Abstract
Crry is a rodent membrane–bound inhibitor of complement activation and is a structural and functional analog of the human complement inhibitors decay-accelerating factor and membrane cofactor protein. We found previously that expression of rat Crry on a human tumor cell line enhances tumorigenicity in nude rats. In this study, we investigated the effect that rat Crry expressed on tumor cells has on rat cell–mediated cytotoxicity and antibody-dependent cell-mediated cytotoxicity (ADCC). The expression of rat Crry on the surface of different human tumor cell lines inhibited ADCC mediated by rat natural killer (NK) cells. C3 opsonization is known to enhance NK cell–mediated cytolysis, and a potential mechanism for Crry-mediated inhibition of NK cell lysis is through Crry modulation of C3 deposition on target cells. However, the transfection of tumor cell lines with Crry enhanced their resistance to NK cell–mediated lysis in the absence of exogenous complement. The resistance of Crry-expressing tumor cells to NK cell–mediated ADCC could be reversed by treatment with anti–Crry F(ab)2. In addition, anti–Crry F(ab)2 enhanced the susceptibility of 13762 rat mammary adenocarcinoma cells (that endogenously express Crry) to ADCC mediated by allogeneic rat NK cells in the absence of added complement. We found no evidence that rat NK cells were a source of complement for target cell deposition during the in vitro cytolysis assay. These data suggest a novel function for rat Crry in tumor immune surveillance that may be unrelated to complement inhibition.
Introduction
Complement activation leads to the generation of C3 convertases that cleave serum C3 into C3a and C3b, the latter of which binds covalently to the activating surface and leads to the further generation of C3 convertase (thus resulting in amplification of the cascade). The activation product C3b is rapidly degraded at the cell surface by proteases with the aid of cofactors to yield iC3b and further degradation products. iC3b can no longer function in the activation of complement, but it is a ligand for complement receptor 3 (CR3, CD11b/CD18) found on phagocytes and NK cells, and iC3b opsonization of target cells can promote and enhance cell-mediated cytotoxicity.1-5 iC3b opsonization alone is not usually sufficient to activate a cell-mediated cytolytic reaction against tumor cells, unless a second distinct lectin domain specific for β-glucan on CR3 is ligated.6,7 iC3b can, however, enhance FcR-mediated cytotoxicity (antibody-dependent cell cytoxicity [ADCC]).2,3 5
Normal and tumor cells are protected from complement by membrane-bound complement inhibitory proteins. In humans, decay-accelerating factor (DAF) and membrane cofactor protein (MCP) are the most important proteins for controlling complement activation and amplification of the cascade. CD59 is a different class of complement inhibitor that acts later in the pathway and inhibits assembly of the terminal cytolytic membrane attack complex. Various studies have reported an up-regulation of complement inhibitors on tumor cells,8-11 implying that complement inhibition may play a role in tumor escape from immune system–mediated clearance. An inhibitor of complement activation termed Crry is expressed on rodent, but not human, cell membranes. Crry is broadly distributed and is a functional and structural analog of human DAF and MCP.12-15 Rodent cells also express DAF and MCP homologues, although the tissue distribution of MCP is restricted.16-18
We are studying the role of complement inhibitory proteins in facilitating tumor growth and the rat is an important model for studying tumor immunology and immune effector mechanisms, especially with regard to complement. Human complement inhibitory proteins are species selective and therefore we have been using human tumor cell lines transfected with rat complement inhibitors to study complement in rat models of human cancer. We have shown that the expression of rat Crry, but not rat CD59, on a human breast tumor cell line (MCF7) significantly enhances tumorigenicity in nude rats.19Crry, but not CD59, will inhibit C3 deposition, and our finding suggested a role for C3 opsonization and cell-mediated cytotoxicity in the modulation of tumor growth in this model. In this context, natural killer (NK) cells and phagocytes are thought to play an important role in immune resistance to tumors. Therefore, we investigated the effect of rat Crry on rat cell–mediated cytolytic activity. We found that rat Crry expression on target cells lowered their susceptibility to rat NK cell–mediated ADCC in the presence of a complement source. This effect may be due to Crry-mediated modulation of C3 deposition. However, we found that Crry also inhibited rat NK cell–mediated ADCC in the absence of exogenous complement, and our data indicate a novel function for rat Crry. These findings share some similarities to data previously reported for human DAF in which DAF was found to inhibit cytotoxicity of human NK cells when incorporated into target or NK cell membranes.20
Materials and methods
Cells
The human breast cancer cell lines MCF7 and SKBR3 and the murine YAC1 cell line were obtained from the American Type Culture Collection (Rockville, MD). The human neuroblastoma cell line LAN-1 was a gift from Dr N.-K. Cheung (Memorial Sloan-Kettering Cancer Center, New York). The rat mammary tumor cell line 13762 was described previously.21 MCF7 cells were grown in Eagle modified essential medium (EMEM) supplemented with 0.1% nonessential amino acids and 1% human insulin. All other cells were grown in RPMI 1640 medium. All media contained 10% heat-inactivated fetal calf serum (FCS) (Hyclone, Logan, UT), 2 mM glutamine, 100 μg/mL penicillin, and 100 μg/mL streptomycin. Cells were incubated in a humidified chamber at 37°C with 5% CO2.
