Abstract
Acute graft-versus-host disease (GVHD) after allogeneic stem cell transplantation is associated with impaired deletion and anergy of host-reactive T cells. To elucidate the immunoregulatory events that may contribute to such dysregulated T-cell responses in GVHD, we studied superantigen (SAg) responses after adoptive T-cell transfer into severe combined immunodeficient (SCID) mice. SAg responses are normally regulated by mechanisms involving deletion and anergy, with SAg-reactive T cells typically being deleted rapidly in vivo. In a SCID mouse model of GVHD, however, allogeneic host SAg-reactive T cells were not deleted rapidly, but rather persisted in increased numbers for several months. Moreover, depending on the timing of SAg stimulation and the numbers of T cells transferred, dysregulation (impaired deletion and anergy) of SAg responses could be demonstrated following the adoptive transfer of syngeneic T cells into SCID mice as well. Transgenic T-cell receptor-bearing KJ1-26.1+ T cells were then used to determine the fate of weakly reactive T cells after adoptive transfer and SAg stimulation. When transferred alone, KJ1-26.1+ T cells demonstrated impaired deletion and anergy. In the presence of more strongly staphylococcal enterotoxin B (SEB)–reactive T cells, however, KJ1-26.1+ T cells were regulated normally, in a manner that could be prevented by inhibiting the effects of more strongly SEB-reactive cells or by increasing the level of activation of the KJ1-26.1+ T cells themselves. We suggest that the control mechanisms that normally regulate strongly activated T cells in immunocompetent animals are lost following adoptive transfer into immunodeficient hosts, and that this impairment contributes to the development of GVHD.
Introduction
Graft-versus-host disease (GVHD) is the major complication of allogeneic stem cell transplantation1(allo-SCT) and prevents this potentially curative treatment2 from being offered to a wider range of patients.3,4 GVHD is caused by T cells in the stem cell graft that recognize the antigenic differences that result from genetic disparities between the donor and host.1 Such antigens are distributed systemically on host antigen-presenting cells (APCs), and their numbers likely exceed by far those of the transplanted donor T cells. In other situations in which T cells encounter antigens in such excess (eg, overwhelming viral infections), the resultant immune responses rapidly become exhausted.5Accordingly, one might also expect that the antihost immune responses that mediate GVHD should exhaust rather quickly as well. That they do not suggests that the rules that govern T-cell responses in the immunocompetent state may be distinct from those that regulate T-cell behavior following their transfer into lymphopenic hosts.
In this paper, we have used superantigen (SAg) responses as a model to study T-cell immunoregulation after adoptive transfer. Immunoregulation of T-cell responses to SAgs has been well characterized7,8 and comprises 2 major phenomena—deletion and anergy. The SAgs stimulate subsets of T cells that use a commonVβ gene to form their T-cell receptor (TCR). After several days of rapid proliferation, a proportion of SAg-activated CD4+ T cells becomes deleted by Fas-mediated apoptosis.9 By 10 days after initial stimulation, the percentage and absolute numbers of SAg-reactive cells have decreased to around 50% of starting values and remain at this level for several months, in the absence of new thymic emigrants.7,10 The remaining cells are anergic because they do not proliferate or secrete interleukin-2 (IL-2) in response to restimulation by the SAg or the cognate antigen,12,13 either in vitro8 or in vivo.11 Such anergy may be cell intrinsic or may be maintained by multiple extrinsic mechanisms involving regulatory or suppressor T14-17 or B cells.18-20
In the studies described here, we have used a previously characterized model in which the transfer of major histocompatibility complex (MHC)–disparate T cells from C57BL/6J (B6; H-2b) donor mice into C.B-17-scid/scid mice (SCID; H-2d) recipients results in severe GVHD.21-23 Severe combined immunodeficient (SCID) mice cannot rearrange their B-cell receptor or TCR genes and are consequently lymphopenic, allowing host-reactive donor T cells to be tracked easily after adoptive transfer.24 Using this model, we have observed that the deletion and anergy of allogeneic T cells reactive to endogenous host SAgs are markedly impaired during GVHD and have demonstrated further that the regulation of T-cell responses to exogenous SAg following the adoptive transfer of syngeneic T cells is impaired as well.
Materials and methods
Mice
C57BL/6J [B6] (H-2b,Mtv-6−) and BALB/c (H-2d, IgHa, Mtv-6+) mice were from the Jackson Laboratories (Bar Harbor, ME). C.B-17 scid/scid[SCID] and C.B-17 OVA-SCID26 (H-2d, IgHb, Mtv-6+) mice were bred and maintained in the defined flora animal colony at the Ontario Cancer Institute (Toronto, ON, Canada). The latter bear a transgenic chicken ovalbumin-specific TCR and thus provide a source of monoclonal CD4+Vβ8+ T cells.26
Antibodies, reagents, and cell lines
Phycoerythrin (PE)– and fluorescein isothiocyanate (FITC)–conjugated Vβ8β, Vβ6, CD3, CD4, and CD8 antibodies and biotinylated Vβ3 antibodies were purchased from Pharmingen (Mississauga, ON); staphylococcal enterotoxin B (SEB), 7-amino actinomycin D (7-AAD), streptavidin-PE, chicken ovalbumin, and complete Freund adjuvant (CFA) were purchased from Sigma Chemical (St Louis, MO). The hybridomas 2.4G2 (antimurine Fc RIII-α)27 and R4-6A2 (antimurine interferon γ [IFN-γ])28 were from the American Type Culture Collection (ATCC; Manassas, VA). Anticlonotypic antibodies from the KJ1-26.1 hybridoma12 (gift of Dr J. Kappler, Denver, CO) that recognize the OVA-SCID TCR29 (derived from the D0-11.10 helper T-cell clone30) were purified and labeled with FITC in our laboratory. Recombinant soluble human tumor necrosis factor receptor (rhuTNFR:Fc)31 and ovalbumin peptides (amino acids 323-339) were gifts of Dr M. Widmer (Immunex, Seattle, WA) and Dr J. Withers (Toronto, ON), respectively. Supernatants from mouse IL-2 cDNA transfected X63Ag8-653 cells (gift of H. Karasuyama32) were titered on CTLL-2 cells (from the ATCC) and used as the source of IL-2.
Cell preparation
Suspensions of spleen and peripheral lymph node cells (PLNCs) were prepared by passage through metal screens. Peritoneal washings were collected following 2 infusions of 6 mL cold phosphate-buffered saline (PBS) using a 10-mL syringe and an 18-gauge needle. A small cut was made in the peritoneal membrane and the infusate was collected using a sterile Pasteur pipette.
Syngeneic transfers
Although BALB/c and C.B-17 mice are congenic, differing only in the Ig heavy-chain allele,24 in the context of a SCID transfer they are functionally syngeneic. Spleen and PLNCs from BALB/c and PLNCs from OVA-SCID mice were mixed in a 1:1 ratio and injected into the tail veins of unirradiated SCID hosts. Cell concentrations were adjusted so that approximately 2 × 106KJ1-26+ cells were transferred. Memory KJ1-26.1+ cells were isolated on nylon wool columns33 from spleen cell suspensions from OVA-SCID mice that had been immunized 10 days earlier with chicken ovalbumin in CFA.
Induction of GVHD
Inguinal lymph node cells (3 × 104) from B6 donor mice were injected into the tail veins of host SCID mice that had been irradiated with 275 cGy from a γ source (137Cs; Gammacell 40 Exactor, Nordion International, Kanata, ON) on the day of the injection. Major toxicities were not observed when these low numbers of donor T cells were used to initiate GVHD.34
SEB injection protocol
Control, or reconstituted mice received intraperitoneal injections of SEB (50 μg), or as indicated in the text.
Proliferation assays
In vitro proliferation.
