Abstract
Multiple myeloma (MM) cells cause devastating bone destruction by activating osteoclasts in the bone marrow milieu. However, the mechanism of enhanced bone resorption in patients with myeloma is poorly understood. In the present study, we investigated a role of C-C chemokines, macrophage inflammatory protein (MIP)–1α and MIP-1β, in MM cell-induced osteolysis. These chemokines were produced and secreted by a majority of MM cell lines as well as primary MM cells from patients. Secretion of MIP-1α and MIP-1β correlated well with the ability of myeloma cells to enhance osteoclastic bone resorption both in vitro and in vivo as well as in MM patients. In osteoclastogenic cultures of rabbit bone cells, cocultures with myeloma cells as well as addition of myeloma cell-conditioned media enhanced both formation of osteoclastlike cells and resorption pits to an extent comparable to the effect of recombinant MIP-1α and MIP-1β. Importantly, these effects were mostly reversed by neutralizing antibodies against MIP-1α and MIP-1β, or their cognate receptor, CCR5, suggesting critical roles of these chemokines. We also demonstrated that stromal cells express CCR5 and that recombinant MIP-1α and MIP-1β induce expression of receptor activator of nuclear factor-κB (RANK) ligand by stromal cells, thereby stimulating osteoclast differentiation of preosteoclastic cells. These results suggest that MIP-1α and MIP-1β may be major osteoclast-activating factors produced by MM cells.
Introduction
Multiple myeloma (MM) is a plasma cell malignancy characterized by an almost exclusive accumulation in the bone marrow, secretion of monoclonal immunoglobulin, and a suppression of normal immunoglobulin production and hematopoiesis, especially that of the erythroid lineage.1,2 Another important clinical feature of MM is a marked stimulation of osteoclastic bone resorption, which causes the most debilitating clinical symptoms including intractable bone pain, disabling multiple fractures, and hypercalcemia. The aggressive bone destruction has significantly contributed to its poor prognosis, which has barely been improved since the introduction of the melphalan and prednisolone therapy in the 1960s despite the development of potent chemotherapeutic regimens.1,3 In our laboratory, new antitumor agents are now under development including immunotherapies with MM-specific monoclonal antibodies (mAbs).4 5 However, elucidation of the molecular mechanism of bone destruction is critical to develop an effective way to improve survival as well as quality of life of patients with MM.
Cytokines with potent bone-resorbing activity such as interleukin 6 (IL-6) and IL-1β have been implicated as potential mediators of osteoclastic bone resorption in MM.6-10 IL-6 up-regulates stromal cell expression of receptor activator of nuclear factor-κB (RANK) ligand through a STAT-3 pathway and thereby osteoclast formation,11 whereas IL-1 stimulates osteoclastogenesis probably through induction of prostaglandin-E2 production by stromal cells12,13 and also directly acts on the osteoclast to potentiate its activity and survival.14However, none of these factors were found to be accumulated in the bone marrow plasma of severe combined immunodeficiency (SCID) mice implanted with a human cell line derived from myeloma patients, ARH77, which recapitulates human MM bone disease.15 In addition, neither anti–IL-6 nor anti–IL-1β blocking antibody was able to inhibit osteoclastogenic effects of secreted factors by ARH-77 in vitro.15 These observations suggest that unidentified soluble factors secreted by MM cells play a causative role in the development of MM bone disease.
To clarify the mechanism of development of MM bone lesions, we searched for MM-derived factors responsible for enhancement of osteoclastic bone resorption. We hereby present evidence that C-C chemokines, macrophage inflammatory protein (MIP)–1α and MIP-1β play critical roles in enhanced bone resorption by MM cells. Both MIP-1α and MIP-1β were produced by MM cell lines including ARH77 as well as a majority of primary MM cells from patients. These chemokines stimulated formation of osteoclastlike cells (OCLs) and their resorbing activity in rabbit bone cell cultures as potently as MM cell-conditioned media. Furthermore, blocking antibodies against MIP-1 were able to almost completely suppress the stimulatory effects of MM cell-conditioned media and those of cocultures with MM cells on osteoclastogenesis as well. Thus, MIP-1α and MIP-1β may be among leading candidates for a soluble factor responsible for development of lytic bone lesions in MM.
Materials and methods
Chemicals
Recombinant human (rh) MIP-1α and MIP-1β, mouse IgG1, and neutralizing mouse antihuman MIP-1α and anti–MIP-1β mAbs; goat IgG, neutralizing goat antihuman MIP-1α and MIP-1β polyclonal antibodies; blocking mouse anti–human VLA-4 and CCR5 polyclonal antibodies; and rh vascular cell adhesion molecule–1 (rhVCAM-1) were obtained from R & D Systems (Minneapolis, MN). Antibodies against CCR5 (anti–hCKR-5 no. sc-8283 and anti–mCKR-5 no. sc-6129) for fluorescence-activated cell sorter (FACS) analysis were from Santa Cruz Biotechnology (Santa Cruz, CA). Neutralizing mouse anti–human IL-6 and anti–IL-1β mAbs were purchased from Genzyme (Cambridge, MA). Fluorescein isothiocyanate (FITC)–conjugated mouse anti–human VLA-4, VLA-5, CD44, and VCAM-1 antibodies were from Pharmingen (San Diego, CA). Mouse anti–human CD3, CD4, CD5, CD8, CD11b, CD14, CD16, CD19, and CD33 mAbs were from Nichirei (Tokyo, Japan). The rhRANK ligand was from Pepro Tech (London, England), and rh osteoprotegerin (OPG) was a kind gift from Dr Tadaaki Higashio at Snow Brand Milk Products (Tochigi, Japan).
Cells and cultures
Human myeloma-derived cell lines, ARH77, U266, RPMI8226, IM9, and HS-Sultan, a human monocytic leukemia cell line, U937, and a murine bone marrow stromal cell line, ST-2, were obtained from the American Type Culture Collection (Rockville, MD). Human myeloma cell lines, TSPC-1, OPC, MPC, and NPC, were established in our laboratory, which showed plasmacytoid morphology, strongly expressed CD38, CD56, syndecan 1, myeloma-specific HM1.24 antigen,16 and VLA-4 on their surface, and secreted monoclonal BJPκ, IgGλ, IgAκ, and IgGλ, respectively. Neither CD19 nor CD20 were expressed on these cells. TSPC-1 and OPC were found to be negative for Epstein-Barr virus (EBV) infection using an in situ hybridization probe for EBV RNA (Daco Japan, Kyoto, Japan). A human bone marrow stromal cell line, KM102,17 was kindly provided by Dr Harigaya (Chiba University, Chiba, Japan). A mouse preosteoclast cell line, C7,18 was a generous gift of Dr Shin-ichi Hayashi (Tottori University, Tottori, Japan).