Antibodies
Rabbit antisera to tumor cell membranes were prepared by standard techniques.22 Cell membranes used for inoculation were prepared by Dounce homogenization of cells in hypotonic media (10 mM sodium phosphate, pH 8) followed by subcellular fractionation to remove nuclei and mitochondria, and collection of cell membranes by centrifugation. Anti–MCF7 polyclonal antibodies were purified by protein A affinity chromatography. Anti–GD2 monoclonal antibody (mAb) 3F8 was the gift of Dr N.-K. Cheung. The hybridoma producing mouse anti–rat CD59 (6D1), mouse anti–rat Crry (512), and rat anti–mouse Crry (5D5) cell lines were the gifts of Drs. B. P. Morgan (University of Wales, Cardiff, United Kingdom), Dr H. Okada (Nagoya City University, Nagoya, Japan) and Dr V. M. Holers (University of Colorado Health Sciences Cener, Denver, CO), respectively. Hybridomas were grown in RPMI containing 10% FCS, and antibodies were purified from culture supernatants by affinity chromatography using protein G–sepaharose. F(ab′)2 fragments of mAb 512 (anti–rat Crry) were obtained by incubating antibody with Ficin (Pierce, Rockford, IL), as per manufacturer's instructions) followed by removal of Fc fragments on a protein G column (Pierce). F(ab′)2were subsequently checked for Fc contamination by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis. Affinity purified F(ab′)2 fragments of polyclonal anti–rat Crry were a gift of Dr R. Quigg (University of Chicago, Chicago, IL). Goat anti–rat C3 and anti–mouse C3 antibodies, and horseradish peroxidase (HRP)– or fluorescein isothiocyanate (FITC)–labeled goat anti–C3 antibodies were purchased from ICN Pharmaceuticals (Aurora, OH). Rabbit anti–goat IgG FITC-conjugated antibody was purchased from Sigma (St Louis, MO). C6-deficient rat serum was kindly provided by Dr W. M. Baldwin (Johns Hopkins University, Baltimore, MD) and Dr B. P. Morgan.
Transfection of tumor cell lines
cDNA encoding rat CD59 and rat Crry were the gifts of Drs B. P. Morgan and H. Okada, respectively. cDNA encoding mouse Crry was the gift of Dr M. V. Holers. Rat CD59 was subcloned into the multiple cloning sites of the mammalian expression vector pCDNA3, and rat and mouse Crry cloned into pCDNA3.1 (Invitrogen, Carlsbad, CA). DNA was transfected into 50% to 75% confluent LAN-1, MCF7, or SKBR3 cells using Lipofectamine Plus according to the manufacturer's instructions (Life Technologies, Grand Island, NY). Stably transfected cells were selected following the cultivation of cells in the presence of G418 (PCDNA3) or zeocin (PCDNA3.1). Populations of cells expressing uniform levels of either rat CD59, rat Crry, or mouse Crry were isolated by several rounds of cell sorting using either anti–rat CD59 (mAb 6D1), anti–rat Crry (mAb 512), or anti–mouse Crry (mAb5D5) as described previously.23 Expression of Crry on transfected cells lines and the 13762 cell line was analyzed by flow cytometry as described.23
Preparation of effector cells
Except where indicated, all cytotoxocity assays were performed using effector cells isolated from Sprague Dawley rats. Sprague Dawley, Wistar Kyoto, or Fisher 344 rats (Taconic Farms, Germantown, NY) were treated with 10 mg/kg poly IC (Calbiochem, San Diego, CA). After 24 hours, rats were killed and their spleens removed. Splenocytes were gently isolated using a plunger and then passed through a 70-μm filter, washed, and isolated by low-speed centrifugation through Ficoll as described. B cells were depleted by incubation with Biomag magnetic beads conjugated to goat anti–rat IgG (PerSeptive Biosystems, Framingham, MA). After applying a magnet for 10 minutes, supernatants were removed and reapplied to the magnet 2 more times. Cells were then washed by centrifugation and resuspended in RPMI 1640. For NK cell depletion, splenocyte preparations were incubated (4°C for 30 minutes) with mouse anti–rat CD161 (Serotec, Raleigh, NC), washed and further incubated (4°C for 30 minutes) with magnetic beads conjugated to goat anti–mouse IgG (Dynall, Lake Success, NY). NK cells were then removed by applying a magnet. For NK cell purification, splenocyte preparations were incubated with mouse anti–rat CD4, CD8, ED1, and B-cell antigen (4°C for 30 minutes) (Serotec), washed, and further incubated (4°C for 30 minutes) with magnetic beads conjugated to goat anti–mouse IgG. A magnet was applied and NK cells were isolated and washed by centrifugation in phosphate-buffered saline (PBS). Preparations were checked for appropriate depletions by flow cytometric analysis. Mouse NK cells were purified from splenocyte preparations using anti–NK cell mAb DX5-coated microbeads and LS type separation columns on a MACS separator according to manufacturer's instructions (Miltenyi Biotec, Auburn, CA). Mouse splenocytes were prepared as described above for rat splenocytes. Purified rat and mouse NK cell preparations were checked by flow cytometry.
