Activation of platelets with 2 agonists, collagen and thrombin, reveals a subpopulation of cells referred to as COAT-platelets (collagen and thrombin activated). These cells are enriched in several membrane-bound, procoagulant proteins, including fibrinogen, thrombospondin, factor V, von Willebrand factor, and fibronectin. α-Granule proteins bound to COAT-platelets are derivatized with serotonin by a transglutaminase-mediated process, and the interaction of conjugated serotonins with unidentified serotonin binding sites on the platelet surface enhances retention of these proteins. We now demonstrate that both thrombospondin and fibrinogen provide the requisite serotonin binding sites. Thrombospondin and fibrinogen were identified using photoreactive cross-linking to an albumin-(serotonin)6conjugate during COAT-platelet production. We subsequently verified that biotin-albumin-(serotonin)6 binds in vitro to thrombospondin, fibrinogen, and fibrinogen fragment D in a saturable manner. These data support a model for COAT-platelets where serotonin-derivatized procoagulant proteins interact with their respective receptors (eg, fibrinogen with glycoprotein IIb/IIIa or factor V with phosphatidylserine) as well as serotonin binding sites on fibrinogen and thrombospondin, resulting in a stable, multivalent complex on the cell surface.
Introduction
Platelets perform a critical role in hemostasis by providing a surface both for catalyzing thrombin generation and for binding adhesive proteins.1,2 The various proteins bound to activated platelets interact via integrins,3 exposed phosphatidylserine,4 or one of several other well-characterized membrane receptors.5 It was, therefore, unexpected to observe procoagulant protein interactions with activated platelets, which were stabilized by a supplementary mechanism. Specifically, platelets activated simultaneously with 2 agonists, collagen and thrombin, demonstrated retention of procoagulant proteins on a subfraction of the total platelets; this subpopulation, referred to as COAT-platelets (collagen andthrombin activated), was enriched in surface-bound factor V, fibrinogen, fibronectin, von Willebrand factor, thrombospondin, and α2-antiplasmin.6,7 Each of these proteins is an acyl-donor substrate for transglutaminases, and fibrinogen from COAT-platelets was found to be derivatized with serotonin (5-hydroxy-tryptamine [5-HT]).7 The extent to which other α-granule proteins are derivatized with 5-HT is yet to be determined, although synthetic 5-HT adducts displace all procoagulant proteins from COAT-platelets.7 These results suggest a model where serotonin-conjugated, procoagulant proteins are bound to the surface of COAT-platelets by 2 mechanisms: the conventional protein-receptor interaction, which has been well characterized for each of these procoagulant proteins, and an interaction of the conjugated serotonin with a serotonin binding protein. The result is an increased avidity of procoagulant proteins for the platelet membrane. One critical aspect of this model for COAT-platelet production that has not been clarified is the identity of the serotonin binding site.
Materials and methods
Human fibrinogen, bovine serum albumin (BSA), bovine thrombin, α-chymotrypsin, goat antihuman fibrinogen, rabbit antihuman von Willebrand factor, rabbit antihuman fibronectin, rabbit antigoat IgG peroxidase (RAGG-POx), goat antirabbit IgG peroxidase (GARG-POx), peroxidase-labeled streptavidin (SA-POx), horseradish peroxidase (HRP), serotonin (5-HT), ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDAC), trypsin-chymotrypsin inhibitor, o-phenylene diamine dihydrochloride, 6-μm latex beads (SD-6A), N-acetyl serotonin, and Sepharose CL-2B were purchased from Sigma Chemical, St Louis, MO. Rabbit antihuman thrombospondin and rabbit antihuman α2-antiplasmin were obtained from Calbiochem, La Jolla, CA, and 4-azido-2,3,5,6-tetrafluorobenzoic acid, succinimidyl ester (ATFB, SE) and phycoerythrin streptavidin (PE-SA) were purchased from Molecular Probes, Eugene, OR. Monoclonal antibody HFV237 recognizing the light chain of factor V (FV) was a gift from Dr C. T. Esmon, Oklahoma Medical Research Foundation, Oklahoma City, OK. Convulxin was purified as previously described.8 Fibrinogen fragments D and E were gifts from Dr P. McKee, University of Oklahoma Health Sciences Center. Thrombospondin was purified from human platelets using a modification of a previously described technique.9Conjugation of antibodies with fluorescein isothiocyanate (FITC) or biotin was performed by standard procedures.