All procedures involving human specimens were performed according to the protocol approved by the Institutional Review Board for human protection. To obtain human marrow stromal cells, bone marrow mononuclear cells were isolated by Ficoll-Hypaque density gradient centrifugation (Pharmacia LKB Biotechnology, Uppsala, Sweden) from heparinized bone marrow blood drawn from patients with MM under written informed consents. These marrow cells were depleted of monocytes and myeloid cells by incubation with anti-CD11b, CD14, and CD33 mAb and subsequent addition of Dynabeads M-450 goat anti–mouse IgG (Dynal, Great Neck, NY) according to the manufacturer's instructions, and were resuspended in Iscove modified Dulbecco medium (IMDM) supplemented with 12.5% fetal calf serum (FCS; Whittaker Bioproducts, Walkersville, MD), 12.5% horse serum (Whittaker), 50 U/mL penicillin, and 50 μg/mL streptomycin (Gibco BRL, Rockville, MD). The adherent marrow stromal cells were serially passed at confluency, using 0.05% trypsin/0.53 mM EDTA (ethylenediaminetetraacetic acid; Gibco BRL), to obtain a homogeneous population of spindle-shaped cells. These cells expressed vimentin, collagen type I and III, but not endothelial cell antigens such as collagen type IV or factor VIII antigen, or hematopoietic cell surface antigens (data not shown).
Myeloma cell-rich fractions were prepared from bone marrow mononuclear cells by negative selection using Dynabeads M-450 goat antimouse IgG (Dynal) and a cocktail of mAbs including anti-CD3, CD4, CD8, CD19, CD11b, CD14, CD16, and CD33 mAbs. Highly purified myeloma cells were subsequently prepared by a positive selection using magnetic beads and a human myeloma cell-specific anti-HM1.24 mAb raised in our laboratory.16 Purity of thus obtained myeloma cells was more than 95%. Myeloma cells were cultured in a serum-free medium containing a 1:1 mixture of Ham F-12 medium and Dulbecco modified Eagle medium (GIT medium, Wako Pure Chemicals, Osaka, Japan).
In vitro osteoclastogenesis
For in vitro osteoclastogenesis, osteoclast precursors were first prepared from unfractionated bone cells according to the previously described procedure19 with a slight modification. In brief, minced long bones of 5-day-old white rabbits were agitated by vortexing, and bone particles were removed by sedimentation for 30 seconds in Eagle minimal essential medium (MEM; Life Technologies, Gaithersburg, MD). After centrifugation at 300g for 3 minutes, two thirds of the supernatant from the top was removed. Remaining fractions were used as a source of osteoclast precursors. Thus obtained preosteoclast-rich fractions of rabbit bone cells were seeded in 96-well plates at 5 × 104 cells/well and cultured for 96 hours in MEM containing 3% fetal bovine serum (FBS) in the absence or presence of various factors or conditioned media. For coculture experiments, MM cells at 2 × 103 cells/well were added into the cultures. Cocultures of mouse stromal cell line, ST-2, and a preosteoclastic cell line, C7,18 were performed as previously described. Briefly, 3 × 104 C7 and 1 × 104 ST-2 cells/well were cultured in 96 well-culture plates in αMEM plus 10% FBS in the presence of 100 nM dexamethasone and test reagents. After 8 days, the number of tartrate-resistant acid phosphatase (TRAP)–positive multinucleated cells (MNCs) were counted as described below.
Assays for osteoclastogenesis and bone resorption
To evaluate osteoclastlike cell formation, cells were stained for TRAP using leukocyte acid phosphatase kit (Sigma, St Louis, MO) and the number of TRAP+ MNCs were counted. For resorption assays, cells were cultured on bone slices from calf femur for 96 hours. Cells were then brushed off and bone slices were stained with acid hematoxylin for 5 minutes to visualize resorption pits. For quantification, the number of mesh squares covering the pits was counted using a microscope with a mesh glass installed in the ocular lens.
Collection of conditioned media and measurement of cytokine secretion
The MM cell lines or primary MM cells from patients were cultured at 5 × 105 cells/mL for 2 days and conditioned media were harvested. MIP-1α and MIP-1β levels were measured using Quantikine MIP-1α and MIP-1β enzyme immunoassay kits, respectively (R & D Systems). IL-1β and IL-6 levels were quantified using TiterZyme IL-1β and IL-6 enzyme immunoassay kits, respectively (Perseptive Diagnostic, Cambridge, MA), according to the manufacturer's instructions.
Flow cytometry
Cell preparation and staining for flow cytometry were performed as described previously.19 Approximately 106cells were incubated with saturating concentrations of FITC-conjugated mAbs on ice for 1 hour. For indirect fluorescence staining, cells were incubated first with primary antibodies on ice for 1 hour, washed, and then incubated with FITC-conjugated secondary antibodies on ice for 30 minutes. Samples were analyzed by flow cytometry using EPICS-Profile (Coulter Electronics, Hialeah, FL).
Cell adhesion assays
rhVCAM-1 was applied to 24-well culture plates at 10 μg/mL in Ca/Mg-free phosphate-buffered saline (PBS) and incubated at 4°C overnight. Nonspecific binding sites were subsequently blocked with 3% human serum albumin (HSA) in Ca/Mg-free PBS (Green-Cross, Osaka, Japan) for 2 hours at 37°C. MM cells were labeled with 10 μg/mL fluorescent dye (BCECF-am; Dojindo, Kumamoto, Japan) in GIT medium for 2 hours at 37°C. A total of 1 × 106 MM cells were washed, resuspended in GIT medium with or without the indicated antibodies at a saturating concentration (20 μg/mL), plated onto prewashed rhVCAM-1–coated plates, and incubated at 4°C for 30 minutes, and then rapidly warmed to 37°C and further incubated for 30 minutes. After gently washing 4 times with GIT medium at room temperature, fluorescence intensity of lysed adherent cells was measured as previously described.20
RNA analysis
Total RNA was extracted from various cells using TRIZOL reagent (Gibco BRL, Rockville, MD). For reverse transcription-polymerase chain reaction (RT-PCR), 2 μg total RNA was reverse transcribed with Superscript II (Gibco) in a 20-μL reaction. Two microliters of the 20-μL reaction was used for the subsequent PCR analysis with cycles of 95°C for 30 seconds, 58°C for 30 seconds, and 72°C for 30 seconds. Primers used are as follows: 5′-gaatcatgcaggtctccactg-3′ (nucleotides 79-99) and 5′-ctctaggtcgctgacatatttc-3′ (nucleotides 350-329) for human MIP-1α; 5′-gtgactgtcctgtctctcctc-3′ (nucleotides 121-141) and 5′-gttccaggtcatacacgtactc-3′ (nucleotides 379-358) for human MIP-1β; 5′-agtattcacagggctctatcac-3′ (nucleotides 316-337) and 5′-atggcctggtctagtctattag-3′ (nucleotides 846-825) for mouse CCR5; 5′-agtgtcaagtccaatctatgac-3′ (nucleotides 369-390) and 5′-tatggaaaatgagagctgcagg-3′ (nucleotides 908-887) for human CCR5; 5′-tgtcttcaccaccatggagaagg-3′ (nucleotides 340-362) and 5′-gtggatgcagggatgatgttctg-3′ (nucleotides 672-650) for mouse and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Amplified products were dissolved in a 2% agarose gel and visualized with ethidium bromide staining.