Cytolysis assays
Target cells were detached from plasticware with versene (Gibco BRL, Grand Island, NY), washed twice, and preloaded with sodium51Cr (Amersham, Arlington Heights, IL) at 0.1 mCi (3.7 MBq)/106 cells for 90 minutes at 37°C. Cells were then washed 3 times in complete media (see above) and resuspended to 1 × 106/mL. If indicated, target cells were then incubated with appropriate opsonizing antibody for 30 minutes at 4°C. Antibody incubations were done using anti–membrane rabbit polyclonal antisera (anti-MCF7 and anti-SKBR3) at a 10% final antiserum concentration, or with anti–GD2 mAb 3F8 at 25 μg/mL (for GD2-positve LAN-1 cells). In some experiments, C6-deficient rat serum was added at time of antibody incubations (10% final concentration) as a source of exogenous complement. C6-deficient serum was used to prevent assembly of cytolytic MAC, but permit C3 deposition. In Crry-blocking experiments, target cells were incubated with anti–rat Crry F(ab)2 fragments at indicated concentrations (30 minutes/4°C) before the addition of opsonizing antibody. Following all antibody incubations, cells were washed and incubated with effector cells (5000 cells/well in 96-well plate) for 4 hours at 37°C/5% CO2. Lysis was determined by 51Cr release assay as described.20 For negative controls, target cells were incubated with media alone, and for maximal lysis controls target cells were incubated with 10% SDS. Rat and mouse NK-susceptible YAC1 cells were used as controls for NK cell activity in these assays.
C3 assays
To determine whether NK cells produced C3, and if so whether C3 could bind to MCF7 target cells, NK cells were incubated in microtiter plate wells at 37°C/5% CO2 in medium at the concentration used in cytolysis assays (above). The wells were first coated with either bovine serum albumin (BSA) or purified anti–MCF7 antibodies (10 μg/mL, 4 hours at room temperature). After 0, 4, or 12 hours, culture supernatant was collected. C3 in culture supernatant was assayed by enzyme-linked immunosorbent assay (ELISA) using a standard techniques.22 Briefly, microtiter plates were coated with goat anti–rat C3 antibody (30 μg/mL overnight at 4°C), and plates then blocked with 2% skimmed milk in PBS. Culture supernatant was incubated in wells for 1 hour at room temperature, and bound C3 detected by means of goat anti–rat C3 conjugated to HRP and chromogenic substrate. Mouse C3 was similarly assayed using goat anti–mouse C3 antibodies. Normal pooled rat or mouse serum was used as standard. Deposition on NK cell–derived C3 MCF7 target cells was assayed by flow cytometry. NK cell culture supernatant was incubated with anti-MCF7 opsonized target cells for 1 hour at 37°C. Cells were washed and analyzed by flow cytometry23 after incubation with FITC-conjugated goat anti–rat or anti–mouse C3. For a positive control, antibody opsonized cells were incubated with C6-deficient rat serum (10% final concentration).
Results
Expression of rat Crry on human tumor target cells reduces their susceptibility to rat NK lysis
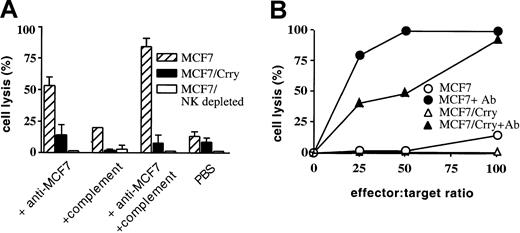
The human breast cancer cell line MCF7 was transfected with rat Crry and a cell population stably expressing the cell surface recombinant protein was isolated by fluorescence activated cell sorting (Figure 1). MCF7 and MCF7/Crry cells were assayed for susceptibility to cell-mediated lysis in vitro using rat splenocytes depleted of B cells. At an effector-to-target (E/T) ratio of 100:1, less than 20% of MCF7 cells were lysed, but lysis was significantly enhanced by the addition of rabbit anti–MCF7 antibody (Figure 2A). When a complement source was added to MCF7 target cells in the presence of specific antibody, lysis of MCF7 was further enhanced. Rat C6–deficient serum was used as a complement source to allow the generation of C3 convertase and C3 deposition on MCF7, but not the formation of the cytolytic membrane attack complex. C6-deficient serum alone (ie, in the absence of added anti–MCF7 antibody) also slightly enhanced cell-mediated lysis, but flow cytometry revealed the presence of “natural” antibodies reactive to MCF7 in C6-deficient rat serum that likely contribute to the observed enhancement of lysis. Crry transfected MCF7 were significantly more resistant to ADCC than untransfected MCF7, even in the presence of a complement source (83% verses 9%) (Figure 2A). The very low level of natural cytotoxicity mediated by splenocyte preparations in the absence of specific antibody was not significantly inhibited by the expression of Crry on target MCF7 cells. Cytotoxicity of the rat splenocyte preparation was mediated by NK cells, since NK cell depletion in vitro resulted in complete abrogation of cell lysis. In addition, purified rat NK cells (Figure 2B) produced similar cytolytic profiles against MCF7 and Crry transfected MCF7 as did B-cell–depleted splenocyte preparations (Figure 2A). Stable expression of rat CD59 on MCF7 cells had no inhibitory effect on NK-mediated cytolysis (not shown), indicating that the isolation procedure did not select an unrelated trait affecting susceptibility to NK lysis.