Buffers
Acid citrate dextrose (ACD), buffered saline glucose citrate (BSGC), and phosphate-buffered saline (PBS) have been detailed previously.6 HEPES/saline and HEPES/saline/BSA were composed of 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 140 mM NaCl, pH 7.5, with or without 1 mg/mL BSA.
Synthesis of b-BSA-(5-HT)6, HRP-HT, and photo cross-linking reagents
Serotonin adducts of BSA and horseradish peroxidase (HRP) were synthesized by reacting BSA (5 mg/mL) or HRP (4 mg/mL) in 20 mM serotonin, 200 mM 2-morpholinoethane sulfonic acid (MES), pH 5.6, with 20 mM ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDAC) for 3 hours at 37°C. The product was dialyzed against HEPES/saline, pH 7.5, and A275 was measured to calculate the 5-HT substitution level (5-HT ε275 = 5700 M−1). BSA-(5-HT)6 was subsequently dialyzed against 50 mM borate, pH 8.5, and biotinylated by reacting with 0.25 mg N-hydroxy succinimido (NHS)–biotin per milligram of protein for 45 minutes at 37°C. The photoreactive cross-linking reagent 4-azido-2,3,5,6-tetrafluorobenzoic acid (0.25 mg/mg protein) was also reacted in 50 mM borate, pH 8.5, for 60 minutes at 37°C in the dark. For HRP derivatization with serotonin, it was necessary to perform the photo cross-linker derivatization prior to 5-HT conjugation to avoid aggregate formation.
Preparation of human platelets
Informed consent was obtained in accordance with local institution review board guidelines. Ten milliliters of whole blood was drawn from the antecubital vein into a plastic syringe containing 1.0 mL ACD. After 1:2 dilution with room temperature (RT) BSGC, pH 7.3, platelet-rich plasma (PRP) was prepared immediately in plastic tubes at 170g (maximum) for 8 minutes at RT. Gel filtration was performed on a column of Sepharose CL-2B, and purified platelets were normalized to a concentration of 4 × 104/μL in BSGC.
Platelet activation for flow experiments
Reactions were 100 μL total volume containing 10 mM HEPES, pH 7.5, 1 mg/mL BSA, 2 mM CaCl2, 1 mM MgCl2, 140 mM NaCl, 4 × 105 platelets and agonist, 500 ng/mL convulxin, and 0.5 U/mL thrombin.6 After 10 minutes at 37°C, the reaction was stopped with 200 μL 1.5% (wt/vol) formalin in HEPES/saline and fixed for 20 minutes at RT. After fixation, 3.5 mL PBS containing 1 mg/mL BSA (PBS/BSA) was added, and the sample was centrifuged at 1500g for 15 minutes. The pellet was resuspended in 200 μL PBS/BSA along with the appropriate detection system. After 25 minutes at RT, platelets were washed as before and resuspended for flow cytometry.
Chymotrypsin treatment of the platelets
Chymotrypsin was dissolved in PBS containing 0.5 mg/mL BSA, and digestion was performed either before or during platelet activation. Before activation, 1 μg/mL (final concentration) of chymotrypsin was added to the platelet suspension for 4 minutes at 37°C, and the proteolytic digestion was stopped by the addition of 50 μg/mL (final concentration) trypsin-chymotrypsin inhibitor. For chymotrypsin digestion during activation, chymotrypsin was added after 3 minutes of activation and stopped at 7 minutes as outlined above. When a monoclonal antibody (mAb) or biotinylated BSA (b-BSA)–HT was required during the assay, it was added only after chymotrypsin was inhibited.
Photoreactive cross-linking of b-BSA-(5-HT)6 to platelets
Ten micrograms per milliliter b-BSA-(5-HT)6derivatized with azidotetrafluorobenzoic acid was coincubated with platelets activated in 2 mM CaCl2, 1 mM MgCl2, 150 mM NaCl, 10 mM HEPES, pH 7.5, with 1 μM A23187 and 0.5 U/mL thrombin to obtain maximal COAT-platelet production.7After 10 minutes at 37°C, the samples were placed on ice and cross-linked for 2 minutes at about 10 cm from a UV lamp (UV Crosslinker, FB-UVXL-1000; Fisher Scientific, Pittsburgh, PA). Before lysing, platelets were treated with 1 μg/mL chymotrypsin for 30 minutes at 37°C. Cells were then lysed with 0.5% Triton X-100 in 1 M NaCl and 50 μg/mL BSA-(5-HT)6. Microtiter plates were coated with antibodies (10 μg/mL) and blocked with 2 mg/mL BSA. Polyclonal antibodies against fibrinogen, thrombospondin, fibronectin, von Willebrand factor, and α2-antiplasmin were utilized in addition to HFV237 monoclonal anti-FV. One hundred–microliter aliquots of platelet lysate were added to the wells for 90 minutes at RT. Bound b-BSA-(5-HT)6 was detected with streptavidin-peroxidase.
