Abstract
Aceruloplasminemia is a recessive disorder characterized by anemia, iron overload, and neurodegeneration, caused by the absence of ceruloplasmin (Cp), a multicopper oxidase important for iron export. Few patients homozygous for loss of function mutations of the Cp gene have been reported. We describe a 62-year-old white woman with heavy liver iron overload, diabetes, anemia, and neurologic symptoms. She was compound heterozygote for 2 novel mutations that result in the absence of hepatocyte Cp: an adenine insertion at nucleotide 2917 causing a truncated protein and a C-G transversion causing a glutamine→glutamic acid substitution at position 146. Although rare in whites, aceruloplasminemia should be considered in the differential diagnosis of unexplained anemia associated with iron overload, because these features anticipate progressive neurologic symptoms. We propose that anemia, secondary to the impaired macrophage iron release, plays a major role in hepatic iron overload through increased absorption mediated by the erythroid regulator.
Introduction
Hereditary aceruloplasminemia is a rare autosomal recessive disease characterized by iron overload and progressive neurodegeneration. The disease is caused by the absence of ceruloplasmin (Cp), a copper-containing ferroxidase, which catalyses the oxidation of ferrous to ferric iron, a change required for release of iron to plasma transferrin.1 It is hypothesized that in reticuloendothelial (RE) cells and hepatocytes Cp cooperates with the iron export protein ferroportin 1 (FPN1).2 Cp deficiency results in iron deposition in the liver, pancreas, basal ganglia, and other organs. Patients develop diabetes mellitus, retinal degeneration, ataxia, and dementia late in life.3,4 A mild-to-moderate degree of anemia with low serum iron and elevated serum ferritin is a constant feature.3-6
The Cp gene maps to chromosome 3q21-24.7 It consists of 19 exons8 and encodes a protein of 1046 amino acids.9 Aceruloplasminemia has been described mainly in Japanese patients3-6,10-17 and rarely in whites.5 18
We report data on a 62-year-old Italian woman, compound heterozygote for 2 novel mutations that hamper Cp expression in hepatocytes. Our findings suggest that the disorder should be considered in the differential diagnosis of anemia associated with unexplained iron overload. Based on the severity of liver iron loading, the mechanisms leading to iron overload in aceruloplasminemia are revisited.
Study design
Case report
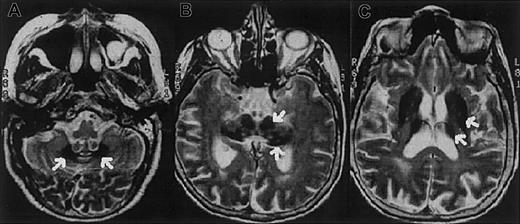
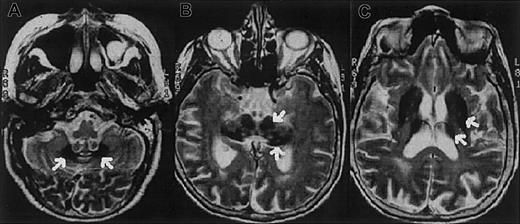
The proband is a 62-year-old Italian woman. No family history of diabetes, iron overload, anemia, or neurologic disorders was recorded. At age 38 years the patient developed insulin-dependent diabetes mellitus. A mild degree of anemia (hemoglobin 9.1 g/L) with normal mean corpuscular volume (MCV; 86 fL) and low-normal values of mean corpuscular hemoglobin (MCH; 27.2 pg) and mean corpuscular hemoglobin concentration (MCHC; 31.7 g/dL) was documented at age 51 years. Serum iron concentration was 33 μg/dL, transferrin saturation 12%, and serum ferritin 819 μg/L. Bone marrow aspirate revealed mild dyserythropoiesis; iron staining showed abundant iron in RE cells and absence of iron granules in erythroblasts. Moderate anemia and abnormal iron parameters without evidence of blood losses or inflammatory diseases were regularly observed at follow-up. Ataxia, dystonia, mild parkinsonism, and dementia became evident at the age of 62 and progressed rapidly. Magnetic resonance imaging of the brain showed a paramagnetic deposition in the basal ganglia, dentate nucleus, thalamus, substantia nigra, and cerebral and cerebellar cortex (Figure1). Serum Cp was undetectable. Liver function tests were normal. No Kayser-Fleischer ring was observed, but the retina showed a pigmentary degeneration.
Magnetic resonance images of the proband showing iron deposition in the brain.
Axial T2-weighted magnetic resonance images of the proband's brain show low-signal areas (arrows) in the dentate nucleus (A), red nucleus and substantia nigra (B), and corpus striatum and thalamus (C), which suggest iron deposition in these regions.
