
Assessment of CCL19-induced dendritic extension in SPDCs.
The SPDCs were generated by culturing splenocytes with GM-CSF and fibroblast supernatant for 14 days. SPDCs were activated by LPS and used as mature SPDCs. The mature SPDCs were incubated with CCL19 (20 ng/mL) or CCL21 (100 ng/mL) for 90 minutes. The morphologic changes were analyzed by invert microscopy (original magnification × 30). The results are representative of 4 independent experiments.
Assessment of CCL19-induced dendritic extension in SPDCs.
The SPDCs were generated by culturing splenocytes with GM-CSF and fibroblast supernatant for 14 days. SPDCs were activated by LPS and used as mature SPDCs. The mature SPDCs were incubated with CCL19 (20 ng/mL) or CCL21 (100 ng/mL) for 90 minutes. The morphologic changes were analyzed by invert microscopy (original magnification × 30). The results are representative of 4 independent experiments.
Discussion
CCL19 and CCL21 are selective agonists for CCR7 and drive the migration of T cells and mature DCs into the LNs.12-16,34,35 The role of CCL19 and CCL21 in the cellular traffic into and within secondary lymphoid tissues is well documented.15,16,35 Both CCL19 and CCL21 induce integrin-dependent adhesion and chemotaxis.15,16,34,35Using in situ hybridization in mice, previous studies show that CCL21 is expressed in the high-endothelial venules (HEVs) and stromal cells of the T-cell zone of the LNs, whereas CCL19 is expressed in the stromal cells of the T-cell zone but not in the HEVs.41-43These findings suggest that CCL21 is involved in T-cell arrest via integrin activation on the HEVs and the subsequent recruitments of the T cells into the T-cell area. On the other hand, CCL19 appears to be involved in the T-cell recruitment within LNs but not the T-cell arrest on the HEVs. Similarly, it seems that mature DCs are recruited into the T-cell area of LNs by either CCL19 or CCL21. From these observations, it has been considered that CCL19 and CCL21 play different roles in recruitment of T cells and mature DCs into the T-cell area. However, apart from the cellular traffic, functional differences between CCL21 and CCL19 have been poorly understood.
In the present study, we have investigated functional roles of CCL19 and CCL21 especially in the morphologic change of murine DCs. It was clearly demonstrated that CCL19 but not CCL21 rapidly and markedly induced morphologic changes of mature DCs. It should be noted that the marked dendritic extension of DCs was observed in the presence of more than 20 ng/mL CCL19 (Figures 1 and 3). On the other hand, migration of mature DCs was vigorously promoted by a broad concentration (0.8-100 ng/mL) of CCL19 with a peak at 4 ng/mL (Figure 1). Thus, high concentrations of CCL19 appeared to be needed to induce the dendritic extension compared to those required for chemotactic response. These findings suggest that low concentrations of CCL19 mainly participate in the recruitment of mature DCs to the T-cell areas of the draining LNs. It appears that marked dendritic extensions of DCs are then promoted in the T-cell area where large amounts of CCL19 produced by the stromal cells are present.41 On the other hand, the CCL19-induced migration of mature DCs may be decreased in the presence of high concentrations of CCL19. The morphologic changes induced by the high concentration of CCL19 seem to be related to the diminished migration of DCs.
CCL21 and CCL19 bind specifically to CCR7 with a similar affinity and exert similar effects on integrin activation and chemotaxis of T cells and mature DCs.15,16,34,35 In agreement with previous reports,12-14 we found that both CCL19 and CCL21 vigorously promoted migration of mature DCs (Figure 1). In contrast, concerning morphologic changes, CCL19 at 20 ng/mL (2.13 nM) markedly induced rapid dendritic extension of mature DCs, whereas CCL21 up to 1 μg/mL (83.3 nM) induced no rapid dendritic extension (Figures 2, 3, and 5). In addition, the CCL19-induced dendritic extension was almost completely inhibited by pretreatment with a high concentration of CCL21 (Figure 5). It has been reported that certain chemokine receptors have natural antagonistic ligands.44,45 For instance, monocyte chemotactic protein 3 (MCP-3) binds to CCR5, but the MCP-3–CCR5 binding leads to no functional activation and competitively inhibits the binding of other ligands.44 Thus, although the interaction between CCL21 and CCR7 induced enhanced migratory response, the interaction might result in no functional influences on rapid dendritic extension of mature DC. It seemed that CCL19 and CCL21 exhibited phenotypically agonistic and antagonistic properties in the morphologic regulation of mature DCs, respectively. At present time, we consider that CCL19-CCR7 interaction may introduce at least 2 different signals that are involved in the chemotaxis or the rapid dendritic extension, respectively. On the other hand, the intracellular signals induced by CCL21-CCR7 interaction may be involved in the chemotaxis but not in the rapid dendritic extension of mature DCs. However, at the later stages, CCL21 also induced modest dendritic extension of DCs as compared with that induced by CCL19 at the same later stages. Although we consider that the dendritic extension of DCs at the later stages is also mainly regulated by CCL19, the distinct role of CCL19 and CCL21 in the dendritic extension should be carefully analyzed in future investigations.