Spleen and PLNCs were purified with Lympholyte separation medium (Cedarlane Laboratories, Hornsby, ON), washed, and then resuspended in complete medium (α-minimal essential medium [MEM], 10% fetal calf serum [FCS], 5 × 10−5 M 2-mercaptoethanol [2-ME], 15 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 2 mM glutamine) at various concentrations. The percentages of CD4+, KJ1-26.1+, and Vβ8+ cells were first measured by flow cytometry to determine the number of T cells to be used subsequently in culture. Responder T cells were plated onto 2 × 105 irradiated (2000 cGy) BALB/c spleen filler cells and stimulated with OVA peptide (1 μM) or SEB (5 μg/mL) for 48 hours at 37°C in 5% CO2. These antigen doses resulted in optimal stimulation in preliminary experiments (data not shown). Proliferation was measured after 48 hours by 3H-thymidine uptake.22 IL-2 (25 U/mL) was included in some cultures.
In vivo proliferation.
Groups of SCID mice were reconstituted from a single pool of syngeneic T cells. After 10 to 12 days, individual mice were injected intraperitoneally with chicken ovalbumin (3 mg) to study the responses of KJ1-26.1+ T cells (Figure 5B) or with SEB (50 μg) to study the responses of CD4+Vβ8+KJ1-26.1− T cells (Figure 6). Mice were killed 48 hours later and the average number of KJ1-26.1+ or CD4+Vβ8+KJ1-26.1− T cells in the spleens and lymph nodes was determined by flow cytometry and manual counting with a hemocytometer. Control mice that had been subjected to the same initial experimental manipulations, but were not subsequently injected with chicken ovalbumin or SEB on days 10 to 12, were used to provide baseline preinjection numbers of KJ1-26.1+ or CD4+Vβ8+KJ1-26.1− T cells. Because total cell numbers did not change significantly following adoptive transfer (Figure 2A), the results from these mice accurately reflected cell numbers present 48 hours earlier in the rechallenged mice. The ratio of the average number of T cells in mice 48 hours after injection of chicken ovalbumin or SEB, relative to the average number from the control group, was used to calculate a proliferative index as a measure of in vivo antigen responsiveness.
Immunofluorescence
Cells were harvested, incubated with antibody, and analyzed by flow cytometry as previously described.22
Statistical analysis
The P values, comparing experimental groups, were obtained using Student t test.
Results
Prolonged survival of SAg-reactive T cells in GVHD
In a previous study34 we showed that although at least 5 × 105 allogeneic (H-2b) B6 T cells caused severe GVHD in sublethally irradiated C.B-17 SCID (H-2d) mice, low numbers (3 × 104) of B6 T cells did not, but rather persisted long-term in an unresponsive state. The fate of specific host-reactive T cells was not determined in that study, however. To follow host-reactive cells specifically, we took advantage of the fact that C.B-17 and B6 mice also differ by the presence and absence, respectively, of the mouse mammary tumor provirusMtv-6,25 which encodes a SAg recognized preferentially by Vβ3+ T cells. B6 CD4+Vβ3+ T cells therefore represent host-reactive T cells in this model of GVHD.35
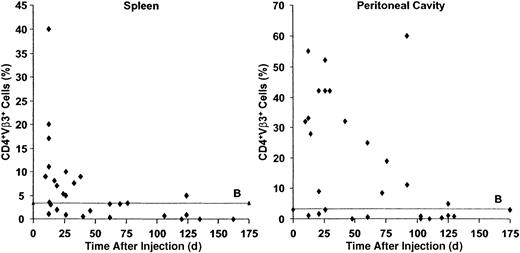
The numbers of CD4+Vβ3+ T cells in the spleens and peritoneal cavities of host SCID mice were determined at various times after the induction of GVHD. The spleens contained the majority of donor T cells34 and the peritoneal cavity was used to represent tertiary lymphoid organs.36 As shown in Figure 1, splenic and peritoneal CD4+Vβ3+ T cells increased strikingly early in the course of GVHD, but eventually decreased to low baseline levels, likely by deletion, consistent with other SAg models.37,38 Notably, however, the time to deletion was in the order of months, rather than of weeks, as in other systems of chronic SAg activation (Figures 1 and 2A, left panel).37 38 Some host mice demonstrated elevated numbers of CD4+Vβ3+ T cells as late as 120 days after injection (Figure 1).
Prolonged survival of SAg-reactive T cells during GVHD.
B6 PLNCs (3 × 104) were injected into sublethally irradiated C.B-17 SCID mice. The percentages of CD4+Vβ3+ T cells (responding to theMtv-6 SAg expressed by SCID cells) in the spleens (left panel) and peritoneal cavities (right panel) of host mice were determined by flow cytometry after transfer. Whereas SAg-reactive T cells are normally deleted within days to weeks, transferred allogeneic SAg-reactive cells persist in immunodeficient hosts for many months. ♦ indicates a single SCID recipient; B, baseline CD4+Vβ3+ T cells (%) in the absence of adoptive transfer (n = 24).
Prolonged survival of SAg-reactive T cells during GVHD.
B6 PLNCs (3 × 104) were injected into sublethally irradiated C.B-17 SCID mice. The percentages of CD4+Vβ3+ T cells (responding to theMtv-6 SAg expressed by SCID cells) in the spleens (left panel) and peritoneal cavities (right panel) of host mice were determined by flow cytometry after transfer. Whereas SAg-reactive T cells are normally deleted within days to weeks, transferred allogeneic SAg-reactive cells persist in immunodeficient hosts for many months. ♦ indicates a single SCID recipient; B, baseline CD4+Vβ3+ T cells (%) in the absence of adoptive transfer (n = 24).
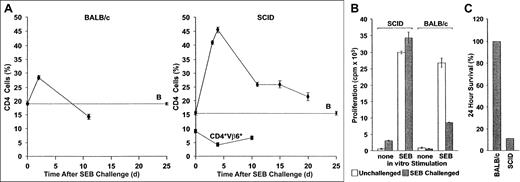
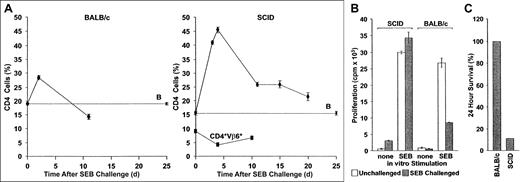
Aberrant immunoregulation of transferred T cells in SCID mice reconstituted with BALB/c lymphocytes and challenged with SEB.
(A) Deletion: BALB/c (left panel) or sublethally irradiated SCID mice reconstituted with 2 × 107 BALB/c spleen cells and PLNCs (right panel) were injected with SEB. The percentage CD4+Vβ8+ and CD4+Vβ6+ cells in pooled axillary, mesenteric, and inguinal lymph node preparations were then determined at various times. Whereas SEB-reactive cells increase rapidly in both BALB/c and SCID recipients, they are deleted much more rapidly in the former. In contrast, CD4+Vβ6+ T cells, which are not SAg responsive, do not respond to SAg injection. The mean ± SE from n separate animals are shown (CD4+Vβ8+ T cells in SCID mice, n = 2, 6, 18, 2, and 5 for days 3, 4, 11, 15, and 20, respectively; CD4+Vβ6+ T cells in SCID mice, n = 3 for each time point; CD4+Vβ8+ T cells in BALB/c mice, n = 10 for each time point). B: The percentage of CD4+Vβ8+ T cells in SCID mice that were reconstituted with BALB/c spleen cells but were not injected with SEB, n = 16. (B) Anergy: CD4+V,8+ PLNC T-cell percentages in BALB/c and BALB/c reconstituted SCID mice (both naive and challenged with SEB 10 days earlier), were quantified flow cytometrically and were adjusted to 2 × 105 cells/mL in complete medium. Irradiated (2000 cGy) BALB/c spleen filler cells and CD4+Vβ8+ T cells (2 × 106cells/mL each), were cultured together in the presence of 1 μg/mL SEB. Proliferation of SEB-challenged cultures (filled bars) was measured 72 hours later and compared to control cultures without added SEB (open bars). Decreased SEB responses were observed in vitro using cells from BALB/c mice previously challenged with SEB, but not if such BALB/c cells were first transferred into SCID hosts. The means ± SE of a representative experiment (n = 4 for each bar) are shown. The experiment was repeated 3 times with similar results. (C) Mortality: BALB/c mice (n = 6) and reconstituted SCID mice (n = 9; 2 × 107 BALB/c spleen cells and PLNCs transferred) were injected with SEB immediately following transfer and again 48 hours later. Mortality was scored 2 days after the second injection.