Solid phase binding assays
Ninety-six–well microtiter plates were coated with 100 μL of 10 μg/mL thrombospondin (Tsp), fibrinogen (Fbg), fragment D, or fragment E in PBS containing 0.5 mM ethylenediaminetetraacetic acid (PBS/EDTA) for 2 hours at RT. Wells were then blocked with 2 mg/mL BSA in PBS/EDTA for 3 hours and then washed with 0.05% (wt/vol) Tween 20 in PBS. Increasing concentrations of b-BSA-(5-HT)6 in PBS/EDTA/Tween 20 (PBS with 0.5 mM EDTA and 0.02% Tween 20) were added for 90 minutes at RT. After washing, bound b-BSA-(5-HT)6 was detected with streptavidin-peroxidase by standard techniques.
Kinetic assay for FITC-BSA-(5-HT)6 binding to fibrinogen
One hundred microliters of 6.2 μm latex beads (10% suspension; 7 × 108 beads per milliliter) was incubated with 1.5 mL 3 mg/mL fibrinogen in HEPES/saline overnight at 4°C with rocking. Beads were then washed 2 times with saline and resuspended at 2 × 106 /mL in HEPES/saline/BSA. For binding experiments, 120 μL of bead suspension was incubated with shaking in 330 μL HEPES/saline/BSA with graded doses of N-acetyl serotonin (500 mM stock in dimethyl sulfoxide [DMSO]) and 4.4 μg/mL FITC-BSA-(5-HT)6. Fifty-microliter aliquots were removed at various times, diluted into 0.4 mL HEPES/saline/BSA, and immediately analyzed for bound FITC by flow cytometry.
Results
Chymotrypsin treatment of COAT-platelets
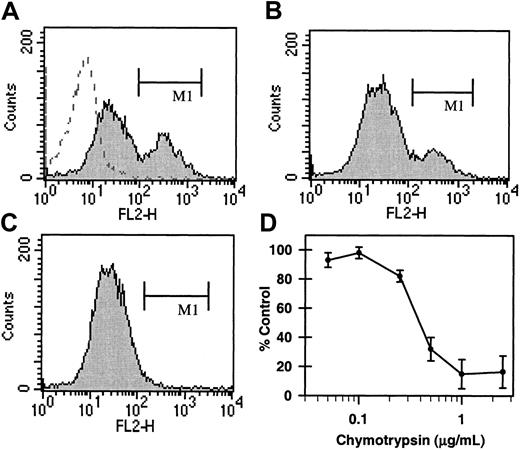
Previous experiments7 demonstrated that biotin-albumin-(serotonin)6 (b-BSA-(5-HT)6) binds to COAT-platelets in a manner similar to α-granule procoagulant proteins (Figure1A). Therefore, b-BSA-(5-HT)6 binding was used as a probe to investigate parameters that affect retention of serotonin conjugates on COAT-platelets. Figure 1C demonstrates that a brief treatment of activated COAT-platelets with 1 μg/mL chymotrypsin eliminates b-BSA-(5-HT)6 binding; however, chymotrypsin treatment of platelets prior to activation does not significantly impede b-BSA-(5-HT)6 binding upon subsequent activation (Figure1B). These observations suggest that the serotonin binding site on COAT-platelets is a protein that is exposed on the cell surface only after activation, making α-granule proteins likely candidates.
Chymotrypsin sensitivity of COAT-platelets.
Gel-filtered platelets were activated with both convulxin and thrombin in the presence of b-BSA-(5-HT)6 and stained with phycoerythrin-streptavidin.7 (A) COAT-platelets6 bind b-BSA-(5-HT)6 (filled histogram; region M1) while resting platelets do not (dashed histogram). (B) A brief exposure of platelets to chymotrypsin prior to activation has only a modest effect on b-BSA-(5-HT)6binding. However, chymotrypsin treatment after activation essentially eliminates retention of b-BSA-(5-HT)6 (C; region M1). (D) The concentration dependence for chymotrypsin (abscissa) inhibition of COAT-platelet production (ordinate).