Chemokine receptors are coupled with Gi proteins, and PTX that inhibits specifically Gi/Go proteins but not other G-proteins such as Gq, Gs, or G12/G13 proteins46 abrogates cell migrations induced by chemokines.46-48 Indeed, CCL19-induced chemotaxis of mature DCs is inhibited by treatment with PTX.14 In the present study, it was shown that PTX also inhibited CCL19-induced dendritic extension of mature DCs (Figure 6). Thus, Gi proteins coupled with CCR7 appear to be involved in signal pathways related to not only chemotaxis but also regulation of dendritic morphology in mature DCs. It has been reported that Gi proteins are involved in Ras activation in mammalian cells.49 Ras regulates activation of extracellular signal-related kinase (ERK).49 In the present study, we found that CCL19-induced dendritic extension was unaffected by treatment with PD98059, a specific inhibitor of ERK pathway (data not shown). Thus, it seems unlikely that the ERK pathway is involved in the CCL19-induced morphologic change. Recently, it has been demonstrated that Gi proteins are also involved in activation of Rac and Cdc42 in mouse fibroblasts.50 We showed herein that the CCL19-induced dendritic extension of mature DCs was completely inhibited by toxin B, a specific inhibitor for Rho GTPase proteins including Rac and Cdc42 (Figure 7). Thus, it seems that activation of Rac or Cdc42 or both by Gi proteins is involved in the CCL19-induced morphologic change.
Rho, Rac, and Cdc42 are responsible for reorganization of the actin cytoskeleton and subsequent morphologic changes in various cellular functions.18 Cross-talk between these proteins has been well documented in various cell culture systems. Cdc42 is a strong activator of Rac,21,22 whereas the actions of Rac and Rho counteract each other.51,52 Thus, cell morphology appears to result from complex reciprocal actions of these proteins. Recently, Yamaguchi et al53 have shown that nerve growth factor induces neurite outgrowth mediated via Rac activation, whereas Rho negatively regulates Rac activation through Rho-associated kinase-dependent pathway in PC12 rat pheochromocytoma cells. Similar results have been shown in neuroblastoma cells.38 In these studies, Y-27632 blocked negative regulation of neurite outgrowth mediated via Rho-associated kinase.38,52 On the other hand, it has been reported that rapid dendritic extension of chicken ganglion cells is inhibited by a dominant-negative form of Rac, but enhanced by a dominant-negative form of Rho.54 These findings suggest that the rapid dendritic extension is positively or negatively regulated by Rac or Rho, respectively. Although DCs possess a number of dendrites as ganglion cells do, no such reciprocal actions of Rac and Rho have been reported in the regulation of dendritic morphology of DCs.
In the present study, we could show similar reciprocal actions of Rho GTPase proteins in the regulation of the dendritic extension of DCs. CCL19-induced dendritic extension of mature DCs was slightly but significantly enhanced by Y-27632 (Figure 7), a specific inhibitor for Rho-associated kinase.36 In addition, Y-27632 alone induced a significant dendritic extension. These observations suggest that the signal pathway mediated via Rho and Rho-associated kinase is involved in negative regulation of dendritic extension of mature DCs. On the contrary, the CCL19-induced dendritic extension of mature DCs was completely inhibited by toxin B, a specific inhibitor for Rho GTPase proteins (Figure 7). In this case, both CCL19-induced positive signals mediated via Rac and Cdc42 and negative signals mediated via Rho and Rho-associated kinase for dendritic extension seem to be completely inhibited by toxin B. We would like to postulate that the CCL19-induced dendritic extension is positively regulated by Rac or Cdc42 (or both), but negatively regulated by Rho.
In the last experiment, we showed that not only BC1 cell line but also SPDCs that had been generated by culturing splenocytes in the presence of GM-CSF and fibroblast supernatant for 14 days exhibited CCL19-induced dendritic extension (Figure 8). We also tried to detect dendritic extension in freshly isolated DCs. However, a significant proportion of the freshly isolated DCs underwent apoptosis during the short-term culture (data not shown). We could not detect CCL19-induced dendritic extension in these DCs. It has been reported that more than 50% of freshly isolated DCs from lymphoid organs undergo apoptosis by only short-term culturing.55 Thus, it seems to us that physiologic dendritic extension is no more anticipated in the presence of massive cell death.
We showed herein a novel role of CCL19 in the regulation of dendritic morphology of DCs. Because extended dendrites of DCs are thought to be of great advantage to interaction with T cells, CCL19 in the T-cell area of LNs appears to be an important component for the efficient antigen presentation. Thus, elucidation of the rather complex pathways controlling the CCL19-induced dendritic extension may lead to development of the new regulation system in various immune disorders in the clinical applications.
Prepublished online as Blood First Edition Paper, May 24, 2002; DOI 10.1182/blood-2002-01-0260.
Supported in part by a Grant-in-Aid for Scientific Research (B) by the Ministry of Education, Science, Japan. This study was also supported by grants from the Hokkaido Foundation for the Promotion of Scientific and Industrial Technology, and The Tomakomai East Hospital Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kazunori Onoé, Division of Immunobiology, Institute for Genetic Medicine, Hokkaido University, Kita-15, Nishi-7, Kita-ku, Sapporo, 060-0815 Japan; e-mail: kazunori@imm.hokudai.ac.jp.