For RNase protection assay, a fragment of mouse RANK ligand cDNA (nucleotides 480-1030) obtained by RT-PCR was subcloned into pBluescript SKII(+) (Stratagene, La Jolla, CA). The resultant plasmid was linearized and used for generation of a cRNA probe using MAXIscript in vitro transcription kit (Ambion, Austin, TX). The control probe template for mouse β-actin was purchased from Ambion. RNase protection assays were performed using RPA II kit (Ambion) according to the manufacturer's instructions. Briefly, RNA samples were incubated simultaneously with α-[32P]-labeled cRNA probes for RANK ligand and actin at 42°C for 18 hours and digested with an RNase A/T1 mixture. RNA/RNA hybrids were precipitated, separated on a 6% polyacrylamide gel containing 8M urea, dried, and autoradiographed.
Results
Stimulatory effect of MM cells on osteoclast formation and function in vitro
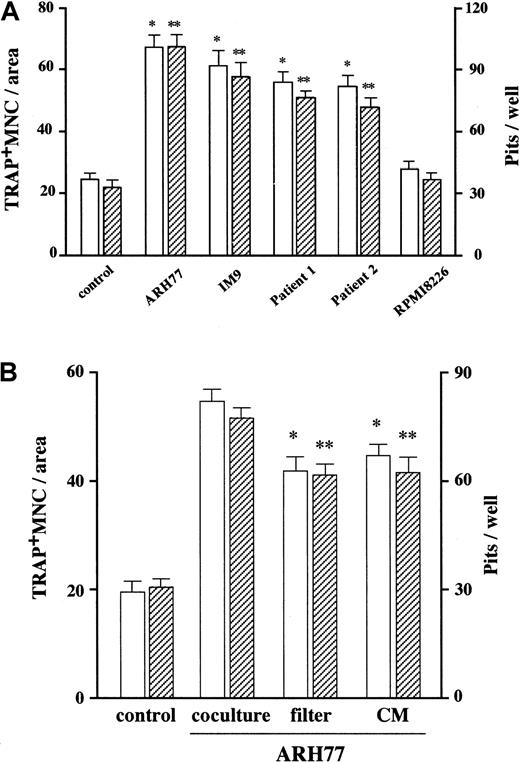
ARH77 is an EBV-transformed human B lymphoblastic cell line that is able to develop MM-like destructive bone lesions when transplanted into SCID mice. As an initial step to investigate the mechanism of MM-induced bone resorption, we examined whether or not ARH77 cells had the ability to stimulate osteoclast formation and function in vitro. When these cells were cocultured with osteoclast precursors from rabbit bone, both formation of TRAP+ multinucleated osteoclastlike cells and their activity to form resorption pits were increased to approximately 3-fold (Figure 1A). The fact that the induction of OCL formation occurred to nearly the same extent as the increase in resorption suggested that the main effect of MM cells was enhancement of osteoclast differentiation rather than direct activation of mature osteoclasts. Similar results were obtained with another human myeloma cell line, IM-9, as well as primary myeloma cells purified from bone marrow samples of 2 patients who had radiographically demonstrated bone lesions. In contrast, addition of RPMI8226 cells that were incapable of forming bone lesions in the SCID mouse model (data not shown) had no effects (Figure 1A). Thus, we have established an in vitro model system where MM cells activate osteoclastic bone resorption in the bone marrow milieu.
Osteoclast formation and function.
(A) Effect of MM cells on osteoclast formation and function in vitro. MM cell lines as well as patients' MM cells were cocultured with rabbit bone cells on dentine slices as described in “Materials and methods.” The data represent the number of TRAP+ MNCs (open bars) and pits on dentine slices (hatched bars). Results were expressed as a mean of 5 samples with an error bar of SD. * and ** indicate significantly different from the control culture in the number of TRAP+ MNCs and pits, respectively (P < .05, ANOVA with Scheffe post hoc tests). (B) Contact- and soluble factor–dependent stimulation of osteoclast formation and function by MM cells. ARH77 cells were cocultured with rabbit bone cells on dentine slices. Membrane filters were used to prevent cell-cell contact between MM cells and rabbit bone cells. Conditioned media (CM) from ARH77 cells were added at a dilution of 1:2 into rabbit bone cell cultures. Results were shown as a mean of 5 samples with an error bar of SD. * and ** indicate significantly different from cocultures in the number of TRAP+ MNCs and pits, respectively (P < .05, ANOVA with Scheffe post hoc tests).
Osteoclast formation and function.
(A) Effect of MM cells on osteoclast formation and function in vitro. MM cell lines as well as patients' MM cells were cocultured with rabbit bone cells on dentine slices as described in “Materials and methods.” The data represent the number of TRAP+ MNCs (open bars) and pits on dentine slices (hatched bars). Results were expressed as a mean of 5 samples with an error bar of SD. * and ** indicate significantly different from the control culture in the number of TRAP+ MNCs and pits, respectively (P < .05, ANOVA with Scheffe post hoc tests). (B) Contact- and soluble factor–dependent stimulation of osteoclast formation and function by MM cells. ARH77 cells were cocultured with rabbit bone cells on dentine slices. Membrane filters were used to prevent cell-cell contact between MM cells and rabbit bone cells. Conditioned media (CM) from ARH77 cells were added at a dilution of 1:2 into rabbit bone cell cultures. Results were shown as a mean of 5 samples with an error bar of SD. * and ** indicate significantly different from cocultures in the number of TRAP+ MNCs and pits, respectively (P < .05, ANOVA with Scheffe post hoc tests).
Soluble factor– and contact-dependent stimulation of osteoclast formation and function by MM cells
We next determined whether soluble factors secreted by MM cells contribute to the ability of these cells to enhance OCL formation and function. As shown in Figure 1B, conditioned media from ARH77 myeloma cell cultures increased OCL formation and function to more than 2-fold. Consistent with the potent osteoclastogenic activity of the conditioned media, inhibition of direct cellular interactions between MM and rabbit bone cells by membrane filters resulted in only 20% reduction in the MM cell effect compared with cocultures of these 2 cell populations (Figure 1B). Therefore, although cell-cell contacts with bone cells may contribute to some extent, a large part of the MM cell effect is accounted for by soluble factors secreted by MM cells at least in this in vitro system.