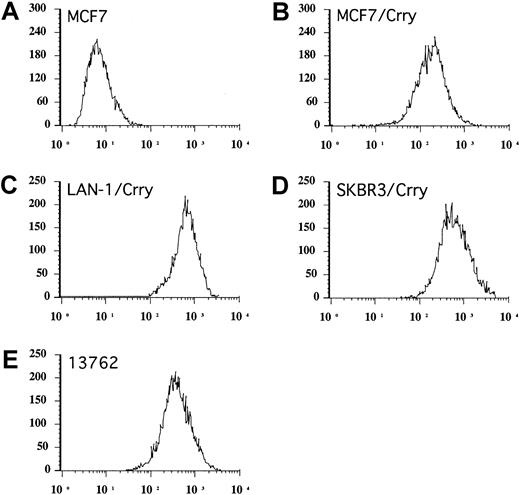
Flow cytometric analysis of Crry expression.
Stably transfected populations of human tumor cell lines expressing rat Crry were isolated by several rounds of cell sorting. Figure shows analysis of sorted populations used in experiments (A-C). Endogenous expression of Crry on 13762 rat mammary tumor cells is also shown (D). Cells were stained by immunofluorescence using anti–rat Crry monoclonal antibody 5I2. Untransfected MCF7 cells are shown as control, but the same levels of fluorescence were seen with other untransfected cell lines.
Flow cytometric analysis of Crry expression.
Stably transfected populations of human tumor cell lines expressing rat Crry were isolated by several rounds of cell sorting. Figure shows analysis of sorted populations used in experiments (A-C). Endogenous expression of Crry on 13762 rat mammary tumor cells is also shown (D). Cells were stained by immunofluorescence using anti–rat Crry monoclonal antibody 5I2. Untransfected MCF7 cells are shown as control, but the same levels of fluorescence were seen with other untransfected cell lines.
Expression of rat Crry on MCF7 cells inhibits rat cell–mediated cytoxicity.
MCF7 cells and Crry transfected MCF7 cells were exposed to either a B-cell–depleted rat splenocyte preparation at an E/T ratio of 100:1 (panel A) or to purified rat NK cells at various E/T ratios (panel B). In the experiment shown in panel A, target cells were preincubated in the presence of either anti–MCF7 antibody, C6-deficient rat serum complement source, both antibody and complement, or PBS. Panel A also shows the effect of NK cell depletion on cytolytic activity of the splenocyte preparation. In the experiment shown in panel B, target cells were preincubated with either anti–MCF7 antibody or PBS as indicated. Lysis was determined by 51Cr release assay after a 4-hour incubation. Shown are the experimental means ± SD (n = 3); data are representative of 3 (panel A) or 2 (panel B) separate experiments.
Expression of rat Crry on MCF7 cells inhibits rat cell–mediated cytoxicity.
MCF7 cells and Crry transfected MCF7 cells were exposed to either a B-cell–depleted rat splenocyte preparation at an E/T ratio of 100:1 (panel A) or to purified rat NK cells at various E/T ratios (panel B). In the experiment shown in panel A, target cells were preincubated in the presence of either anti–MCF7 antibody, C6-deficient rat serum complement source, both antibody and complement, or PBS. Panel A also shows the effect of NK cell depletion on cytolytic activity of the splenocyte preparation. In the experiment shown in panel B, target cells were preincubated with either anti–MCF7 antibody or PBS as indicated. Lysis was determined by 51Cr release assay after a 4-hour incubation. Shown are the experimental means ± SD (n = 3); data are representative of 3 (panel A) or 2 (panel B) separate experiments.
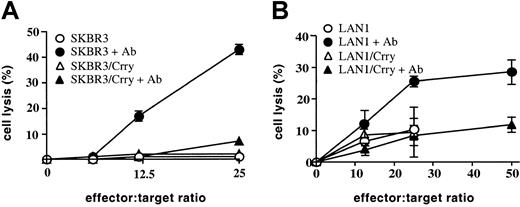
Two other human tumor cell lines, SKBR3 (breast carcinoma) and LAN1 (neuroblastoma), were similarly transfected with rat Crry and their susceptibility to NK-mediated lysis determined. These 2 cell lines were also susceptible to NK-mediated ADCC and, similar to MCF7, expression of rat Crry conferred resistance to NK-mediated ADCC (Figure3). SKBR3 and LAN1 cell lines were inherently more resistant than MCF7 to NK-mediated cytolysis in the absence of specific antibody (SKBR3 was totally resistant; compare Figures 2B, 3A-B), and the expression of Crry on these targets also had no detectable effect on natural NK-mediated cytolysis.
Expression of rat Crry on SKBR3 and LAN1 tumor cells inhibits NK-mediated antibody-dependent cell cytotoxicity.