Chymotrypsin sensitivity of COAT-platelets.
Gel-filtered platelets were activated with both convulxin and thrombin in the presence of b-BSA-(5-HT)6 and stained with phycoerythrin-streptavidin.7 (A) COAT-platelets6 bind b-BSA-(5-HT)6 (filled histogram; region M1) while resting platelets do not (dashed histogram). (B) A brief exposure of platelets to chymotrypsin prior to activation has only a modest effect on b-BSA-(5-HT)6binding. However, chymotrypsin treatment after activation essentially eliminates retention of b-BSA-(5-HT)6 (C; region M1). (D) The concentration dependence for chymotrypsin (abscissa) inhibition of COAT-platelet production (ordinate).
Identification of serotonin binding sites by photo cross-linking
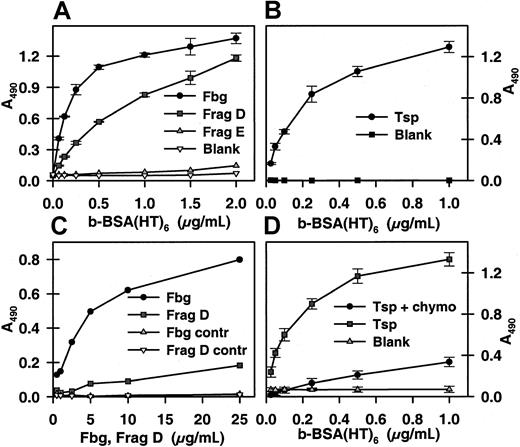
Biotin-BSA-(5-HT)6, derivatized with a photoreactive probe, 4-azido-2,3,5,6-tetrafluorobenzoic acid,10 was coincubated with platelets during activation with ionophore A23187 and thrombin to maximize the number of COAT-platelets produced.7 After activation, cells were irradiated with ultraviolet light for 2 minutes and then lysed for analysis. Preliminary experiments indicated that photo cross-linking resulted in large macromolecular complexes that were refractory to examination; however, partial digestion with chymotrypsin did allow subsequent analysis by enzyme-linked immunosorbent assay (ELISA). Therefore, partially digested samples were applied to microtiter plates coated with polyclonal antibodies against several α-granule proteins, including fibrinogen (Fbg), thrombospondin (Tsp), fibronectin, von Willebrand factor, and α2-antiplasmin; a monoclonal antibody against factor V was also utilized. Biotin-BSA-(5-HT) was coupled almost exclusively to Fbg and Tsp (Figure2A). Further examination demonstrated that photo cross-linking and dual-agonist activation were both required for significant cross-linking of the serotonin probe to occur (Figure2B), although thrombin activation alone resulted in a modest retention of the biotin probe. Similar results were obtained with horseradish peroxidase derivatized with 5-HT and 4-azido-2,3,5,6-tetrafluorobenzoic acid (data not shown).
Photo cross-linking of a serotonin conjugate to COAT-platelets.
B-BSA-(5-HT)6 conjugated with 4-azido-2,3,5,6-tetrafluorobenzoic acid was used in COAT-platelet production7 as described in “Materials and methods.” (A) After activation, cells were UV irradiated, lysed, partially digested with chymotrypsin, and added to microtiter plates coated with antibodies against platelet α-granule proteins: fibrinogen (Fbg), thrombospondin (Tsp), fibronectin (Fn), von Willebrand factor (VWF), factor V, and α2-antiplasmin (AP). Bound biotin was detected with streptavidin-peroxidase. (B) A more extensive examination of b-BSA-(5-HT)6 cross-linking to Fbg and Tsp. Ionophore/thrombin (A/T)–activated, thrombin (T)–activated, and control platelets were analyzed with and without photo cross-linking. Biotin bound to either anti-Fbg or anti-Tsp was detected as in the previous panel.
Photo cross-linking of a serotonin conjugate to COAT-platelets.