Production of osteoclastogenic chemokines MIP-1α and MIP-1β by MM cells
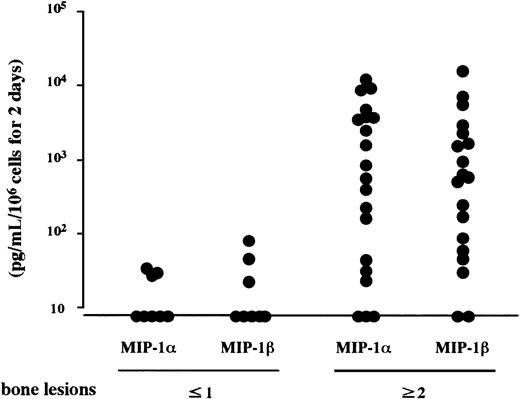
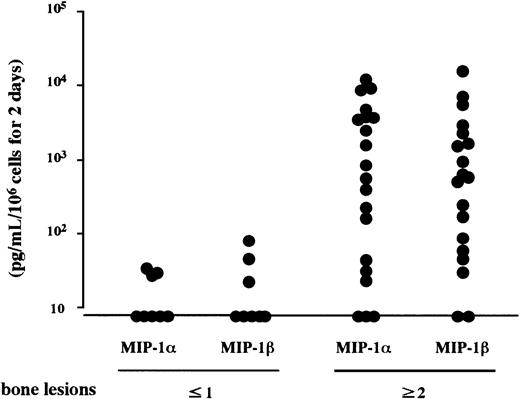
In search of soluble factors that mediate the effect of MM cells on osteoclastogenesis, we first measured amounts of IL-1β and IL-6, well-known stimulators of osteoclast differentiation, in the MM cell-conditioned media. However, these factors were not produced at detectable levels by ARH77, IM-9, or primary cells from the 2 patients (data not shown), all of which potently stimulated osteoclastogenesis in cocultures with marrow cells under our experimental conditions. We then turned to C-C chemokines, MIP-1α and MIP-1β, both of which dose-dependently up-regulated osteoclast formation and function when added to the rabbit bone cell cultures (Figure2). We found that 7 MM cell lines including ARH77, IM9, TSPC-1, MPC, NPC, OPC, and U266 secreted large amounts of both MIP-1α and MIP-1β in culture supernatants (Table1). Expression of MIP-1α and MIP-1β by these cell lines except MPC and NPC were also confirmed at the mRNA levels (Figure 3). In contrast, RPMI8226 cells, which are incapable of enhancing bone resorption in vitro or forming bone lesions in vivo, did not produce detectable amounts of MIP-1α and MIP-1β (Table 1 and Figure 3). MIP-1α and MIP-1β were also secreted by MM cells freshly isolated from 20 and 21 of 28 patients, respectively. Furthermore, MM cells from patients with multiple bone lesions secreted an apparently higher amount of MIP-1 than those from patients with less advanced bone involvement (Figure4), demonstrating a clinical correlation between severity of bone lesions and MIP-1 production by MM cells. On the average, concentrations of MIP-1α and MIP-1β in culture supernatants of isolated MM cells were 14 ± 7 (mean ± SD) and 24 ± 13 pg/mL, respectively, in patients with no or a single bone lesion (n = 8); and 2417 ± 822 and 1462 ± 845, respectively, in those with 2 or more bone lesions (n = 20). Collectively, these results suggested that osteoclastogenic chemokines MIP-α and MIP-1β are constitutively secreted by a majority of MM cells and further implied that they may play a role in the development of MM bone lesions because MIP-1 production correlated well with the ability of MM cells to enhance osteoclastic bone resorption in the in vitro cocultures with rabbit bone cells (Figure 1) and to cause bone destruction in a mouse myeloma model (Table 1 and Figure 3) and in MM patients (Figure4).
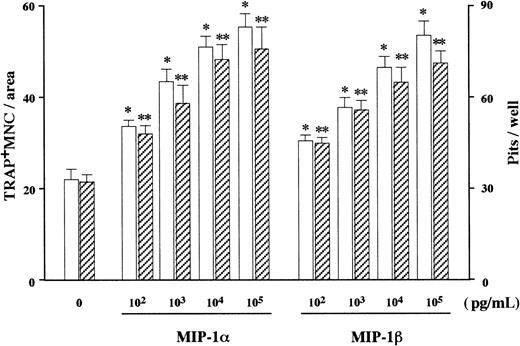
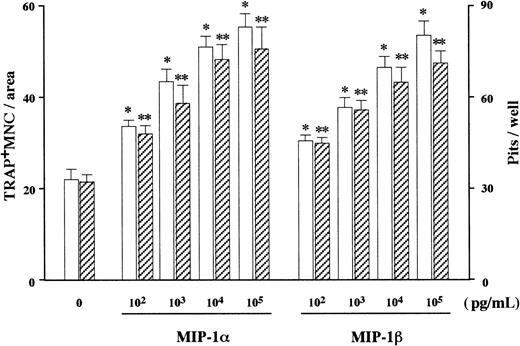
Effect of rh MIP-1α and MIP-1β on OCL formation and function in vitro.
rhMIP-1α and MIP-1β were added at the indicated doses to rabbit bone cell cultures. The data represent the number of TRAP+MNCs (open bars) and pits on dentine slices (hatched bars). Results were expressed as a mean of 5 samples with an error bar of SD. * and ** indicate significantly different from the control culture in the number of TRAP+ MNCs and pits, respectively (P < .05, ANOVA with Scheffe post hoc tests).
Effect of rh MIP-1α and MIP-1β on OCL formation and function in vitro.
rhMIP-1α and MIP-1β were added at the indicated doses to rabbit bone cell cultures. The data represent the number of TRAP+MNCs (open bars) and pits on dentine slices (hatched bars). Results were expressed as a mean of 5 samples with an error bar of SD. * and ** indicate significantly different from the control culture in the number of TRAP+ MNCs and pits, respectively (P < .05, ANOVA with Scheffe post hoc tests).
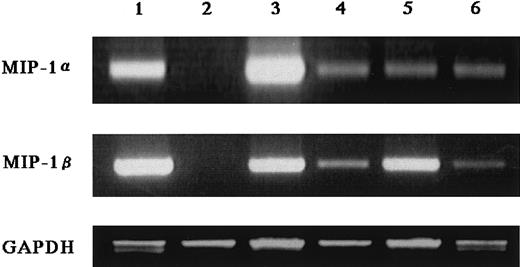
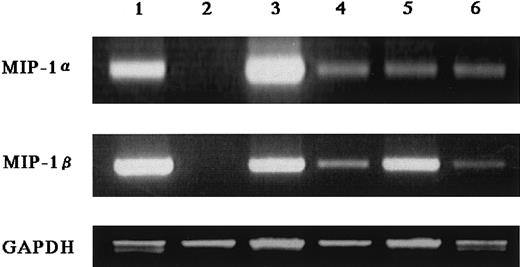
Expression of MIP-1α and MIP-1β mRNA by MM cells.