Control transfected or Crry-transfected SKBR3 cells (A) or LAN1 cells (B) were preincubated either in the presence or absence of target cell–specific antibody as indicated (either anti–SKBR3 membrane polyclonal antibody or anti–GD2 mAb for LAN-1 cells). Cells were then washed and exposed to purified rat NK cells. Lysis was determined by51Cr release assay after a 4-hour incubation. Figures show means ± SD (n = 3) and are representative of 2 (panel A) or 4 (panel B) experiments.
Expression of rat Crry on SKBR3 and LAN1 tumor cells inhibits NK-mediated antibody-dependent cell cytotoxicity.
Control transfected or Crry-transfected SKBR3 cells (A) or LAN1 cells (B) were preincubated either in the presence or absence of target cell–specific antibody as indicated (either anti–SKBR3 membrane polyclonal antibody or anti–GD2 mAb for LAN-1 cells). Cells were then washed and exposed to purified rat NK cells. Lysis was determined by51Cr release assay after a 4-hour incubation. Figures show means ± SD (n = 3) and are representative of 2 (panel A) or 4 (panel B) experiments.
Anti–Crry F(ab)2 modulates NK inhibitory effects of Crry expression
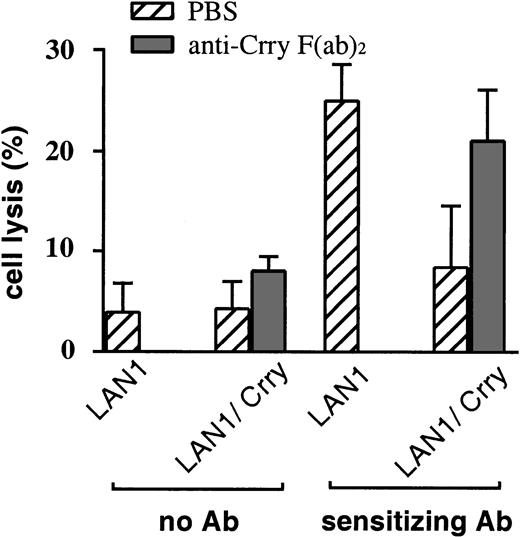
Further confirmation that rat Crry expressed on target cells can modulate NK cytotoxicity was obtained from experiments using anti–Crry F(ab)2 to block Crry-mediated NK inhibitory activity. Anti–Crry F(ab)2 increased susceptibility of Crry-expressing MCF7 to NK cell–mediated ADCC in a dose-dependent manner (Figure 4). Anti–Crry F(ab)2 had no significant effect on NK lysis of untransfected MCF7 either in the presence or absence of anti–MCF7 sensitizing antibody, indicating that the F(ab)2preparation was not alone responsible for enhancing NK cell–mediated lysis. NK cell susceptibility of Crry-transfected LAN-1 was similarly enhanced by the addition of anti–Crry F(ab)2 (Figure5). In these antibody blocking experiments, similar results were obtained with F(ab)2preparations derived from either polyclonal or monoclonal anti–Crry antibodies. Data shown in Figure 4 were obtained using a polyclonal anti-Crry preparation together with a polyclonal anti–MCF7 sensitizing antibody. Data shown in Figure 5 were obtained using a monoclonal anti–Crry antibody (mAb 5I2) together with a monoclonal sensitizing antibody (anti–GD2 mAb 3F8). Both monoclonal14 and polyclonal (not shown) Crry F(ab)2 preparations block the complement inhibitory function of Crry.
Anti–Crry F(ab)2 fragments reverse the inhibitory effect of Crry expressed on MCF7 cells.
Parental MCF7 or Crry-transfected MCF7 cells were preincubated with PBS or the indicated concentration of anti–Crry F(ab)2. Cells were then incubated either in the absence or presence of anti–MCF7 polyclonal antibody, washed and used as target cells in cytolysis assays with rat splenocytes enriched for NK cells as effector cells (E/T ratio of 100:1). Lysis was determined by 51Cr release assay after a 4-hour incubation. Data shown are representative of 6 separate experiments.
Anti–Crry F(ab)2 fragments reverse the inhibitory effect of Crry expressed on MCF7 cells.
Parental MCF7 or Crry-transfected MCF7 cells were preincubated with PBS or the indicated concentration of anti–Crry F(ab)2. Cells were then incubated either in the absence or presence of anti–MCF7 polyclonal antibody, washed and used as target cells in cytolysis assays with rat splenocytes enriched for NK cells as effector cells (E/T ratio of 100:1). Lysis was determined by 51Cr release assay after a 4-hour incubation. Data shown are representative of 6 separate experiments.
Anti–Crry F(ab)2 fragments reverse the inhibitory effect of Crry expressed on LAN1 cells.
Target LAN1 or Crry-transfected LAN1 cells were preincubated with PBS or 10 μg/mL anti–Crry F(ab)2. Cells were then incubated either in the absence or presence of anti–GD2 monoclonal antibody as indicated, washed and tested for susceptibility to lysis by enriched NK cells (E/T ratio of 100:1). Lysis was determined by 51Cr release assay after a 4-hour incubation. Figure shows means ± SD (n = 3) and is representative of 5 separate experiments.