B-BSA-(5-HT)6 conjugated with 4-azido-2,3,5,6-tetrafluorobenzoic acid was used in COAT-platelet production7 as described in “Materials and methods.” (A) After activation, cells were UV irradiated, lysed, partially digested with chymotrypsin, and added to microtiter plates coated with antibodies against platelet α-granule proteins: fibrinogen (Fbg), thrombospondin (Tsp), fibronectin (Fn), von Willebrand factor (VWF), factor V, and α2-antiplasmin (AP). Bound biotin was detected with streptavidin-peroxidase. (B) A more extensive examination of b-BSA-(5-HT)6 cross-linking to Fbg and Tsp. Ionophore/thrombin (A/T)–activated, thrombin (T)–activated, and control platelets were analyzed with and without photo cross-linking. Biotin bound to either anti-Fbg or anti-Tsp was detected as in the previous panel.
Thrombospondin and fibrinogen bind serotonin conjugates
Tsp, Fbg, and Fbg fragments D and E11 were adsorbed onto microtiter plates, and the binding of graded concentrations of b-BSA-(5-HT)6 was determined. Fbg and Fbg fragment D both bound b-BSA-(5-HT)6 while fragment E did not (Figure3A); Tsp also bound b-BSA-(5-HT)6 (Figure 3B). The apparent median effective concentration (EC50) values for b-BSA-(5-HT)6 binding to Fbg, fragment D, and Tsp were 2.2, 8.2, and 2.3 nM, respectively. Although this appears to be a relatively strong interaction, it is likely to represent the product of multiple binding interactions rather than a high affinity of Fbg or Tsp for 5-HT. This is supported by the observation that the reverse ELISA experiment, binding of solution phase Fbg and fragment D to immobilized b-BSA-(5-HT)6, demonstrated that intact Fbg was retained while fragment D bound poorly (Figure 3C). This suggests that the 2 binding sites of intact Fbg were sufficient to stabilize it on the immobilized b-BSA-(5-HT)6 while the single binding site on fragment D was not. Finally, the binding of b-BSA-(5-HT)6to immobilized Tsp was found to be sensitive to chymotrypsin (Figure3D), suggesting that Tsp may be responsible for the chymotrypsin sensitivity observed in Figure 1. Binding of b-BSA-(5-HT)6to immobilized Fbg is only modestly affected by chymotrypsin (data not shown).
Binding of a serotonin conjugate to fibrinogen and thrombospondin.
(A) Microtiter plates coated with fibrinogen, fragment D, and fragment E (10 μg/mL) were incubated with graded concentrations of b-BSA-(5-HT)6. Bound biotin was detected with streptavidin-peroxidase. (B) Plates were coated with thrombospondin and analyzed as in the previous panel. (C) B-BSA-(5-HT)6adsorbed to the plate was incubated with graded levels of fibrinogen and fragment D. Bound ligand was detected with goat antifibrinogen antibody and peroxidase-conjugated rabbit antigoat immunoglobulin G (IgG). (D) Thrombospondin was bound to the plate and then treated with chymotrypsin or buffer as described in “Materials and methods.” B-BSA-(5-HT)6 binding was then determined as detailed for panel A.
Binding of a serotonin conjugate to fibrinogen and thrombospondin.
(A) Microtiter plates coated with fibrinogen, fragment D, and fragment E (10 μg/mL) were incubated with graded concentrations of b-BSA-(5-HT)6. Bound biotin was detected with streptavidin-peroxidase. (B) Plates were coated with thrombospondin and analyzed as in the previous panel. (C) B-BSA-(5-HT)6adsorbed to the plate was incubated with graded levels of fibrinogen and fragment D. Bound ligand was detected with goat antifibrinogen antibody and peroxidase-conjugated rabbit antigoat immunoglobulin G (IgG). (D) Thrombospondin was bound to the plate and then treated with chymotrypsin or buffer as described in “Materials and methods.” B-BSA-(5-HT)6 binding was then determined as detailed for panel A.
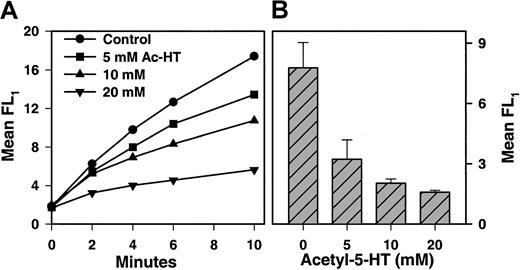
A kinetic assay for monitoring binding of FITC-BSA-(5-HT)6to immobilized Fbg was also established. Specifically, Fbg was bound to 6-μm nylon beads, and the time-dependent binding of FITC-BSA-(5-HT)6 was monitored by flow cytometry. A typical experiment is shown in Figure 4A, where the binding of 4.4 μg/mL FITC-BSA-(5-HT)6 was followed in the presence of graded doses of an inhibitor, N-acetyl serotonin. Figure 4B presents cumulative data on the effect of N-acetyl serotonin and indicates a concentration-dependent inhibition of FITC-BSA-(5-HT)6 binding. Additional experiments showed that neither serotonin nor 5-hydroxyindoleacetic acid were effective inhibitors of FITC-BSA- (5-HT)6 binding to immobilized Fbg (data not shown). It is likely that the positive charge of serotonin and the negative charge of 5-hydroxyindoleacetic acid prevent them from being effective mimetics of the uncharged, conjugated serotonin on FITC-BSA-(5-HT)6.