Two micrograms of total RNA extracted from various myeloma cell lines was reverse transcribed, and 2 μL of the 20 μL reaction was used for PCR analysis of MIP-1α and MIP-1β, and GAPDH mRNA expression as described in “Materials and methods.” Cell lines used in these experiments were U266 (lane 1), RPMI8226 (lane 2), ARH77 (lane 3), IM-9 (lane 4), TSPC-1 (lane 5), and OPC (lane 6).
Expression of MIP-1α and MIP-1β mRNA by MM cells.
Two micrograms of total RNA extracted from various myeloma cell lines was reverse transcribed, and 2 μL of the 20 μL reaction was used for PCR analysis of MIP-1α and MIP-1β, and GAPDH mRNA expression as described in “Materials and methods.” Cell lines used in these experiments were U266 (lane 1), RPMI8226 (lane 2), ARH77 (lane 3), IM-9 (lane 4), TSPC-1 (lane 5), and OPC (lane 6).
Constitutive secretion of MIP-1α and MIP-1β by MM cells.
MM cells were isolated from bone marrow MNCs of patients with MM who had no or a single radiographically demonstrated osteolytic lesion or 2 or more lesions. MIP-1α and MIP-1β levels in the culture supernatants of the cells were measured by enzyme-linked immunosorbent assay (ELISA) as described in “Materials and methods.”
Constitutive secretion of MIP-1α and MIP-1β by MM cells.
MM cells were isolated from bone marrow MNCs of patients with MM who had no or a single radiographically demonstrated osteolytic lesion or 2 or more lesions. MIP-1α and MIP-1β levels in the culture supernatants of the cells were measured by enzyme-linked immunosorbent assay (ELISA) as described in “Materials and methods.”
Inhibition of the MM cell-induced osteoclastogenesis by neutralizing antibodies against MIP-1α and MIP-1β or their receptor CCR5
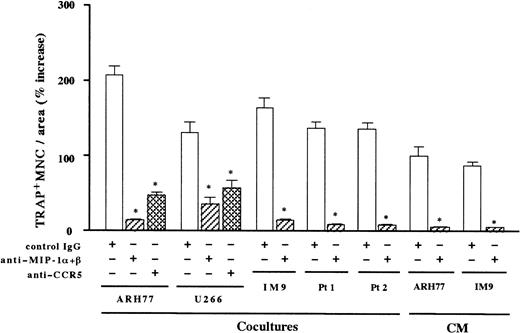
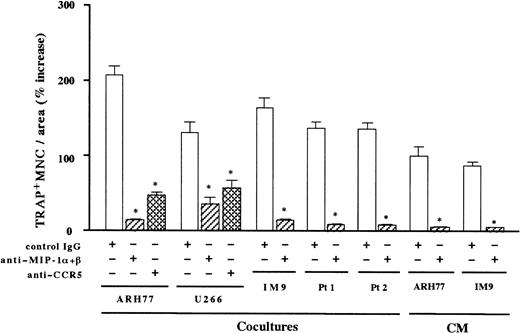
To further determine the contribution of MIP-1α and MIP-1β to the MM-induced bone resorption, we next examined the effect of neutralizing antibodies against MIP-1α and MIP-1β on the osteoclast induction by MM cells. As shown in Figure5, the stimulatory effect of MM cell-conditioned media on OCL formation was almost completely abrogated by the addition of antibodies against MIP-1α and MIP-1β in combination. Near-total inhibition of the osteoclast induction by these antibodies was also observed in cocultures with MM cells including ARH77, IM-9, and the same populations of primary MM cells from the 2 patients shown in Figure 1A (Figure 5). Similar results were also obtained with another EBV− myeloma cell line, U266. In contrast, neutralizing antibodies against IL-1β or IL-6 had no effects on the osteoclastogenic activities of these MM cells (data not shown). These results suggested that osteoclastogenic effects of MM cells were largely dependent on MIP-1α and MIP-1β in the bone marrow milieu. To examine if the effects of these chemokines were mediated by a specific chemokine receptor, we tested the ability of a blocking antibody against a chemokine receptor, CCR5. CCR5 has been shown to bind both MIP-1α and MIP-1β and is likely to be a major receptor for these chemokines.21 An anti-CCR5 blocking antibody also inhibited ARH77- and U266-induced OCL formation to a large extent (Figure 5). However, the suppression of OCL formation was significantly less than that by antibodies against MIP-1α and MIP-1β, implying that other chemokine receptors may also mediate the effect of MIP-1α and MIP-1β on OCL formation. Taken together, these results suggest that osteoclast induction by MM cells is in large part mediated by MIP-1α and MIP-1β, which are secreted by MM cells and which mainly act via a chemokine receptor, CCR5.
Inhibition of contact- and soluble factor–dependent MM cell-induced osteoclastogenesis by antibodies against MIP-1α and MIP-1β or CCR5.
Rabbit bone marrow cells were cocultured with MM cells or cultured with MM cell-conditioned media (CM). CM were added at a final dilution of 1:2. Anti–MIP-1α and anti–MIP-1β neutralizing antibodies were used in combination at 20 μg/mL for each. Goat IgG was used as a control. Results were shown as a percent increase in the number of TRAP+ MNCs from the control culture with rabbit bone marrow cells alone and expressed as a mean of 5 samples with an error bar of SD. * indicates significantly different from the control IgG-treated culture in the number of TRAP+ MNCs (P < .05, ANOVA with Scheffe post hoc tests).
Inhibition of contact- and soluble factor–dependent MM cell-induced osteoclastogenesis by antibodies against MIP-1α and MIP-1β or CCR5.
Rabbit bone marrow cells were cocultured with MM cells or cultured with MM cell-conditioned media (CM). CM were added at a final dilution of 1:2. Anti–MIP-1α and anti–MIP-1β neutralizing antibodies were used in combination at 20 μg/mL for each. Goat IgG was used as a control. Results were shown as a percent increase in the number of TRAP+ MNCs from the control culture with rabbit bone marrow cells alone and expressed as a mean of 5 samples with an error bar of SD. * indicates significantly different from the control IgG-treated culture in the number of TRAP+ MNCs (P < .05, ANOVA with Scheffe post hoc tests).