Anti–Crry F(ab)2 fragments reverse the inhibitory effect of Crry expressed on LAN1 cells.
Target LAN1 or Crry-transfected LAN1 cells were preincubated with PBS or 10 μg/mL anti–Crry F(ab)2. Cells were then incubated either in the absence or presence of anti–GD2 monoclonal antibody as indicated, washed and tested for susceptibility to lysis by enriched NK cells (E/T ratio of 100:1). Lysis was determined by 51Cr release assay after a 4-hour incubation. Figure shows means ± SD (n = 3) and is representative of 5 separate experiments.
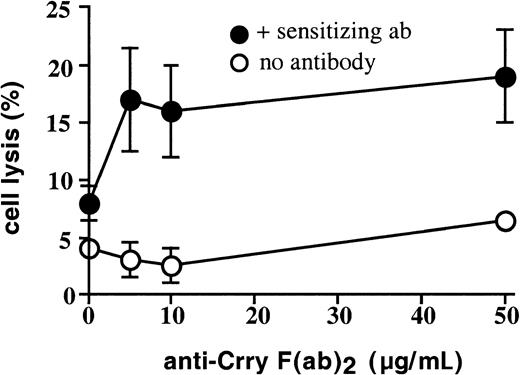
Anti–Crry F(ab)2 enhances NK-mediated lysis of allogeneic but not syngeneic target cells
We investigated whether Crry expressed endogenously on rat target cells may play a role in modulating cell-mediated cytotoxicity. First, the susceptibility of rat mammary adenocarcinoma 13762 cell line to ADCC mediated by either syngeneic or allogeneic splenocyte preparations was determined. 13762 cells express endogenous Crry (Figure 1) and CD59 (not shown). Not surprisingly, 13762 cells were completely resistant to lysis by syngeneic Fisher 344–derived splenocytes, both in the presence and absence of rabbit anti–13762 specific antibody (not shown). In contrast, 13762 cells were sensitive to ADCC mediated by allogeneic splenocyte preparations, but the percent lysis was low; specific cytolysis of splenocytes prepared from Sprague Dawley or Wistar Kyoto rats ranged between 8% (Figure 6) and 12% (not shown), respectively, at an E/T ratio of 100:1 in the presence of specific antibody. However, the susceptibility of 13762 cells to ADCC by allogeneic splenocytes was significantly enhanced by their preincubation with anti–Crry F(ab)2 from about 8% to 19% specific lysis (Figure 6). Anti-Crry had no significant effect on natural cell-mediated cytoxicity mediated by allogeneic splenocytes (Figure 6), and did not enhance ADCC mediated by syngeneic Fisher 344–derived splenocytes (not shown). Splenocyte preparations used in these experiments were depleted of B cells and, similarly as shown above with experiments using xenogeneic targets, the depletion of NK cells from splenocyte preparations completely abrogated lytic activity (data not shown).
Anti–Crry F(ab)2 fragments enhance antibody-dependent rat cell–mediated cytotoxicity of allogeneic target cells.
13762 rat mammary tumor cells were preincubated with PBS or increasing concentrations of anti–Crry F(ab)2. The target cells were then incubated in the absence or presence of anti–13762 polyclonal antibody as indicated, washed, and exposed to B-cell–depleted rat splenocytes (E/T ratio of 100:1). Lysis was determined by51Cr release assay after a 4-hour incubation. Figure shows means ± SD and is representative of 5 separate experiments.
Anti–Crry F(ab)2 fragments enhance antibody-dependent rat cell–mediated cytotoxicity of allogeneic target cells.
13762 rat mammary tumor cells were preincubated with PBS or increasing concentrations of anti–Crry F(ab)2. The target cells were then incubated in the absence or presence of anti–13762 polyclonal antibody as indicated, washed, and exposed to B-cell–depleted rat splenocytes (E/T ratio of 100:1). Lysis was determined by51Cr release assay after a 4-hour incubation. Figure shows means ± SD and is representative of 5 separate experiments.
Deposition of NK-derived C3 on target cells
Crry inhibits complement activation and limits C3 deposition and opsonization of target cells. Although Crry inhibited NK-mediated ADCC in the absence of exogenously added complement (see above), it is possible that C3 synthesized by NK cells during the assay can become cleaved and deposited on the target cell, thus enhancing NK cytolysis. Crry expression on target cells could inhibit the deposition of NK-derived C3 and, therefore, potentially inhibit C3-enhanced NK-mediated cytolysis. In this context, it is known that human lymphocytes and NK cells synthesize C3.20,24 Therefore, we determined whether rat NK cells secrete C3. NK cell preparations were cultured the absence of target cells under assay conditions, and at various time points the culture supernatant was removed and assayed for C3 by ELISA. Since it is possible that the cross-linking of Fc receptors on rat NK cells mediated by antibody-coated target cells may induce C3 secretion, in some experiments Fc receptors were engaged on NK cells by incubating the NK cells in the presence of immobilized purified anti–MCF7 antibody (the same antibody used to sensitize MCF7 target cells to ADCC). The data in Table1 show that rat NK cells secrete C3, and at levels similar to those reported for human NK cells.20Interestingly, the presence of immobilized anti–MCF7 antibody caused an initial enhancement of C3 secretion. To determine whether C3 secreted by NK cells could opsonize target tumor cells, NK cell preparations were cultured for either 4 or 12 hours in the presence of immobilized anti–MCF7 antibody, the culture supernatant removed and added to antibody-sensitized MCF7 target cells. Target cells were then assayed for C3 deposition by flow cytometry. No C3 was detected on MCF7 target cells that were incubated in either 4- or 12-hour NK cell culture supernatant (Figure 7). The duration of NK cell cytolytic assay is 4 hours. Together these data indicate that rat NK cells produce C3, but not at levels sufficient to modulate ADCC.