Binding of FITC-BSA-(5-HT)6 to fibrinogen beads.
(A) Latex beads (6 μm) coated with Fbg were incubated with shaking at room temperature with 4.4 μg/mL FITC-BSA-(5-HT)6. At various times, aliquots were diluted and immediately analyzed for bound fluorescence by flow cytometry. Mean FL1 fluorescence was plotted. Various concentration of N-acetyl serotonin (Ac-HT) were included in the assay and found to inhibit binding of FITC-BSA-(5-HT)6. (B) Data from multiple experiments similar to that presented in panel A were analyzed at 10 minutes (mean ± 1 SD; n = 4) and are plotted as a function of N-acetyl serotonin concentration.
Binding of FITC-BSA-(5-HT)6 to fibrinogen beads.
(A) Latex beads (6 μm) coated with Fbg were incubated with shaking at room temperature with 4.4 μg/mL FITC-BSA-(5-HT)6. At various times, aliquots were diluted and immediately analyzed for bound fluorescence by flow cytometry. Mean FL1 fluorescence was plotted. Various concentration of N-acetyl serotonin (Ac-HT) were included in the assay and found to inhibit binding of FITC-BSA-(5-HT)6. (B) Data from multiple experiments similar to that presented in panel A were analyzed at 10 minutes (mean ± 1 SD; n = 4) and are plotted as a function of N-acetyl serotonin concentration.
Discussion
COAT-platelets are a recently described subpopulation of dual-agonist–stimulated cells characterized by high levels of membrane-bound, strongly retained, procoagulant proteins.6,7 We previously postulated7 that COAT-platelet production involves a transglutaminase-mediated conjugation of serotonin to several of these procoagulant proteins and stabilization of the serotonin-derivatized proteins on the platelet surface by an unidentified serotonin binding protein in addition to the specific known receptors (eg, glycoprotein [GP] IIb/IIIa for Fbg, phosphatidylserine [PS] for factor Va). This report demonstrates that thrombospondin (Tsp) and fibrinogen (Fbg) provide the serotonin binding sites necessary for COAT-platelet formation.
The binding of serotonin conjugates to Fbg is likely to represent a relatively low-affinity interaction, as indicated by the poor binding of soluble fragment D compared with soluble, intact Fbg (Figure3C). This does not indicate, however, that the physiologic importance of this interaction is diminished. The inherent stability of multiple, low-affinity interactions has been well documented with polyvalent antibodies,12,13 and the avidity produced by even bivalent interactions12 14 can increase the apparent affinity by a factor of 103 to 104 while higher valency interactions can be potentiated by factors of 106. Therefore, the potential stability of the multiple interactions proposed in the following model can be significant even though any single Fbg/conjugated serotonin contact is of modest strength.
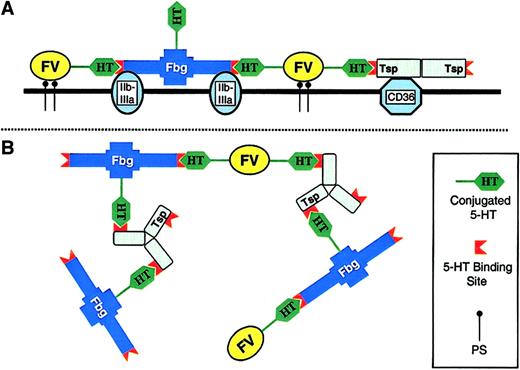
A proposed model of COAT-platelets is shown in Figure5. In this model, serotonin-derivatized proteins bind to both their respective receptors and to the serotonin binding sites on either Fbg or Tsp (Figure 5A). The result is a stabilization of each procoagulant protein on the cell surface due to increased avidity provided by multiple binding interactions. In fact, extensive cross-linking of surface proteins as shown in Figure 5B would result in a 2-dimensional meshwork reminiscent of an immune complex.