Aberrant immunoregulation of transferred T cells in SCID mice reconstituted with BALB/c lymphocytes and challenged with SEB.
(A) Deletion: BALB/c (left panel) or sublethally irradiated SCID mice reconstituted with 2 × 107 BALB/c spleen cells and PLNCs (right panel) were injected with SEB. The percentage CD4+Vβ8+ and CD4+Vβ6+ cells in pooled axillary, mesenteric, and inguinal lymph node preparations were then determined at various times. Whereas SEB-reactive cells increase rapidly in both BALB/c and SCID recipients, they are deleted much more rapidly in the former. In contrast, CD4+Vβ6+ T cells, which are not SAg responsive, do not respond to SAg injection. The mean ± SE from n separate animals are shown (CD4+Vβ8+ T cells in SCID mice, n = 2, 6, 18, 2, and 5 for days 3, 4, 11, 15, and 20, respectively; CD4+Vβ6+ T cells in SCID mice, n = 3 for each time point; CD4+Vβ8+ T cells in BALB/c mice, n = 10 for each time point). B: The percentage of CD4+Vβ8+ T cells in SCID mice that were reconstituted with BALB/c spleen cells but were not injected with SEB, n = 16. (B) Anergy: CD4+V,8+ PLNC T-cell percentages in BALB/c and BALB/c reconstituted SCID mice (both naive and challenged with SEB 10 days earlier), were quantified flow cytometrically and were adjusted to 2 × 105 cells/mL in complete medium. Irradiated (2000 cGy) BALB/c spleen filler cells and CD4+Vβ8+ T cells (2 × 106cells/mL each), were cultured together in the presence of 1 μg/mL SEB. Proliferation of SEB-challenged cultures (filled bars) was measured 72 hours later and compared to control cultures without added SEB (open bars). Decreased SEB responses were observed in vitro using cells from BALB/c mice previously challenged with SEB, but not if such BALB/c cells were first transferred into SCID hosts. The means ± SE of a representative experiment (n = 4 for each bar) are shown. The experiment was repeated 3 times with similar results. (C) Mortality: BALB/c mice (n = 6) and reconstituted SCID mice (n = 9; 2 × 107 BALB/c spleen cells and PLNCs transferred) were injected with SEB immediately following transfer and again 48 hours later. Mortality was scored 2 days after the second injection.
Absence of SEB-induced deletion and anergy in T-cell reconstituted SCID mice
To explain the impaired deletion of SAg-reactive T cells during GVHD in SCID mice, we first considered the effects of sublethal irradiation and concomitant stimulation by host peptide alloantigens, because both of these factors are known to increase the survival of activated T cells.39,40 Syngeneic BALB/c T cells were therefore injected into unirradiated SCID mice and were then challenged with another SAg, SEB, which stimulates CD4+Vβ8+ T cells. Consistent with previous observations in immunocompetent BALB/c mice,7,8CD4+Vβ8+ T cells were reduced in number by about 40% 10 days after challenge with SEB (Figure 2A, left panel), and the remaining cells were anergic (Figure 2B). In contrast, in SCID mice that had been reconstituted with syngeneic BALB/c spleen cells and challenged immediately with SEB, the increase in CD4+Vβ8+ T cells was much greater and remained significantly elevated for at least 20 days (Figure 2A, right panel). Preferential expansion of CD4+Vβ8+ T cells during the reconstitution could not account for this result, because the percentage of CD4+Vβ8+ T cells remained constant in the absence of SEB stimulation (Figure 2A, right panel, B: baseline CD4+Vβ8+ cells). Moreover, BALB/c-derived cells surviving in SCID hosts were not anergic because they proliferated as well in vitro in response to SEB as did naive CD4+Vβ8+ T cells (Figure 2B). Impaired immunoregulation following SAg stimulation was also demonstrated by the response in vivo to a second challenge with SEB. Although serial injections of SEB into BALB/c mice had no deleterious effects, as previously reported,37 over 80% of rechallenged T cell-reconstituted SCID mice died within 24 hours (Figure 2C). Taken together, therefore, these data illustrate that SEB-induced deletion and anergy were absent in T cell–reconstituted SCID mice.
Weak SEB reactivity of KJ1-26.1+ cells relative to BALB/c T cells
Because CD4+Vβ8+ T cells in BALB/c spleens are polyclonal due to variability in TCR-α chain usage,41 it is likely that they demonstrate a range of responsiveness to SEB.42 To determine more precisely the fate of individual SEB-activated T cells after adoptive transfer into SCID mice, we therefore used a monoclonal population of KJ1-26.1+CD4+ T cells from OVA-SCID transgenic mice. KJ1-26.1+CD4+ T cells30recognize chicken ovalbumin peptides, but can also be stimulated and tolerized by the SAg SEB, because their TCR uses the Vβ8 gene product.12
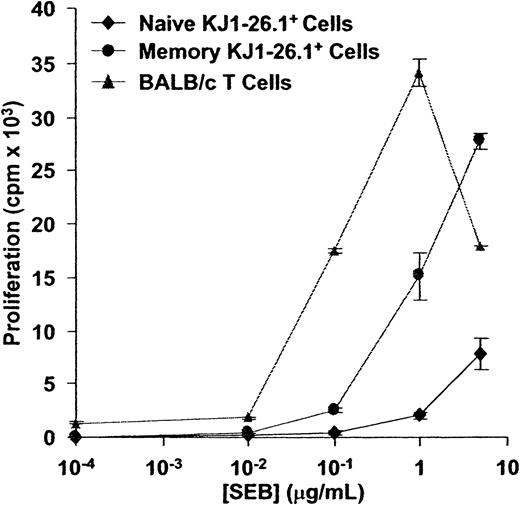
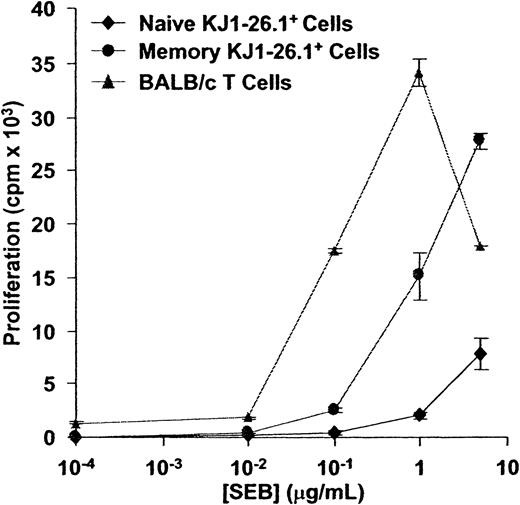
The response of KJ1-26.1+ T cells to SEB was first compared to that of syngeneic BALB/c T cells. In an in vitro assay in which irradiated BALB/c filler cells were used to ensure that observed differences were intrinsic to T cells, memory and naive KJ1-26.1+ T cells required at least 10-fold more SEB for maximal proliferation than did BALB/c T cells (Figure3). We also observed that KJ1-26.1+ T cells were always outnumbered by CD4+Vβ8+KJ1-26.1− T cells within 3 days after adoptive transfer into SCID mice and challenge with SEB, regardless of the initial starting ratio of BALB/c and OVA-SCID cells (data not shown). Taken together, these results indicated that KJ1-26.1+ T cells were relatively weakly reactive to SEB, compared to other splenic BALB/c CD4+Vβ8+ T cells.