Expression of CCR5 on bone marrow stromal and myeloma cells
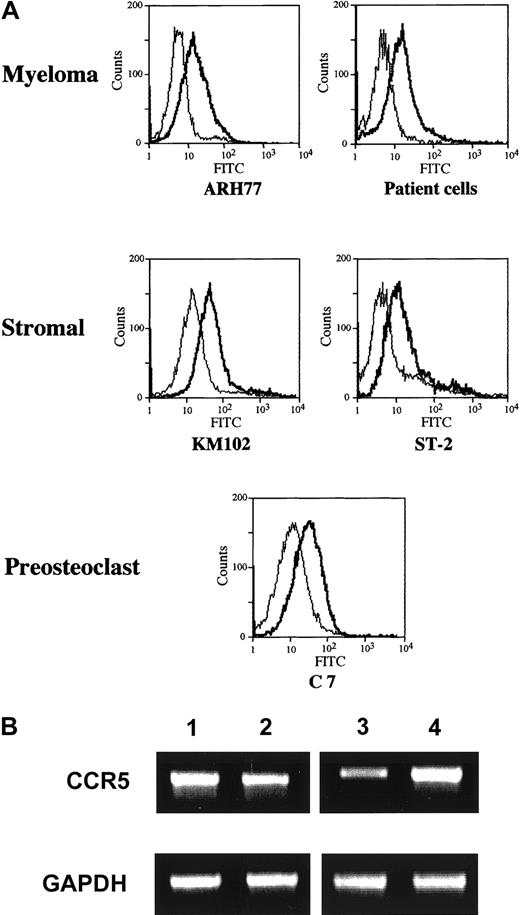
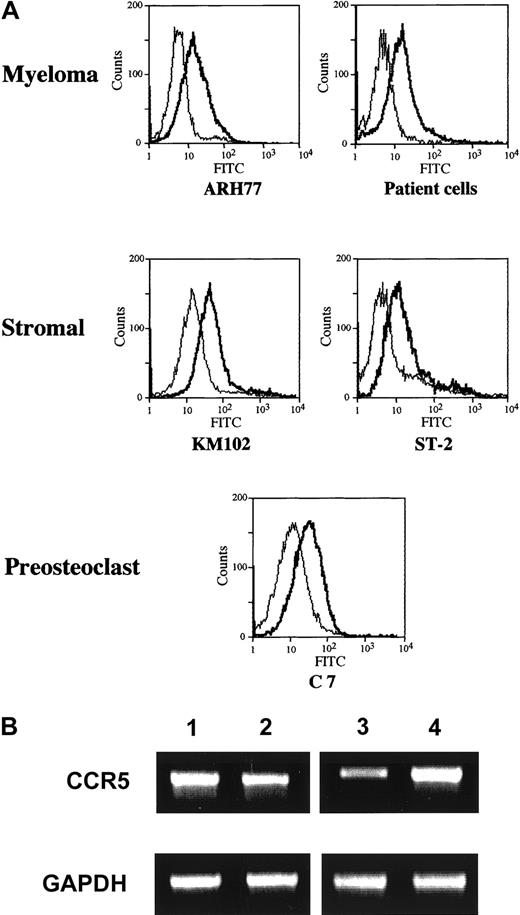
Given the fact that the effect of MM cells is largely dependent on CCR5, we next examined its expression on the cell surface of marrow stromal and myeloma cells by FACS analysis. The results indicated that CCR5 was expressed in both a human marrow stromal cell line KM102 and a myeloma cell line ARH77 as well as primary MM cells from patients (Figure 6A). As shown in Figure 6B, the expression of CCR5 in these cells was also confirmed at the mRNA level by RT-PCR analysis. Therefore, both marrow stromal and myeloma cells are potential targets of MIP-1α and MIP-1β.
CCR5 expression by MM cells.
(A) Surface expression of CCR5 on human MM cells and marrow stromal cells. A human MM cell line ARH77, primary MM cells from patients, and a marrow stromal cell line KM102, and a mouse stromal cell line ST-2 and a preosteoclastic cell line C7 were incubated first with mouse antihuman or mouse CCR5 antibodies or control IgG and then with an FITC-labeled secondary antibody, and were analyzed for the surface expression of CCR5 by flow cytometry as described in “Materials and methods.” (B) Expression of CCR5 mRNA. Expression of CCR5 mRNA as well as well an internal control GAPDH by ARH77 (lane 1), KM102 (lane 2), ST-2 (lane 3), and C7 (lane 4) was determined by RT-PCR analysis as described in “Materials and methods.”
CCR5 expression by MM cells.
(A) Surface expression of CCR5 on human MM cells and marrow stromal cells. A human MM cell line ARH77, primary MM cells from patients, and a marrow stromal cell line KM102, and a mouse stromal cell line ST-2 and a preosteoclastic cell line C7 were incubated first with mouse antihuman or mouse CCR5 antibodies or control IgG and then with an FITC-labeled secondary antibody, and were analyzed for the surface expression of CCR5 by flow cytometry as described in “Materials and methods.” (B) Expression of CCR5 mRNA. Expression of CCR5 mRNA as well as well an internal control GAPDH by ARH77 (lane 1), KM102 (lane 2), ST-2 (lane 3), and C7 (lane 4) was determined by RT-PCR analysis as described in “Materials and methods.”
Dependency of MM cell-induced osteoclastogenesis on RANK ligand expression by stromal cells
Expression of CCR5 on the surface of marrow stromal cells led us to hypothesize that the osteoclastogenic effect of MIP-1α and MIP-1β is mediated by stromal cells. Critical roles of stromal/osteoblastic cells in osteoclast differentiation have been established. Recent studies have identified RANK ligand, a tumor necrosis factor family member, as a key molecule mediating osteoclastogenic effects of a large repertoire of bone resorptive cytokines and hormones such as parathyroid hormone (PTH), 1α,25-dihydroxyvitamin D, and IL-6.22-24 They first stimulate stromal/osteoblastic cells to express RANK ligand, which then interacts with its cognate receptor RANK expressed on the cell surface of osteoclast progenitors to induce their differentiation. Therefore, we next examined whether or not the MM cell effect was dependent on RANK ligand expression by stromal cells by testing inhibitory effects of OPG,25 26 a decoy receptor of RANK ligand on MM cell-induced OCL formation. As shown in Figure7, the osteoclastogenic activity of ARH77 cells as well as MIP-1α and MIP-1β was almost completely inhibited by OPG in rabbit bone cell cultures. Furthermore, MIP-1α and MIP-1β directly induced RANK ligand expression by a mouse marrow stromal cell line, ST-2, which expressed CCR5 in the presence of a suboptimal concentration (0.1 nM) of 1α,25-dihydroxyvitamin D3(Figure 8A). The induction of RANK ligand in ST-2 cells was in parallel with the stimulation of osteoclast differentiation in cocultures with a mouse preosteoclast cell line, C7 (Figure 8B), confirming its functional significance. Neither MIP-1α nor MIP-1β was able to induce osteoclast differentiation when C7 cells were cultured alone (data not shown), although CCR5 was expressed not only by ST-2 but also C7 cells (Figure 6). Taken together, these results suggest that up-regulation of stromal cell RANK ligand expression by MIP-1α and MIP-1β, probably via CCR5, may be involved in the mechanisms whereby MM cells enhance osteoclastic bone resorption.