Deposition of rat C3 on MCF7 cells following incubation in NK cell culture supernatant.
Culture supernatant derived from cultured rat NK cells in the presence of immobilized anti–MCF7 antibodies was removed at time 0 (A) 4 hours (B) and 12 hours (C) and incubated with MCF7 cells opsonized with complement activating anti–MCF7 antibody. Deposition of C3 on MCF7 cells was detected by flow cytometry after staining with anti–rat C3. For a positive control, antibody-coated MCF7 cells were incubated in 10% C6-deficient rat serum (D). Experiments were performed using cell concentrations and culture conditions that were used for cytoxicity assays. Histograms with relative mean fluorescence values are shown from a representative experiment of 2.
Deposition of rat C3 on MCF7 cells following incubation in NK cell culture supernatant.
Culture supernatant derived from cultured rat NK cells in the presence of immobilized anti–MCF7 antibodies was removed at time 0 (A) 4 hours (B) and 12 hours (C) and incubated with MCF7 cells opsonized with complement activating anti–MCF7 antibody. Deposition of C3 on MCF7 cells was detected by flow cytometry after staining with anti–rat C3. For a positive control, antibody-coated MCF7 cells were incubated in 10% C6-deficient rat serum (D). Experiments were performed using cell concentrations and culture conditions that were used for cytoxicity assays. Histograms with relative mean fluorescence values are shown from a representative experiment of 2.
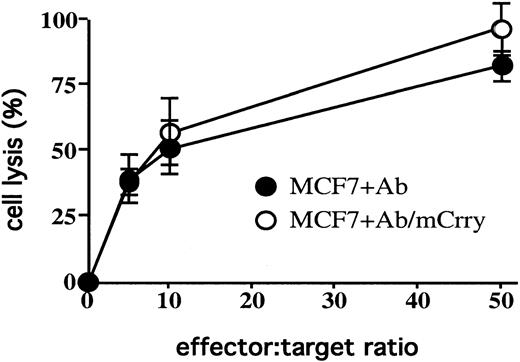
The use of NK cells derived from C3-deficient mice would allow us to more directly determine whether C3 produced by NK cells was involved in modulating cytolysis. We therefore transfected MCF7 cells with mouse Crry, isolated a stably expressing population, and determined the effect of mouse Crry on mouse NK cell–mediated lysis. Surprisingly, mouse Crry had no effect on cytolysis of MCF7 cells mediated by purified mouse NK cells (Figure 8); nevertheless, mouse NK cells did secrete C3 at similar levels to rat NK cells, and no C3 was detected on MCF7 target cells that were incubated in 12-hour mouse NK cell culture supernatant (data not shown).
Expression of mouse Crry on MCF7 cells does not effect susceptibility to mouse NK cell–mediated cytolysis.
MCF7 cells and mouse Crry-transfected MCF7 cells were exposed to purified mouse NK cells at various E/T ratios. Target cells were preincubated in the presence of anti–MCF7 antibody (in the absence of a complement source). Lysis was determined by 51Cr release assay after a 4-hour incubation. The figure shows the experimental means ± SD (n = 3) and is representative of 3 separate experiments.
Expression of mouse Crry on MCF7 cells does not effect susceptibility to mouse NK cell–mediated cytolysis.
MCF7 cells and mouse Crry-transfected MCF7 cells were exposed to purified mouse NK cells at various E/T ratios. Target cells were preincubated in the presence of anti–MCF7 antibody (in the absence of a complement source). Lysis was determined by 51Cr release assay after a 4-hour incubation. The figure shows the experimental means ± SD (n = 3) and is representative of 3 separate experiments.
Discussion
Innate recognition by NK cells is mediated by opposing effects of various activation and inhibitory receptors that are less well defined in the rat than in humans and mice (for review, see Lanier25 and Lanier26). In addition to receptors involved in innate recognition, NK cells express CD16 (FcγRIII), a low-affinity receptor for IgG that is responsible for mediating ADCC in the absence of effective inhibition.27Macrophages and polymorphonuclear cells also express Fcγ receptors, and the role of these immune effector cells in mediating ADCC is well characterized in vitro. Although the physiologic significance of ADCC has been questioned, Clynes et al recently demonstrated that engagement of Fcγ receptors (both activation and inhibitory) on effector cells is an important component of in vivo activity of antibodies against tumors in various mouse models of cancer.28
Another activation receptor expressed on NK cells is complement receptor 3 (CR3 or CD11b/CD18). The principal complement ligand for CR3 is iC3b, a C3 degredation product covalently bound to the cell surface at a site of complement activation. Enhancement of phagocyte and NK cell–mediated ADCC by complement opsonization of the target cell is mediated via the iC3b/CR3 interaction.1-5 However, although CR3 can mediate cell adhesion to tumor cells, the binding of iC3b alone to CR3 does not trigger phagocyte or NK cell cytotoxicity. A signaling mechanism to trigger cytolytic function is provided by the membrane association of CR3 with Fcγ receptors.5,29-31The additional ligation of a lectin domain on CR3 that is distinct from the iC3b binding site can also induce degranulation in the absence of antibody and Fcγ ligation.7
Our experiments show that expression of Crry inhibits NK-mediated target cell lysis in vitro, a phenotype which we suggest may be related to tumor escape from immune clearance in vivo.