CD4+KJ1-26.1+ T cells are weakly responsive to SEB in vitro.
Naive or memory KJ1-26.1+ cells (104), or Vβ8+ BALB/c T cells (104), were cultured together with 2 × 105 BALB/c filler cells in the presence of varying concentrations of SEB. 3H-thymidine uptake was determined 48 hours later. The response of BALB/c cells to SEB exceeded that of both naive and memory KJ1-26.1+ cells. The means ± SE of 3 replicate cultures are shown.
CD4+KJ1-26.1+ T cells are weakly responsive to SEB in vitro.
Naive or memory KJ1-26.1+ cells (104), or Vβ8+ BALB/c T cells (104), were cultured together with 2 × 105 BALB/c filler cells in the presence of varying concentrations of SEB. 3H-thymidine uptake was determined 48 hours later. The response of BALB/c cells to SEB exceeded that of both naive and memory KJ1-26.1+ cells. The means ± SE of 3 replicate cultures are shown.
BALB/c T-cell requirement for SEB-induced deletion and anergy of KJ1-26.1+ T cells after adoptive transfer
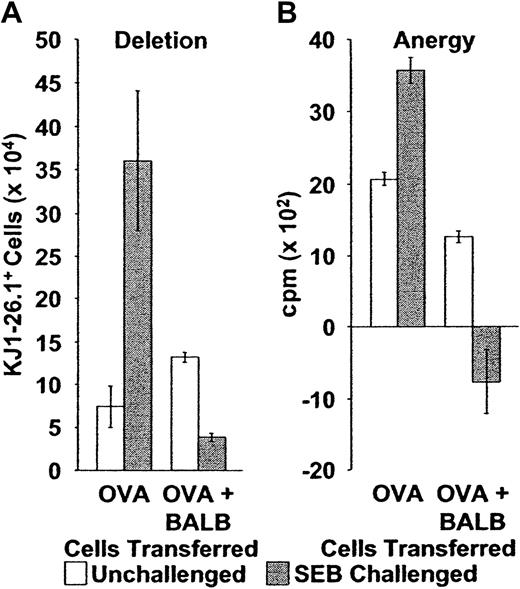
When naive monoclonal KJ1-26.1+ T cells were transferred alone into SCID mice and stimulated with SEB, their behavior recapitulated that previously observed for similarly transferred BALB/c cells. Specifically, KJ1-26.1+ cells increased in number about 400% by 10 days (Figure4A, OVA), and cells present at this time also proliferated strongly in vitro in response to OVA peptides (Figure4B, OVA) and to the SAg SEB (not shown). Because impaired proliferation to both SEB (Figure 2B) and to cognate antigens12 are properties of anergic CD4+Vβ8+ T cells, these data suggested that the transferred KJ1-26.1+ cells were not subject to the normal control mechanisms of deletion and anergy. Similar observations were made after the transfer of memory KJ1-26.1+ CD4+ T cells into SCID mice as well (not shown).
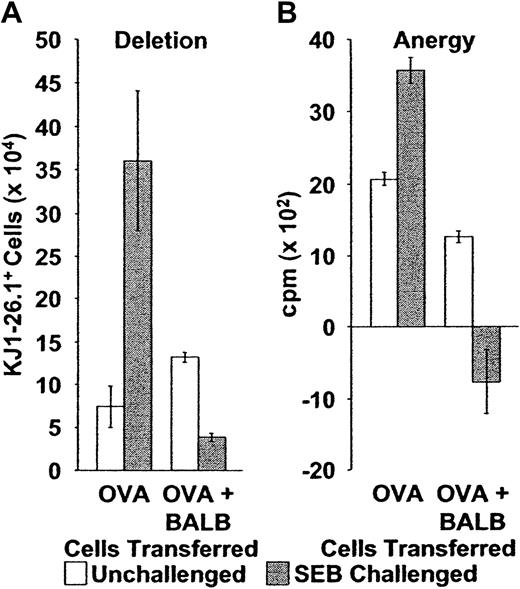
Deletion and anergy of adoptively transferred KJ1-26.1+ T cells following stimulation with SEB is restored by the cotransfer of BALB/c cells.
(A) Deletion: Naive or memory KJ1-26.1+ PLNCs were transferred into SCID mice, either alone (OVA, n = 4), or in a 1:1 ratio with 1 × 106 BALB/c spleen cells (OVA + BALB, n = 8). Reconstituted SCID mice were then observed (open bars) or injected immediately with SEB (filled bars). Splenic KJ1-26.1+ cell numbers were determined 10 to 12 days later. The presence of BALB/c cells during adoptive transfer and SEB stimulation markedly decreased the subsequent yield of KJ1-26.1+ cells. Means ± SE are shown. The transfer of naive and memory KJ1-26.1+ T cells yielded similar results. Similar trends were observed in recipient lymph nodes. OVA − SEB, n = 4; OVA + SEB, n = 4; OVA + BALB − SEB, n = 12; OVA + BALB + SEB, n = 8. (B) Anergy: OVA: 104 KJ1-26.1+ cells from SCID mice, injected 10 days earlier with OVA-SCID cells with or without SEB (open bars, no SEB; closed bars, SEB), were plated at a concentration of 1 to 2 × 106 cells/mL (determined by the percentage of KJ1-26.1+ cells) onto 105 irradiated (2000 cGy) BALB/c filler cells. OVA + BALB: 3 × 103KJ1-26.1+ cells from SCID mice, injected 10 days earlier with a mixture of OVA-SCID and BALB/c cells with or without SEB (open bars, no SEB; closed bars, SEB), were similarly plated. KJ1-26.1+ cells were then stimulated with OVA peptide (1 μM) and proliferation (as determined by 3H-thymidine incorporation) was measured 48 hours later. The presence of BALB/c cells during adoptive transfer and SEB stimulation dramatically decreased the subsequent proliferative response of KJ1-26.1+ cells to OVA peptide. The means ± SE of 3 separate experiments, each performed in triplicate are shown.
Deletion and anergy of adoptively transferred KJ1-26.1+ T cells following stimulation with SEB is restored by the cotransfer of BALB/c cells.
(A) Deletion: Naive or memory KJ1-26.1+ PLNCs were transferred into SCID mice, either alone (OVA, n = 4), or in a 1:1 ratio with 1 × 106 BALB/c spleen cells (OVA + BALB, n = 8). Reconstituted SCID mice were then observed (open bars) or injected immediately with SEB (filled bars). Splenic KJ1-26.1+ cell numbers were determined 10 to 12 days later. The presence of BALB/c cells during adoptive transfer and SEB stimulation markedly decreased the subsequent yield of KJ1-26.1+ cells. Means ± SE are shown. The transfer of naive and memory KJ1-26.1+ T cells yielded similar results. Similar trends were observed in recipient lymph nodes. OVA − SEB, n = 4; OVA + SEB, n = 4; OVA + BALB − SEB, n = 12; OVA + BALB + SEB, n = 8. (B) Anergy: OVA: 104 KJ1-26.1+ cells from SCID mice, injected 10 days earlier with OVA-SCID cells with or without SEB (open bars, no SEB; closed bars, SEB), were plated at a concentration of 1 to 2 × 106 cells/mL (determined by the percentage of KJ1-26.1+ cells) onto 105 irradiated (2000 cGy) BALB/c filler cells. OVA + BALB: 3 × 103KJ1-26.1+ cells from SCID mice, injected 10 days earlier with a mixture of OVA-SCID and BALB/c cells with or without SEB (open bars, no SEB; closed bars, SEB), were similarly plated. KJ1-26.1+ cells were then stimulated with OVA peptide (1 μM) and proliferation (as determined by 3H-thymidine incorporation) was measured 48 hours later. The presence of BALB/c cells during adoptive transfer and SEB stimulation dramatically decreased the subsequent proliferative response of KJ1-26.1+ cells to OVA peptide. The means ± SE of 3 separate experiments, each performed in triplicate are shown.