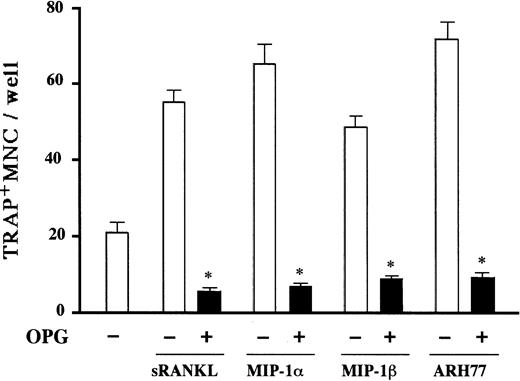
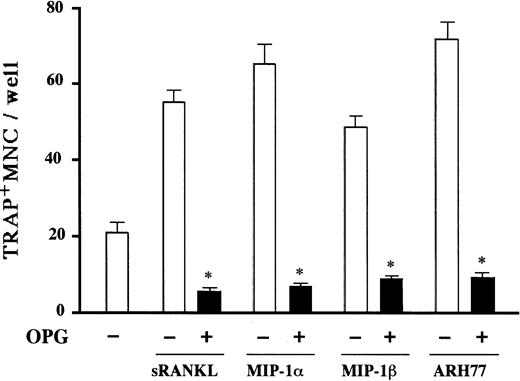
Inhibitory effects of OPG on osteoclastogenesis induced by MIP-1α, MIP-1β, and MM cells.
Rabbit bone marrow cells were cultured with rh soluble RANK ligand (sRANKL) at 50 ng/mL, rhMIP-1α at 10 ng/mL, and rhMIP-1β at 10 ng/mL, and cocultured with ARH77 cells in the presence (filled bars) or absence (open bars) of OPG at 1 mg/mL. The data represent the number of TRAP+ MNCs and are expressed as a mean of 5 samples with an error bar of SD. * indicates significantly different from the cultures without OPG (P < .05, ANOVA with Scheffe post hoc tests).
Inhibitory effects of OPG on osteoclastogenesis induced by MIP-1α, MIP-1β, and MM cells.
Rabbit bone marrow cells were cultured with rh soluble RANK ligand (sRANKL) at 50 ng/mL, rhMIP-1α at 10 ng/mL, and rhMIP-1β at 10 ng/mL, and cocultured with ARH77 cells in the presence (filled bars) or absence (open bars) of OPG at 1 mg/mL. The data represent the number of TRAP+ MNCs and are expressed as a mean of 5 samples with an error bar of SD. * indicates significantly different from the cultures without OPG (P < .05, ANOVA with Scheffe post hoc tests).
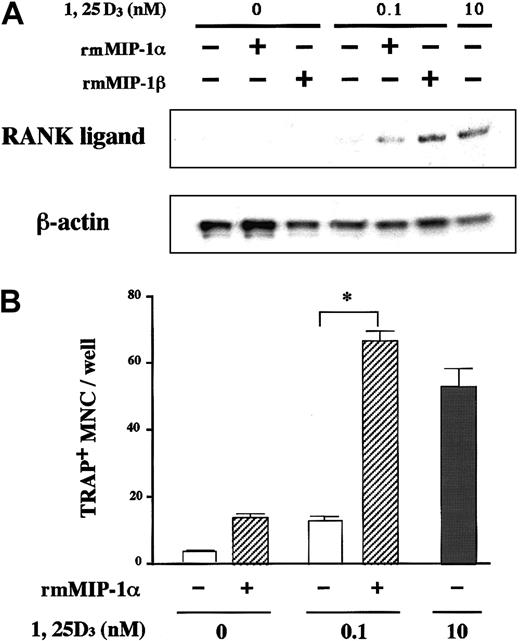
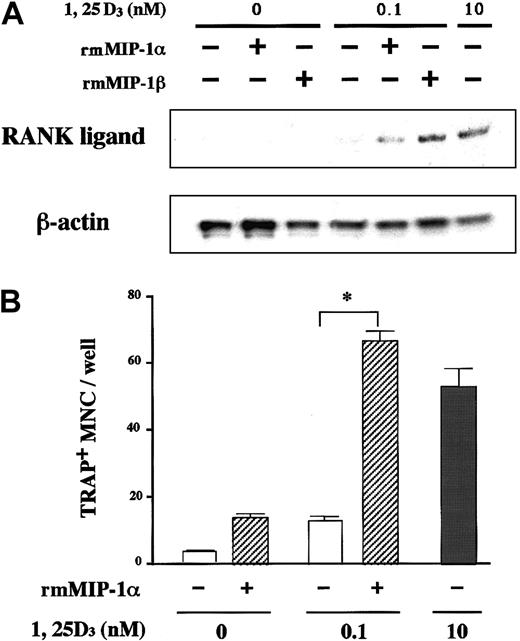
MIP-1 induces stromal cell RANK ligand expression and osteoclastogenesis.
(A) MIP-1 induction of RANK ligand mRNA expression by a mouse marrow stromal cell line, ST-2. ST-2 cells were cultured with or without recombinant mouse (rm) MIP-1α or MIP-1β at 10 ng/mL in the presence or absence of a suboptimal concentration (0.1 nM) of 1α,25-dihydroxyvitamin D3. RANK ligand mRNA expression was determined by RNase protection assay as described in “Materials and methods.” The lower panel shows β-actin mRNA expression as a loading control. (B) MIP-1 induction of osteoclast differentiation of C7 in cocultures with ST-2 cells. A mouse preosteoclast cell line, C7, was cocultured with ST-2 cells with or without rmMIP-1α at 10 ng/mL in the presence or absence of 0.1 nM 1α,25-dihydroxyvitamin D3. Osteoclast differentiation was examined by counting the number of TRAP+ MNCs after 8 days of culture as described in “Materials and methods.” Results represent a mean of 5 samples with an error bar of SD. * indicates significantly different from the control culture without treatment (P < .05, ANOVA with Scheffe post hoc tests).
MIP-1 induces stromal cell RANK ligand expression and osteoclastogenesis.
(A) MIP-1 induction of RANK ligand mRNA expression by a mouse marrow stromal cell line, ST-2. ST-2 cells were cultured with or without recombinant mouse (rm) MIP-1α or MIP-1β at 10 ng/mL in the presence or absence of a suboptimal concentration (0.1 nM) of 1α,25-dihydroxyvitamin D3. RANK ligand mRNA expression was determined by RNase protection assay as described in “Materials and methods.” The lower panel shows β-actin mRNA expression as a loading control. (B) MIP-1 induction of osteoclast differentiation of C7 in cocultures with ST-2 cells. A mouse preosteoclast cell line, C7, was cocultured with ST-2 cells with or without rmMIP-1α at 10 ng/mL in the presence or absence of 0.1 nM 1α,25-dihydroxyvitamin D3. Osteoclast differentiation was examined by counting the number of TRAP+ MNCs after 8 days of culture as described in “Materials and methods.” Results represent a mean of 5 samples with an error bar of SD. * indicates significantly different from the control culture without treatment (P < .05, ANOVA with Scheffe post hoc tests).