Model of COAT-platelets.
This proposed model for COAT-platelets is not meant to be detailed or precise; there are currently too many unknown variables for a comprehensive model. The intent is to fit current observations into a feasible scheme. Basic observations include the following: COAT-platelets require serotonin derivatization of procoagulant proteins; serotonin binding sites are present on fibrinogen (Fbg) and thrombospondin (Tsp); traditional platelet receptors (eg, GP IIb/IIIa for Fbg) are occupied on COAT-platelets; and Fbg is bound to COAT-platelets with exceptional avidity. (A) A cross-section of the COAT-platelet membrane. Fbg, factor V (FV), and Tsp are bound to glycoprotein IIb/IIIa, phosphatidylserine (PS), and CD36, respectively. In addition, conjugated serotonin on FV interacts with serotonin binding sites on Fbg and/or Tsp, resulting in an enhanced stability for all 3 proteins. (B) A broader view of the cell surface showing some of the possible interactions present on COAT-platelets. For example, Fbg is not only binding GP IIb/IIIa and FV-5-HT, but it is also conjugated with 5-HT itself. Interactions between conjugated 5-HT on various proteins and fibrinogen or thrombospondin result in a 2-dimensional matrix with increased avidity for the cell surface. In the interest of simplicity, no membrane receptors are depicted in panel B.
Model of COAT-platelets.
This proposed model for COAT-platelets is not meant to be detailed or precise; there are currently too many unknown variables for a comprehensive model. The intent is to fit current observations into a feasible scheme. Basic observations include the following: COAT-platelets require serotonin derivatization of procoagulant proteins; serotonin binding sites are present on fibrinogen (Fbg) and thrombospondin (Tsp); traditional platelet receptors (eg, GP IIb/IIIa for Fbg) are occupied on COAT-platelets; and Fbg is bound to COAT-platelets with exceptional avidity. (A) A cross-section of the COAT-platelet membrane. Fbg, factor V (FV), and Tsp are bound to glycoprotein IIb/IIIa, phosphatidylserine (PS), and CD36, respectively. In addition, conjugated serotonin on FV interacts with serotonin binding sites on Fbg and/or Tsp, resulting in an enhanced stability for all 3 proteins. (B) A broader view of the cell surface showing some of the possible interactions present on COAT-platelets. For example, Fbg is not only binding GP IIb/IIIa and FV-5-HT, but it is also conjugated with 5-HT itself. Interactions between conjugated 5-HT on various proteins and fibrinogen or thrombospondin result in a 2-dimensional matrix with increased avidity for the cell surface. In the interest of simplicity, no membrane receptors are depicted in panel B.
It is not yet known whether Fbg and Tsp bind first to their respective receptors and then to serotonin-conjugated proteins or whether the binding order is reversed. Also, it is not clear why only a fraction of platelets become COAT-platelets upon stimulation with 2 agonists6,7; further elucidation of the mechanism involved with COAT-platelet production will be required to answer these questions. These observations, however, do provide evidence of an unexpected role for Tsp in platelet function. While Tsp has been documented to bind many adhesive proteins and several platelet receptors,15,16 this role in COAT-platelets was not anticipated. Knock-out mice deficient in Tsp-1 do exhibit a diffuse alveolar hemorrhage,17 though limited analysis of platelet function has not demonstrated any obvious abnormalities. Our report would suggest that a further evaluation of these mice is warranted. In addition, COAT-platelets offer a new role for 5-HT in hemostasis, and the possible involvement of COAT-platelets in physiological and pathologic processes must be considered. For example, recent observations concerning the protective effect of antidepressants (selective serotonin reuptake inhibitors [SSRIs]) against myocardial infarction remain unexplained.18,19 Because platelet 5-HT levels are lowered by SSRI drugs,20involvement of COAT-platelets in these clinical observations deserves investigation.
Prepublished online as Blood First Edition Paper, June 14, 2002; DOI 10.1182/blood-2002-02-0354.
Supported by the National Institutes of Health (HL53585 and HL68129) and the Warren Medical Research Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
George L. Dale, Department of Medicine, BSEB-330, OU Health Sciences Center, 941 Stanton Young Blvd, Oklahoma City, OK 73104; e-mail: george-dale@ouhsc.edu.