Notably, however, the outcome was quite different when BALB/c T cells were transferred into SCID mice together with KJ1-26.1+ cells. As before (Figure 2), BALB/c T cells (CD4+Vβ8+KJ1-26.1−) were not deleted and did not become anergic 10 to 12 days after adoptive transfer and stimulation by SEB in vivo (Figure5A, right panel, control and SEB only; 5C, upper and middle right panels). However, in contrast to when transferred alone (Figure 4A, OVA), KJ1-26.1+ T cell numbers in the spleen and lymph nodes decreased about 300% (Figure 4A, OVA+BALB; Figure 5A, left panel, control and SEB only; 5C, upper and middle left panels). KJ1-26.1+ T cell numbers were also decreased in the peritoneal cavity and bone marrow (not shown), suggesting that deletion following SEB, and not altered trafficking of activated cells, accounted for the changes in the secondary lymphoid organs in the presence of BALB/c T cells. Similar results were obtained with memory KJ1-26.1+ T cells (not shown). Moreover, KJ1-26.1+ T cells from OVA-scid/+ mice behaved similarly (not shown), indicating that the scid mutation itself was not responsible for the restored deletion of KJ1-26.1+ T cells.
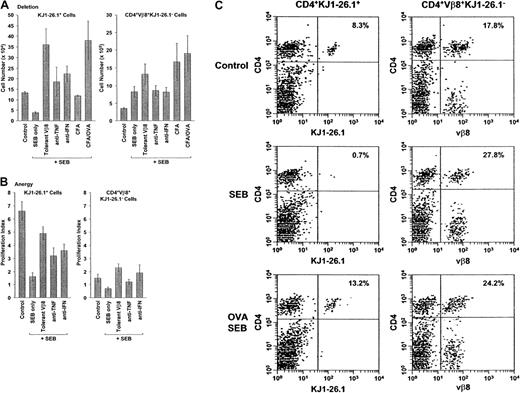
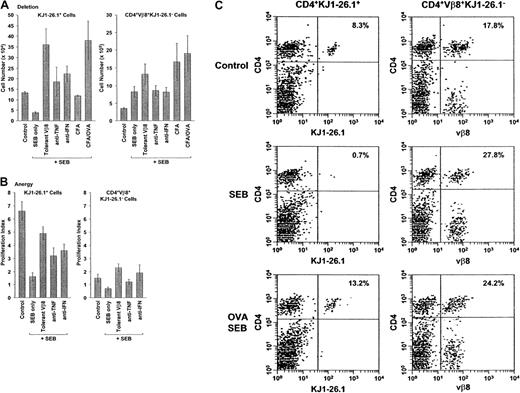
Effect of BALB/c cells, cytokine blockade, and cognate antigen on the deletion and anergy of transferred SEB-activated KJ1-26.1+ T cells.
(A) Deletion: Mixtures of OVA-SCID PLNCs and naive BALB/c spleen cells were transferred into SCID mice. Experimental mice were then observed (control, n = 12), or injected with SEB alone (n = 8) or in combination with rhuTNFR:Fc (anti-TNF, n = 5), anti–IFN-γ (anti-IFN, n = 3), CFA (CFA, n = 3), or CFA + ovalbumin (CFA/OVA, n = 3) (see below). SCID mice reconstituted with OVA-SCID PLNCs and about 1 × 106 SEB-tolerant BALB/c spleen cells (tolerant Vβ8, n = 3) were injected with SEB in parallel. Ten days after adoptive transfer, the percentage and absolute numbers of KJ1-26.1+ and CD4+Vβ8+KJ1-26.1− cells in the spleens and lymph nodes (the latter defined as the difference between CD4+Vβ8+ cells and KJ1-26.1+cells) were determined. Circulating TNF-α and IFN-γ were blocked by intraperitoneal injections of rhuTNFR:Fc31 (200 μg) or anti–IFN-γ ascites28 61 (250 μL) every 2 days from day −1. The effect of simultaneous stimulation with cognate antigen was determined in SCID mice immunized 24 hours earlier with CFA with or without chicken ovalbumin. Deletion of KJ1-26.1+ cells in the presence of BALB/c cells was prevented by decreasing the posttransfer SEB responsiveness of cotransferred BALB/c cells by treating donors with SEB prior to cell harvest, by decreasing overall activation levels with anticytokine antibodies, or by increasing the relative strength of activation of KJ1-26.1+ T cells by simultaneous stimulation with SEB and cognate antigen. The means ± SE of n animals are shown. The initial ratio of KJ1-26.1+/CD4+Vβ8+KJ1-26.1−cells at time of transfer was about 1:1, with about 1 × 106 of each of KJ1-26.1+ and CD4+Vβ8+KJ1-26.1− cells injected. Without SEB stimulation, T-cell numbers were independent of the use of anticytokine reagents or of CFA (not shown). (B) Anergy: Mixtures of OVA-SCID PLNCs and naive BALB/c spleen cells were transferred into SCID mice. Ten days later, reconstituted control mice (control, n = 7) and animals injected with SEB alone (n = 7) or in combination with rhuTNFR:Fc (anti-TNF, n = 7), or anti–IFN-γ (anti-IFN, n = 3), were injected intraperitoneally with 3 mg chicken ovalbumin. SCID mice reconstituted with OVA-SCID PLNCs and approximately 1 × 106 SEB-tolerant BALB/c spleen cells (tolerant Vβ8, n = 4) were injected in parallel. Three days later, total numbers of spleen and lymph node KJ1-26.1+ cells were determined and used to calculate the proliferation index (defined as the ratio of the cell numbers before and 48 hours after injection of chicken ovalbumin). OVA-dependent anergy of KJ1-26.1+ cells transferred together with BALB/c cells and stimulated with SEB was decreased in the presence of SEB-tolerant BALB/c cells and by TNF-α and IFN-γ blockade. The means ± SE of n animals are shown. (C) KJ1-26.1+ T cells are rescued from deletion by stimulation with cognate antigen. BALB/c and KJ1-26.1+ cells were transferred into SCID mice, some of which had been injected subcutaneously 24 hours earlier with chicken ovalbumin in CFA (lower panels). Mice were observed (control, upper panels) or injected with SEB (SEB and SEB OVA, middle and lower panels, respectively) and secondary lymphoid organs were analyzed 10 days later. The percentages of CD4+KJ1-26.1+ T cells (left panels) and CD4+Vβ8+KJ1-26.1− T cells (right panels) are shown. The reduction in transferred CD4+KJ1-26.1+ T cells that is observed following SEB stimulation (SEB, 0.7%), is prevented by stimulation with the cognate antigen ovalbumin (OVA SEB, 13.2%). One of 6 representative experiments is shown.
Effect of BALB/c cells, cytokine blockade, and cognate antigen on the deletion and anergy of transferred SEB-activated KJ1-26.1+ T cells.