Discussion
MIP-1α and MIP-1β belong to the C-C chemokine family, which is characterized by the absence of an intervening amino acid between the first 2 of the 4 cysteine residues that are conserved in the chemokine superfamily.27,28 MIP-1α has been suggested to be a positive regulator of osteoclast differentiation and chemotaxis.29-33 In the present study, we have demonstrated that MM cells isolated from a majority of MM patients as well as MM cell lines constitutively secrete MIP-1α and MIP-1β and that both of the chemokines are able to potently stimulate osteoclastogenesis and bone resorption. Furthermore, treatment with neutralizing antibodies against MIP-1α and MIP-1β in combination or those against CCR5, a common receptor for these chemokines, was able to almost completely block the osteoclast induction either by cocultures with MM cells or by MM cell-conditioned media. These results suggest that MIP-1α and MIP-1β may play a role in the pathogenesis of lytic bone lesions in MM.
Critical roles of MIP-1α and MIP-1β in the MM bone disease are further supported by the clinical correlations between the severity of bone lesions and MIP-1 production by MM cells isolated from patients. Similar results were very recently reported by Choi and his colleagues demonstrating that concentrations of MIP-1α in the bone marrow plasma were higher in patients with MM stage III with active disease than in those with stage I or those with stage III with inactive disease.31 It is of note that our data are based on the amount of MIP-1 secretion per MM cell, which has been purified to near homogeneity, in contrast to their results obtained from marrow plasma samples. Our results have ruled out the possibility that the increase in MIP-1 secretion is a mere reflection of an increase in the number of MM cells present in the bone marrow. Therefore, increased expression of MIP-1α and MIP-1β by MM cells is associated with aggressive bone destruction. However, it is currently unknown whether this association is based on clonal variance determined by each clone's intrinsic character or whether it is a result of stage-dependent changes in production of these chemokines within a particular MM clone. Further studies will be necessary to clarify the molecular mechanism of up-regulation of MIP-1α and MIP-1β expression by MM cells with an increased capacity of inducing bone resorption.
We have demonstrated that both MIP-1α and MIP-1β, when added exogenously to culture media, are able to induce RANK ligand expression by stromal cells. Induction of RANK ligand may at least in part account for the osteoclast-inductive effects of these chemokines as a secreted factor. Consistently, osteoclast-inductive activities of MM cells as well as MIP-1α and MIP-1β were mostly blocked by OPG treatment. The fact that osteoclast induction by MM cells is RANK ligand-dependent would provide an important molecular basis for clinical applications of OPG to the treatment of myeloma bone disease. However, contribution of other factors such as PTH-related peptide and IL-6 to RANK ligand induction by MM cells remains to be determined.
Although our results suggest that soluble factors secreted by MM cells play substantial roles in osteoclast induction in the in vitro system used in the present study, direct interactions between MM cells and bone marrow components should also contribute significantly to MM cell-induced bone resorption. It was at first surprising to us that anti–MIP-1α and anti–MIP-1β neutralizing antibodies were able to almost completely abrogate the osteoclast-inductive activities of MM cells even in the cocultures because disruption of direct contacts by membrane filters consistently and considerably weakened the effects of MM cells on the osteoclast induction. These results suggest that MIP-1α and MIP-1β may also play a role in the contact-dependent ability of MM cells to stimulate osteoclastogenesis. In this aspect, it is intriguing that MIP-1α and MIP-1β have been demonstrated to activate integrins to trigger cell adhesion.34,35 Among major integrins abundantly expressed on the surface of MM cells is VLA-4 (α4β1-integrin),36 which can bind to VCAM-1 present on the stromal cell surface. Michigami et al recently reported that a murine myeloma cell line, 5TGM1, binds to stromal cells and induces secretion of osteoclastogenic factors in a VLA-4–dependent manner.37 Because the chemokine receptor, CCR5, is also expressed by MM cells, MIP-1α and MIP-1β may act on MM cells in an autocrine paracrine fashion to induce their adhesion to stromal cells through VLA-4/VCAM-1 interactions. Consistent with this idea, we found that adhesion of MM cells to VCAM-1–coated plates was inhibited by the addition of anti–MIP-1 neutralizing antibodies by approximately 80% (data not shown), suggesting that interactions of MM cells with stromal cell VCAM-1 may require continuous activation of VLA-4 by MIP-1. Taken together, these results suggest that MIP-1α and MIP-1β may also participate in cell-cell contact-dependent induction of osteoclasts by MM cells in the bone marrow. The molecular mechanisms by which MM cells activate osteoclastic bone resorption through direct cell-cell interactions remain to be elucidated.
In addition to their osteoclast-inductive capacity, MIP-1α and MIP-1β have other biologic activities that may be relevant to clinical features of myeloma patients. First, these chemokines have been suggested to be a potent modulator of hematopoiesis. MIP-1α has been shown to suppress hematopoietic stem cell proliferation38 as well as early erythropoiesis probably via one of the MIP-1α receptors, CCR1, which is predominantly expressed in cells of the erythroid lineage in the bone marrow.39 Moreover, MIP-1β has also been reported to induce apoptosis of pre–B cells in the presence of a survival factor, IL-7.40 Therefore, MIP-1α and MIP-1β are pluripotent chemokines that may play important roles in the pathogenesis of multiple clinical features of MM including not only destructive bone lesions, but also suppression of erythropoiesis and immunoglobulin production.
In conclusion, evidence presented herein suggests that MIP-1α and MIP-1β are among leading candidates for MM-derived factors that enhance osteoclast differentiation and function, thereby causing extensive bone destruction. Because the chemokines may also be involved in the development of major clinical features of MM other than bone complications, inhibition of production and activities of MIP-1α and MIP-1β could be a novel, powerful therapeutic maneuver with multiple targets for patients with myeloma.
We would like to thank Dr T. Higashio in Snow Brand Milk Products (Tochigi, Japan) for donating osteoprotegerin, Dr K. Harigaya (Chiba University, Chiba, Japan) for KM102, and Dr S. I. Hayashi (Tottori University, Tottori, Japan) for C7.
Supported by a Grant-in-Aid for Cancer Research (9-22) from the Ministry of Health, Labor and Welfare of Japan, and Grants-in-Aid for Scientific Research on Priority Areas (B) for T.M., for Scientific Research (B) for T.M., and for Encouragement of Young Scientists for D.I. from the Ministry of Education, Science, Sports and Culture of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Daisuke Inoue, First Department of Internal Medicine, University of Tokushima School of Medicine, 3-18-15 Kuramoto-cho, Tokushima 770-8503, Japan; e-mail:inoued@clin.med.tokushima-u.ac.jp.