Since C3 opsonization of target cells is known to enhance NK-mediated ADCC, an inhibitor of complement activation expressed on a target cell might inhibit ADCC by limiting C3 deposition. Such an effect would inhibit target cell susceptibility to lysis. However, in the studies reported here, Crry expressed on target cell surfaces inhibited NK-mediated ADCC in the absence of exogenously added complement. There are 2 plausible mechanisms for this effect of Crry on NK cell lytic function. One possibility is that Crry may directly interact with an NK cell inhibitory receptor and regulate lytic function in spite of target cell recognition. There is currently no direct evidence to support this notion, but this possibility is plausible since DAF, a structurally and functionally related protein, is a cellular ligand for CD97, an activation-induced leukocyte receptor with no apparent link to the complement system.32,33 The biologic significance of CD97-DAF interaction is unknown. In addition, both DAF and Crry can also associate with other membrane proteins and both are involved in signal transduction.34 39
A second possible mechanism for the effect of Crry on NK cell cytotoxicity is that C3 secreted by the NK cells during the in vitro cytotolysis assay becomes bound to the target cells enhancing ADCC, and that the deposition of locally synthesized C3 on the target cell is inhibited by the expression of Crry. Supporting this notion are data demonstrating that human NK cells secrete C3 in vitro.20Here we have shown that rat NK cells also secrete C3, but that NK-secreted C3 does not deposit on antibody opsonized target cells in detectable quantity. This argues against a role for C3 opsonization in the Crry-mediated inhibitory effect on NK cells, but we cannot rule out the possibility that NK cell C3 secretion is polarized resulting in a high local concentration of C3 at the site of NK target cell contact, as has been shown to occur for cytokine secretion and cytolysis of lytic granules. To determine whether C3 was involved in the Crry-NK interaction, we attempted to duplicate our findings using a mouse system. The use of NK cells derived from C3-deficient mice would have allowed us to directly address the role of C3 in NK lysis. However, transfection of human tumor cells with mouse Crry had no effect on their susceptibility to mouse NK lysis, although mouse NK cells did produce C3.
The expression of rat Crry on xenogeneic targets had a negligible effect on rat NK cell–mediated cytoxicity in the absence of target cell–specific antibodies, although the human cell lines used as targets were relatively resistant to natural lysis mediated by either B-cell–depleted splenocyte preparations or purified NK cells. Thus, antibody opsonization of target cells may be required for complement activation and C3 deposition on target cells. In the absence of exogenous complement, in vitro activation of NK-derived C3 may occur by direct binding to the target cell and/or target cell–bound antibody,40,41 by proteolytic cleavage by lymphocyte-derived protease,42 or via classical pathway activation by NK synthesized complement proteins. In any case, in the tumor microenvironment in situ tumor cells would be expected to have substantial complement available owing to abundant vascularization.
It is not immediately apparent why the haplotype of the NK cell should have an effect on ADCC (Figure 6) since there is no direct evidence that ADCC is major histocompatibility complex (MHC) restricted. It is possible that the result may reflect differences in expression levels of receptors or activity among the different NK cell haplotypes. Activation of NK cells is held in balance by various activation and inhibitory signals, and it is certainly conceivable that MHC molecules can contribute to this balance during NK cell–mediated ADCC.
The rat is an important model for studying tumor immunology and immune effector mechanisms, and our findings are relevant to the interpretation of certain studies using rat models of cancer. An earlier study showed that human DAF inhibited the cytotoxicity of human NK cells.20 DAF has structural and functional similarities with Crry, and thus the phenomenon reported here with rat Crry and NK cells is likely of relevance to human immune mechanisms. On the other hand, we found that expression of mouse Crry on target cells did not effect their susceptibility to mouse NK cells. We were surprised by this result, but NK cells have multiple mechanisms of target cell recognition and further research is needed to define related mechanisms in the various systems. It is worthwhile noting that significant differences among human, rat, and mouse NK cell–mediated ADCC of tumor targets has been reported previously.43
Supported by Department of the Army grants DAMD17-01-1-0393 and DAMD12-99-1-9325 and by National Institutes of Health grant AI 34451.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stephen Tomlinson, Medical University of South Carolina, Department of Microbiology and Immunology, BSB 201, 173 Ashley Ave, Charleston, SC 29425; e-mail:tomlinss@musc.edu.