(A) Deletion: Mixtures of OVA-SCID PLNCs and naive BALB/c spleen cells were transferred into SCID mice. Experimental mice were then observed (control, n = 12), or injected with SEB alone (n = 8) or in combination with rhuTNFR:Fc (anti-TNF, n = 5), anti–IFN-γ (anti-IFN, n = 3), CFA (CFA, n = 3), or CFA + ovalbumin (CFA/OVA, n = 3) (see below). SCID mice reconstituted with OVA-SCID PLNCs and about 1 × 106 SEB-tolerant BALB/c spleen cells (tolerant Vβ8, n = 3) were injected with SEB in parallel. Ten days after adoptive transfer, the percentage and absolute numbers of KJ1-26.1+ and CD4+Vβ8+KJ1-26.1− cells in the spleens and lymph nodes (the latter defined as the difference between CD4+Vβ8+ cells and KJ1-26.1+cells) were determined. Circulating TNF-α and IFN-γ were blocked by intraperitoneal injections of rhuTNFR:Fc31 (200 μg) or anti–IFN-γ ascites28 61 (250 μL) every 2 days from day −1. The effect of simultaneous stimulation with cognate antigen was determined in SCID mice immunized 24 hours earlier with CFA with or without chicken ovalbumin. Deletion of KJ1-26.1+ cells in the presence of BALB/c cells was prevented by decreasing the posttransfer SEB responsiveness of cotransferred BALB/c cells by treating donors with SEB prior to cell harvest, by decreasing overall activation levels with anticytokine antibodies, or by increasing the relative strength of activation of KJ1-26.1+ T cells by simultaneous stimulation with SEB and cognate antigen. The means ± SE of n animals are shown. The initial ratio of KJ1-26.1+/CD4+Vβ8+KJ1-26.1−cells at time of transfer was about 1:1, with about 1 × 106 of each of KJ1-26.1+ and CD4+Vβ8+KJ1-26.1− cells injected. Without SEB stimulation, T-cell numbers were independent of the use of anticytokine reagents or of CFA (not shown). (B) Anergy: Mixtures of OVA-SCID PLNCs and naive BALB/c spleen cells were transferred into SCID mice. Ten days later, reconstituted control mice (control, n = 7) and animals injected with SEB alone (n = 7) or in combination with rhuTNFR:Fc (anti-TNF, n = 7), or anti–IFN-γ (anti-IFN, n = 3), were injected intraperitoneally with 3 mg chicken ovalbumin. SCID mice reconstituted with OVA-SCID PLNCs and approximately 1 × 106 SEB-tolerant BALB/c spleen cells (tolerant Vβ8, n = 4) were injected in parallel. Three days later, total numbers of spleen and lymph node KJ1-26.1+ cells were determined and used to calculate the proliferation index (defined as the ratio of the cell numbers before and 48 hours after injection of chicken ovalbumin). OVA-dependent anergy of KJ1-26.1+ cells transferred together with BALB/c cells and stimulated with SEB was decreased in the presence of SEB-tolerant BALB/c cells and by TNF-α and IFN-γ blockade. The means ± SE of n animals are shown. (C) KJ1-26.1+ T cells are rescued from deletion by stimulation with cognate antigen. BALB/c and KJ1-26.1+ cells were transferred into SCID mice, some of which had been injected subcutaneously 24 hours earlier with chicken ovalbumin in CFA (lower panels). Mice were observed (control, upper panels) or injected with SEB (SEB and SEB OVA, middle and lower panels, respectively) and secondary lymphoid organs were analyzed 10 days later. The percentages of CD4+KJ1-26.1+ T cells (left panels) and CD4+Vβ8+KJ1-26.1− T cells (right panels) are shown. The reduction in transferred CD4+KJ1-26.1+ T cells that is observed following SEB stimulation (SEB, 0.7%), is prevented by stimulation with the cognate antigen ovalbumin (OVA SEB, 13.2%). One of 6 representative experiments is shown.
To determine if the SEB-activated KJ1-26.1+ T cells remaining after deletion were anergic, we developed an in vivo anergy assay11 to overcome the technical problems posed by the low KJ1-26.1+ cell numbers and the overwhelming contribution of BALB/c CD4+Vβ8+KJ1-26.1− T cells to in vitro proliferation assays (Figure 2B). This assay was based on the observation that anergic SAg-reactive T cells demonstrate impaired proliferative responses to their cognate antigens as well.12,13 Consequently, anergy of KJ1-26.1+ T cells in reconstituted SCID mice could be determined by measuring the proliferative response to chicken ovalbumin. This response was unlikely to involve significant numbers of BALB/c KJ1-26.1− T cells because the frequency of naive KJ1-26.1− T cells that can respond to a cognate antigen, such as chicken ovalbumin, is less than 1:10,5 43 and should not be selectively increased after priming with SEB. Proliferation of KJ1-26.1+ T cells remaining 10 to 12 days after adoptive transfer was consequently measured by an index defined as the ratio of cell number before and 48 hours after injection of chicken ovalbumin. In this assay, KJ1-26.1+ T cell numbers increased by approximately 600% in control mice that had only been reconstituted with OVA-SCID and BALB/c cells but did not change significantly if similarly reconstituted mice had been previously injected with SEB (Figure 5B, left panel, control and SEB only). These results suggested that stimulation with SEB caused KJ1-26.1+ T cells to become anergic in the presence of BALB/c T cells that could respond strongly to SEB. Consistent with this, proliferation of KJ1-26.1+ T cells in vitro in response to antigenic peptides was also decreased (Figure 4B, OVA+BALB, filled bar) compared to transferred but otherwise unstimulated KJ1-26.1+ T cells (Figure 4B, OVA+BALB, open bar), and to KJ1-26.1+ T cells that were stimulated with SEB after adoptive transfer in the absence of BALB/c spleen cells (Figure 4B, OVA).
Effect of strongly SEB-reactive T cells on deletion and anergy of KJ1-26.1+ T cells in reconstituted SCID mice
The finding that KJ1-26.1+ T cells were less reactive to SEB than were BALB/c cells (Figure 3) led us to wonder if it was the more strongly SEB-reactive T cells in BALB/c spleens that were responsible for the restored deletion and anergy of KJ1-26.1+ T cells observed when both were transferred together into SCID mice and stimulated with SEB. If so, the removal of strongly SEB-reactive T cells from BALB/c spleens before adoptive transfer would be expected to prevent the subsequent deletion and anergy of KJ1-26.1+ T cells. Moreover, by increasing the activation state of SEB-activated KJ1-26.1+ T cells after adoptive transfer, one might allow them to escape regulation by more strongly reactive BALB/c T cells.
We therefore used 3 approaches to overcome the relative effects of the strongly SEB-reactive BALB/c T cells: the physical removal of strongly reactive cells, a decrease in the overall level of T-cell activation, and increased KJ1-26.1+ T-cell activation.
Removal of strongly SEB-reactive T cells.
Intravenous injection of SEB into BALB/c mice (1 injection of 50 μg, 10 days before harvesting, or 5 injections of 10 μg, every 2 days) was used to delete strongly SEB-reactive T cells. CD4+Vβ8+ cells remaining after deletion are known to respond weakly to SEB as assessed by proliferation and cytokine production8,44 and may represent T cells that have not been deleted due to the low affinity of their TCRs for SEB.45 BALB/c mice treated previously with SEB would therefore be “SEB tolerant” donors. SCID mice were reconstituted with a mixture of KJ1-26.1+ T cells and tolerant BALB/c spleen and PLNCs and challenged with SEB. Ten days later, rather than being deleted, KJ1-26.1+ cells had increased by about 300% compared with controls (Figure 5A, left panel, control and tolerant Vβ8 bars). Despite their presumed anergy CD4+Vβ8+ KJ1-26.1− T cells also increased by about 300% in response to SEB (Figure 5A, right panel, control and tolerant Vβ8 bars). And consistent with our prediction, the response of KJ1-26.1+ T cells to chicken ovalbumin was almost completely restored in the presence of tolerant BALB/c T cells (Figure 5B, left panel, control and tolerant Vβ8 bars).
Decreased overall immune activation.
Blockade of cytokines such as TNF-α46 47 that mediate costimulatory signals for T-cell responses in vivo after SEB stimulation and adoptive transfer would be expected to decrease the level of stimulation of strongly reactive T cells without removing them physically. Consistent with this notion, deletion of KJ1-26.1+ T cells was prevented, and proliferation in vivo was restored partially, by TNF-α blockade (Figure 5A,B, left panels, control and anti-TNF bars) even in the presence of BALB/c T cells. Similar results were obtained using blocking antibodies directed against IFN-γ (Figure 5A,B, left panels, control and anti-IFN bars).
Increased KJ1-26.1+ T-cell activation.