Magnetic resonance images of the proband showing iron deposition in the brain.
Axial T2-weighted magnetic resonance images of the proband's brain show low-signal areas (arrows) in the dentate nucleus (A), red nucleus and substantia nigra (B), and corpus striatum and thalamus (C), which suggest iron deposition in these regions.
Two asymptomatic relatives were evaluated. The patient's son, aged 24, had normal iron parameters but reduced serum Cp concentration (10 mg/dL). The proband's sister, aged 65, had normal iron and Cp values.
Informed consent for molecular studies was obtained according to institutional guidelines.
Methods
Biochemical determinations were performed by standard procedures. Liver iron concentration (LIC) and copper concentrations were determined by atomic spectrophotometry on liver biopsy specimens.19 Immunohistochemical (IHC) studies were performed on liver biopsy specimens using a sheep anti-Cp horseradish peroxidase–conjugated antibody (Biogenesis, Poole, United Kingdom).
Oligonucleotide primers were synthesized according to database sequences (http://www.ncbi.nlm.nih.gov). Polymerase chain reaction was carried out on genomic DNA in a thermal cycler using 12.5 pMol primers and 0.5U Taq polymerase in a final volume of 50 μL. Hemochromatosis (HFE) mutations Cys282Tyr and His63Asp were studied as described.20 Direct sequencing was performed using a ThermoSequenase Cy 5.5 sequencing kit in a Seq4 × 4 Apparatus (Amersham-Pharmacia-Biotech, Piscataway, NJ). Single-strand conformation polymorphism was used to screen Cp exon 3 for mutations. Fifty healthy individuals with normal iron parameters served as controls.
Results and discussion
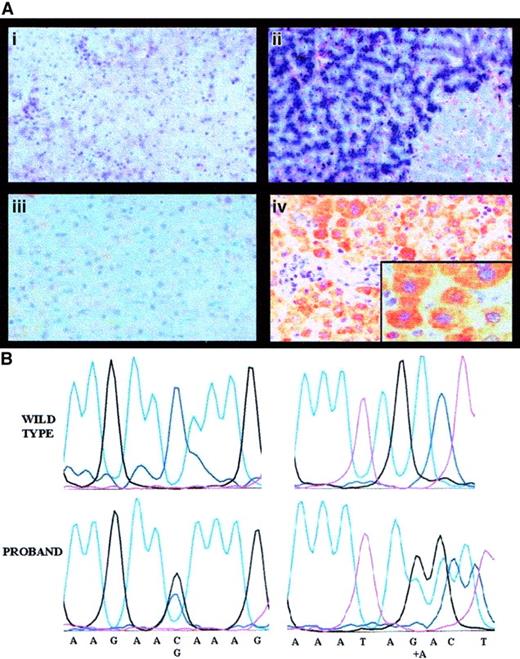
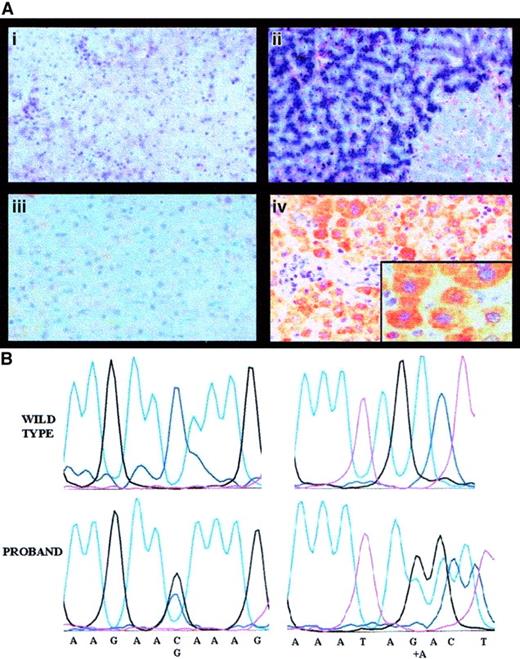
Liver histology, Perls staining, and IHC results for the proband are shown in Figure 2A. LIC was strikingly elevated (535 μmol/g dry weight [dw]; normal, 3-33) and hepatic iron index (LIC divided by years of age = 8.6) was in the range observed in hereditary HFE.18 However, common HFE mutations were negative. Rubeanic acid staining for copper was positive (not shown) and liver copper concentration was remarkably increased (1260 μg/g dw; normal, 20-50 μg/g).
Pathophysiology of aceruloplasminemia in the proband: absence of liver Cp and causal mutations.