We reasoned that the activation state of KJ1-26.1+ T cells should be increased by simultaneous stimulation with SEB and with the cognate antigen, chicken ovalbumin, which may mediate distinct and additive signaling pathways.48 49 Accordingly, SCID mice were injected with chicken ovalbumin in CFA 1 day before adoptive transfer of a mixture of BALB/c and OVA-SCID cells. Notably, KJ1-26.1+ T cells were not deleted when stimulated simultaneously by antigen, even in the presence of nontolerant BALB/c T cells (Figure 5A, left panel, control and CFA/OVA bars; 5C, lower panel). Consistent with the notion that the deletion of KJ1-26.1+ T cells was related to their relative activation state, the injection of CFA alone also prevented deletion of KJ1-26.1+ T cells by SEB, albeit to a lesser extent (Figure5A, left panel, control and CFA bars).
Time dependence of deletion and anergy of SEB-reactive T cells after adoptive transfer
In the 2 systems described above, allogeneic (B6; Figure 1) or syngeneic (BALB/c; Figure 2) T cells were stimulated with SAgs immediately after adoptive transfer. Because the timing of stimulation by the endogenous Mtv-6 SAg could not be altered easily, the SAg SEB was injected at various times after adoptive transfer of syngeneic T cells to determine the effect on deletion and anergy (Figure 6). Consistent with our previous observations (Figure 2A, right panel), the percentage of CD4+Vβ8+ T cells was elevated significantly 10 days after adoptive transfer and immediate stimulation with SEB (Figure 6, left panel). Progressively lower numbers and percentages of CD4+Vβ8+ T cells (indicating increased deletion) were observed when T cells were allowed to reconstitute SCID hosts for 2 or 9 days before stimulation with SEB, compared to cells that were transferred but not stimulated (Figure 6, left panel).
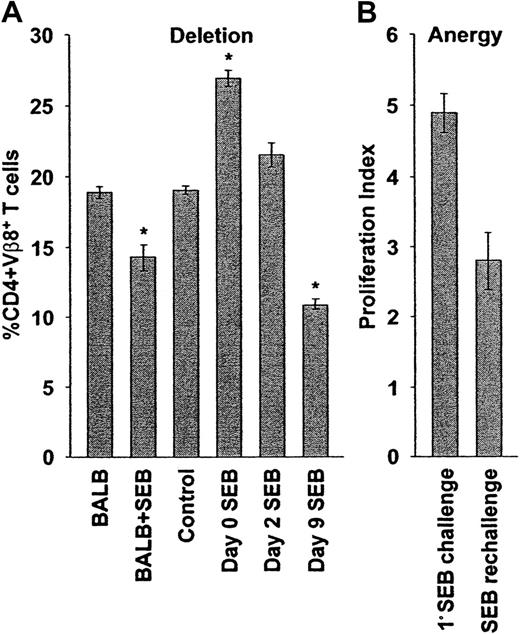
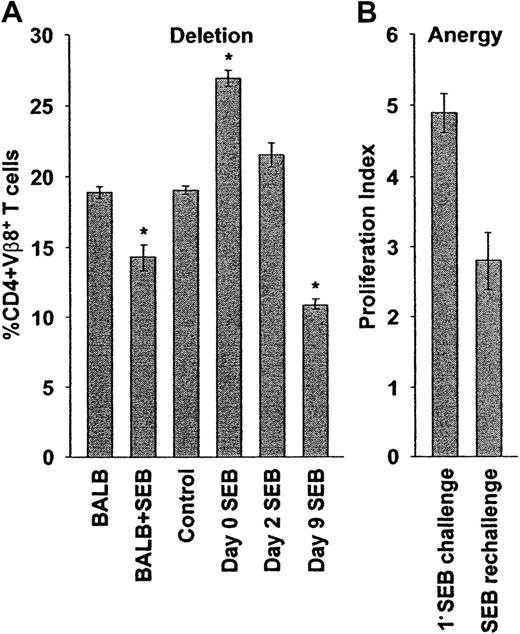
Delayed superantigenic stimulation restores deletion and anergy of adoptively transferred cells.
(A) Deletion: SCID mice reconstituted with BALB/c cells were observed (control, n = 5) or were injected with SEB immediately (day 0 SEB, n = 25), 2 (day 2 SEB, n = 2), or 9 (day 9 SEB, n = 5) days after adoptive transfer. Ten days after SEB stimulation (or 10 days after transfer, control), the percentages of peripheral lymph node CD4+Vβ8+ T cells were determined by flow cytometry. Delayed SEB challenge restores the deletion of SEB responsive cells. The percentages of peripheral lymph node CD4+Vβ8+ T cells in BALB/c mice before (BALB, n = 4) and 10 days after (BALB + SEB, n = 19) injection of SEB are shown for comparison. The percentages plotted reflect absolute cell numbers (not shown), with means ± SE from n animals shown. Asterisk indicates statistically significant difference (P < .02) between control and adoptively transferred mice. (B) Anergy: The SEB-dependent proliferative capacity of transferred CD4+Vβ8+ T cells was determined after delayed SEB challenge. Reconstituted SCID mice were initially challenged with SEB 9 days after adoptive transfer and were then rechallenged 10 days later, and the total numbers of spleen and peripheral lymph node CD4+Vβ8+ T cells were determined by flow cytometry and manual counting 48 hours later. The total number of CD4+Vβ8+ T cells was determined in a similar manner in mice that had been reconstituted for 21 days, but had received only a single injection of SEB 12 days before, and the ratio of these numbers was used to calculate a proliferation index representing the ability of SEB-stimulated T cells to proliferate in response to a second challenge with SEB (SEB rechallenge, n = 5). The proliferation index of CD4+Vβ8+ T cells in response to a primary stimulation with SEB after adoptive transfer was determined in a similar manner (1° SEB challenge, n = 3). Delayed SEB challenge restores SEB-dependent anergy (P < .02). The means ± SE from n animals are reported.
Delayed superantigenic stimulation restores deletion and anergy of adoptively transferred cells.
(A) Deletion: SCID mice reconstituted with BALB/c cells were observed (control, n = 5) or were injected with SEB immediately (day 0 SEB, n = 25), 2 (day 2 SEB, n = 2), or 9 (day 9 SEB, n = 5) days after adoptive transfer. Ten days after SEB stimulation (or 10 days after transfer, control), the percentages of peripheral lymph node CD4+Vβ8+ T cells were determined by flow cytometry. Delayed SEB challenge restores the deletion of SEB responsive cells. The percentages of peripheral lymph node CD4+Vβ8+ T cells in BALB/c mice before (BALB, n = 4) and 10 days after (BALB + SEB, n = 19) injection of SEB are shown for comparison. The percentages plotted reflect absolute cell numbers (not shown), with means ± SE from n animals shown. Asterisk indicates statistically significant difference (P < .02) between control and adoptively transferred mice. (B) Anergy: The SEB-dependent proliferative capacity of transferred CD4+Vβ8+ T cells was determined after delayed SEB challenge. Reconstituted SCID mice were initially challenged with SEB 9 days after adoptive transfer and were then rechallenged 10 days later, and the total numbers of spleen and peripheral lymph node CD4+Vβ8+ T cells were determined by flow cytometry and manual counting 48 hours later. The total number of CD4+Vβ8+ T cells was determined in a similar manner in mice that had been reconstituted for 21 days, but had received only a single injection of SEB 12 days before, and the ratio of these numbers was used to calculate a proliferation index representing the ability of SEB-stimulated T cells to proliferate in response to a second challenge with SEB (SEB rechallenge, n = 5). The proliferation index of CD4+Vβ8+ T cells in response to a primary stimulation with SEB after adoptive transfer was determined in a similar manner (1° SEB challenge, n = 3). Delayed SEB challenge restores SEB-dependent anergy (P < .02). The means ± SE from n animals are reported.
In parallel with cell number, anergy was measured by an in vivo proliferation assay. When the initial SEB injection was delayed by 9 days, CD4+Vβ8+ T cells proliferated less well to a second challenge with SEB in vivo compared to cells that were stimulated immediately after adoptive transfer (Figure 2B), or were transferred but not stimulated initially, with SEB (Figure 6, right panel). These results suggested that anergy was also restored partially when the primary challenge of transferred CD4+Vβ8+ T cells with SEB was delayed.
Effect of transferred T-cell number on the regulation of SAg responses
We also investigated whether the impaired anergy and deletion of SAg-reactive T cells were related to the number of transferred T cells. Varying numbers of BALB/c or B6 T cells were injected into SCID mice before stimulation with SEB or Mtv-6 SAg, respectively (Table 1). The percentages of SAg-reactive T cells in the lymph nodes were then determined 12 days later (Table 1). These percentages were decreased significantly when large numbers (1 × 108 cells) were transferred, in contrast to the impaired deletion observed with the transfer of lower cell numbers (Table 1). T cells remaining in host mice after transfer of high numbers of T cells and superantigenic stimulation also proliferated less well to in vitro restimulation with the SAg (Figure2B, and data not shown), suggesting that anergy had been restored partially.
Discussion
In this paper we report that (1) the normal regulation (deletion and anergy) of SAg responses is markedly impaired when T cells are transferred adoptively into immunodeficient mice immediately before stimulation; (2) the degree of this impairment of deletion and anergy is dependent on the number of T cells transferred, and the timing of SAg stimulation after adoptive transfer; and (3) whether or not T cells will undergo normal or impaired regulation following SAg stimulation is determined by the strength of their individual SAg responses, relative to those of other responding T cells in the transferred population.
These studies were prompted by the unexpectedly prolonged survival ofMtv-6 SAg–reactive allogeneic B6 CD4+Vβ3+ T cells during GVHD in sublethally irradiated SCID mice (Figure 1). This observation was surprising because the chronic administration of exogenous SAgs normally results in the almost complete deletion of reactive T cells within 2 weeks.37 We had expected therefore, that chronic activation by the host-derived endogenous Mtv-6 SAg would also lead to the rapid deletion of SAg-reactive donor T cells during GVHD.
The normal regulation of SAg responses in immunocompetent mice is multifactorial in nature and involves cell-cell contact through TNF and TNF receptor family members,50,51 regulatory CD4+ and CD8+ T cells,15-17 and B-cell–mediated idiotypic networks.18-20 The altered behavior of SAg-activated T cells in immunodeficient mice indicates that these regulatory activities are not present in SCID mice and suggests as well that they are not fully transferable either. For example, although one might expect spleen cell populations from immunocompetent mice treated with SEB to be enriched in regulatory cells, appropriate deletion and anergy were nevertheless not seen after the transfer of such populations into SCID mice (Figure 5A, right panel, control and tolerant Vβ8 bars).
The altered behavior of SAg-activated donor T cells in immunodeficient mice might also relate to differences in the cellular architecture of secondary lymphoid organs in immunocompetent and T cell-reconstituted immunodeficient mice. Cell concentrations in the paracortical T-cell areas of the spleen and lymph nodes normally exceed 108cells/mL.52 Because these concentrations are probably much lower in immunodeficient mice immediately following the adoptive transfer of T cells,53 regulatory cell-cell interactions might be diluted, thereby allowing the unregulated proliferation of responding cells. This explanation is compatible with the observed partial restoration of SAg-dependent deletion and anergy seen as early as 48 hours after adoptive transfer (Figure 6). This short time period is presumably too early for idiotypic networks and suppressor T cells to have developed in host immunodeficient mice, but possibly sufficient for cell proliferation and trafficking54 55 to lead to increased cell concentrations in the paracortical areas of lymphoid organs, thereby permitting increased cell-cell interactions. The partial restoration of deletion following the transfer of larger numbers of donor T cells (Table 1) further supports this notion.
However, if it is merely lymphoid architecture and the initial dearth of regulatory cell-cell interactions that underlie the aberrant regulation of SAg-activated T cells after adoptive transfer into SCID mice, then why are transgenic OVA-SCID KJ1-26.1+ T cells seemingly regulated properly when they are transferred into SCID recipients together with BALB/c spleen cells (Figures 4 and 5)? Our results suggest that a relationship between deletion/anergy and the reactivity of individual T cells to SEB may underlie this observation. Being monoclonal, KJ1-26.1+ T cells would be expected to be uniformly SEB reactive. In contrast, BALB/c spleen cells, being polyclonal, should comprise a spectrum of SEB responsiveness. Consistent with this, as assessed by their SEB-dependent proliferative responses in vitro and in vivo, KJ1-26.1+ T cells were more weakly responsive to SEB than were at least some BALB/c T cells (Figure 3), possibly due to SEB-binding effects of the Vα chain of the transgenic TCR or of cross-reactive peptide-MHC ligands, both of which are known to alter SEB-TCR interactions.41,56 Notably, deletion and anergy of these more weakly reactive KJ1-26.1+ T cells were prevented (1) in the absence of more strongly activated T cells (by transferring KJ1-26.1+ T cells alone; Figure 4); (2) by decreasing the posttransfer SEB responsiveness of cotransferred BALB/c cells by treating donors with SEB prior to cell harvest (Figure 5), or by decreasing overall activation levels with anti–cytokine antibodies (Figure 5); or (3) by increasing the relative strength of activation of KJ1-26.1+ T cells by simultaneous stimulation with SEB and cognate antigen (Figure 5). Thus, the outcome of transferred SAg-reactive cells appears to be related both to their absolute and relative levels of activation, with the behavior of strongly and weakly reactive T cells reflecting seemingly distinct, but possibly overlapping, regulatory mechanisms. We suspect that the impaired regulation of the former is most consistent with the absence of regulatory cells and of cell-cell interactions, as suggested above. In contrast, the latter appear to be regulated specifically in the presence of more strongly reactive cells. Why the behavior of weakly reactive T cells should be altered in the presence of more strongly reactive T cells is not clear. Possibly, strongly reactive T cells merely out-compete weakly reactive T cells for effective activation by APCs.57 Another possibility is that strongly and weakly reactive T cells may respond differentially to cytokines produced following antigenic stimulation. We were unable to demonstrate experimentally, however, that weakly and strongly SEB- reactive cells responded differentially to TNF-α (data not shown).
Although we have focused here on the regulation of SAg responses in an immunodeficiency model of GVHD, we speculate that similar phenomena may define the responses of T cells to MHC-restricted peptides as well. In this light, the observation that immune responses are regulated aberrantly after the adoptive transfer of T cells into immunodeficient hosts may therefore provide insights into the pathogenesis of acute GVHD after allo-SCT. For example, the absence of normal regulation in this clinical setting may contribute to more severe GVHD in a manner analogous to the increased mortality observed after a second SEB challenge in T-cell–reconstituted mice (Figure 2C). Moreover, because the graft-versus-leukemia effect is also mediated by T cells,58 and such tumor-reactive T cells are often only weakly reactive to tumor antigens59 60 (while other allogeneic T cells mediating GVHD may be activated more strongly), the observation that weakly reactive T-cell responses can be down-regulated in the presence of strongly reactive T cells (Figures 4 and 5) may have implications for the therapeutic effects of allo-SCT as well.
We thank J. Wither for OVA-peptides, J. Kappler for the KJ1-26.1 hybridoma, M. Widmer for rhuTNFR:Fc reagents, and R. G. Miller for OVA-SCID mice.
Supported by grants from the Sunnybrook Trust Fund (D.S.), the Canadian Institutes of Health Research (D.S.), and the National Cancer Institute of Canada (A.C.S.). D.S. is a physician scientist supported by Cancer Care Ontario.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David Spaner, Division of Molecular and Cellular Biology, Research Institute, S-116A, S-Wing, Sunnybrook and Women's College Health Sciences Center, 2075 Bayview Ave, Toronto, ON, Canada M4N 3M5; e-mail: spanerd@srcl.sunnybrook.utoronto.ca.