Panel Ai shows the histology of the proband's liver (hematoxylin and eosin, × 250), with normal lobular architecture without evidence of fibrosis; Aii shows heavy iron deposition in hepatocytes (Perls colors, × 250); Aiii shows results of IHC studies with a polyclonal antibody showing absence of Cp in proband's hepatocytes (IHC, × 250); and Aiv shows Cp in normal hepatocytes (IHC, × 250). Insert is a high-power view (× 400). In (B), the left panels show a sequencing chromatograph of the proband's amplified exon 3 in the ceruloplasmin region spanning the mutation, compared with a normal control (wild-type). The right panels are a sequencing chromatograph of the proband's amplified exon 17 in the region encompassing the mutation, compared with a normal control (wild-type).
Pathophysiology of aceruloplasminemia in the proband: absence of liver Cp and causal mutations.
Panel Ai shows the histology of the proband's liver (hematoxylin and eosin, × 250), with normal lobular architecture without evidence of fibrosis; Aii shows heavy iron deposition in hepatocytes (Perls colors, × 250); Aiii shows results of IHC studies with a polyclonal antibody showing absence of Cp in proband's hepatocytes (IHC, × 250); and Aiv shows Cp in normal hepatocytes (IHC, × 250). Insert is a high-power view (× 400). In (B), the left panels show a sequencing chromatograph of the proband's amplified exon 3 in the ceruloplasmin region spanning the mutation, compared with a normal control (wild-type). The right panels are a sequencing chromatograph of the proband's amplified exon 17 in the region encompassing the mutation, compared with a normal control (wild-type).
Sequence analysis of the whole Cp gene revealed 2 heterozygous nucleotide changes: a C-G transversion at nucleotide 436, causing the glutamine→glutamic acid (Gln146Glu) replacement in the protein and an adenine (A) insertion at position 2917, causing a frameshift and a premature stop at amino acid 983 (Figure 2B). The truncated protein resulting from A insertion causes the loss of most copper-binding sites and affects the function of both classic and glycosylphosphatidylinositol-anchored Cp, produced in the brain by an alternative splicing.21 Segregation studies confirmed that the 2 mutations were present in trans. The A insertion was not present in the 2 relatives studied; Gln146Glu was inherited by the proband's son, an obligate disease carrier with reduced Cp levels. This observation and the replacement of a neutral amino acid with a negatively charged one suggest that Gln146Glu is a causal mutation. A common polymorphic change is excluded because Gln146Glu was not found in healthy individuals, but we cannot formally rule out that it is in linkage disequilibrium with other mutations in the promoter or in intronic sequences that were not explored by our sequencing.
Moderate anemia, low serum iron, and high serum ferritin, features shared with anemia of chronic diseases, are constant in aceruloplasminemia. Erythrocyte indices may be either reduced or still within normal range, as in our case. As shown in Cp-deficient mice,22 a decreased efflux from the sites of iron storage may overload RE cells and simultaneously impair iron availability for erythropoiesis. Altered compartmentalization of iron was suggested as the cause of increased hepatic stores in Cp−/−mouse.22 Based on the entity of hepatic stores in our patient, we assume that such a loading cannot result only from iron redistribution, but requires a real increase in intestinal iron absorption. It must also be underlined that, in contrast with patients, Cp−/− mice have no anemia.22 We propose that the hepatic iron loading is mediated by the “erythroid regulator,” a positive determinant of iron absorption in conditions of iron-deficient or expanded erythropoiesis.23 A similar mechanism has been recently taken to explain iron overload in HFE24,25 caused by mutations of FPN1,24,26the protein that is hypothesized to cooperate with Cp to export iron in macrophages. Although anemia is not a constant feature in FPN1-associated HFE, a comparison of the clinical phenotype of patients affected by these 2 “iron export” disorders may provide insights into the role of the 2 proteins. In aceruloplasminemia the accumulation of iron in the basal ganglia is mediated by the lack of expression of Cp in neurons and indicates that, within the central nervous system, Cp is essential for iron efflux from storage sites.21
Although rare, aceruloplasminemia is present in whites and should be included in the differential diagnosis of anemia with high serum ferritin unrelated to chronic diseases.
We thank Dr Ezio David and Prof Leonardo Lopiano for kind collaboration.
Prepublished online as Blood First Edition Paper, May 17, 2002; DOI 10.1182/blood-2002-02-0584.
Partially supported by Telethon grant no. GP00255Y01, European Union contract QLK6-1999-02237, and the Italian Ministry of the University and Research (to C.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Clara Camaschella, Dipartimento di Scienze Cliniche e Biologiche, Università di Torino, Azienda Ospedaliera San Luigi, 10043-Orbassano, Torino, Italy; e-mail:clara.camaschella@unito.it.