Abstract
Cytogenetic clonal evolution (CE) is a known poor prognostic factor in Philadelphia chromosome–positive chronic myelogenous leukemia (Ph-positive CML). However, its prognostic relevance in the era of imatinib therapy is unknown. We investigated the independent prognostic relevance of CE in 498 patients with Ph-positive CML treated with imatinib for chronic or accelerated phases. One hundred twenty-one patients had CE alone (n = 70) or with other accelerated phase criteria (n = 51). Patients were compared in 4 categories: chronic phase (n = 295), CE only (n = 70), accelerated phase without CE (n = 82), and accelerated phase with CE (n = 51). Statistical methods used established methodologies for univariate and multivariate analyses. In chronic and accelerated phases of CML, CE was not associated with significant differences in major or complete cytogenetic response rates, but it was an independent poor prognostic factor for survival by multivariate analyses in both chronic (P = .005) and accelerated phase (P = .03). Multivariate analyses conducted at the 3-month landmark (including the 3-month cytogenetic response) identified the lack of cytogenetic response at 3 months to be a stronger independent poor prognostic factor for survival than CE for both chronic (major cytogenetic response versus other) and accelerated phase (any cytogenetic response versus other). We conclude that cytogenetic CE is not an important factor for achieving major or complete cytogenetic response with imatinib mesylate therapy, but it is an independent poor prognostic factor for survival in both chronic and accelerated phases of CML. The 3-month cytogenetic response to imatinib mesylate refined the prognostic relevance of such studies in patients on imatinib mesylate therapy.
Introduction
Philadelphia chromosome (Ph)–positive chronic myelogenous leukemia (CML) presents and evolves through different phases: chronic, accelerated, and blastic.1-6 The Ph-related molecular abnormalities are causally associated with the pathophysiology of CML.7,8 Therapy of CML has been directed at suppressing the Ph-positive cells or Ph-associated molecular events and has included allogeneic stem cell transplantation (SCT), interferon α (IFN-α), and, more recently, imatinib mesylate (Gleevec, STI571; Novartis, East Hanover, NJ).9-30
Cytogenetic clonal evolution is traditionally considered as a fingerprint of the multistep progression of CML. It was originally described in patients with blastic phase CML, occurring in 50% to 80% of cases, and involving nonrandom chromosomal abnormalities, including double Ph, trisomy 8, isochromosome 17, trisomies of chromosomes 19 and 20, and 20 q− abnormalities.31,32 Cytogenetic clonal evolution was later described in 5% to 10% of patients presenting with chronic phase CML and in 30% of patients developing accelerated phase features, and has been associated with poor prognosis.33-35 Cytogenetic clonal evolution was subsequently considered one of the factors defining accelerated phase CML.5 As more frequent marrow cytogenetic studies were performed in patients in chronic phase CML to monitor their cytogenetic response on IFN or other therapies, it was realized that this event, cytogenetic clonal evolution, was more common than expected and involved cytogenetic abnormalities other than the classical ones.36,37 The prognostic significance of cytogenetic clonal evolution was not uniform and depended on several factors: (1) the particular cytogenetic abnormality, (2) its frequency in metaphase analysis, (3) its association with other accelerated-phase features, (4) the time of occurrence (early versus late), and (5) the therapy of CML (IFN versus others).38,39 About half of the patients with cytogenetic clonal evolution showed suppression of the abnormal clone with IFN therapy.39 Cytogenetic clonal evolution as a single feature of “accelerated” phase CML was associated with good prognosis with allogeneic SCT and a long-term survival rate of 60%.40
The significance of prognostic factors in disease states may be drastically altered with the introduction of new therapies that change prognosis significantly. Imatinib mesylate therapy now appears to be the most effective therapy for CML outside the setting of allogeneic SCT; its efficacy compared with allogeneic SCT will require further maturation of data and longer follow-up of patients on therapy. Cytogenetic clonal evolution is considered an evolution step independent of the Ph-related abnormalities, although arguably still partially related to the primary Ph molecular event. Therefore, the prognostic significance of cytogenetic clonal evolution may be altered in the setting of chronic and accelerated phases of CML with imatinib mesylate therapy. Herein, we analyzed the results of imatinib mesylate therapy in patients with chronic or accelerated phase CML with or without cytogenetic clonal evolution, in relation to response and survival.
Patients and methods
Patients
Patients with Ph-positive CML in chronic or accelerated phases of CML treated on phase 2 studies of imatinib mesylate (Novartis studies 110, 109, 114, and 113) were analyzed. Those studies were approved by the Institutional Review Board, and all patients signed informed consent according to institutional guidelines. Eligibility criteria and pretreatment and follow-up studies have been detailed25-29; marrow cytogenetic analyses were performed every 3 months in the first year and every 3 to 6 months subsequently. Patients in chronic-phase CML received imatinib mesylate at a starting dose of 400 mg orally daily, whereas patients in accelerated-phase CML received imatinib mesylate 400 mg or 600 mg orally daily. Dose escalation for resistant disease and dose reductions for toxicities have been detailed extensively in previous reports.25-29
In the Food and Drug Administration (FDA) pivotal trial in chronic phase (Novartis 110) and the expanded access trial in chronic phase (Novartis 113), patients with cytogenetic clonal evolution with no other criteria of accelerated phase were allowed to be treated on study. Moreover, the expanded access trial in accelerated phase (Novartis 114) allowed entry of patients with more “relaxed” criteria for accelerated phase, including splenomegaly 10 cm or more below the costal margin and the presence of 10% or more blasts. Patient registration by protocol and by the categories used for analysis (chronic phase, clonal evolution only, accelerated phase with or without cytogenetic clonal evolution) are shown in Table1. Only 1 of 495 patients (patient with accelerated-phase criteria registered on Novartis 113) was not eligible by the study criteria.
For the purpose of this analysis, patients were considered to have accelerated phase if they met any of the following criteria: (1) peripheral or marrow blasts 15% or more, (2) peripheral or marrow basophils 20% or more, (3) peripheral or marrow blasts plus promyelocytes 30% or more, or (4) platelets less than 100 × 109/L unrelated to therapy. Patients were then analyzed for outcome of chronic or accelerated phase by the presence or absence of cytogenetic clonal evolution. These are the classical accelerated-phase criteria used in many studies5 but not in the expanded access study (Novartis 114) that allowed patients with less than such accelerated phase criteria to benefit from therapy.29 Thus, patients registered on Novartis study 114 were reclassified according to the above criteria.
Response criteria
Response criteria were as previously described.25 26 A complete hematologic response (CHR) was defined as normalization of peripheral blood counts and differentials (white blood cell [WBC] count < 10 × 109/L; no peripheral blasts or promyelocytes; < 5% myelocytes + metamyelocytes; platelets < 450 × 109/L) and disappearance of signs and symptoms of disease, including palpable splenomegaly. Within CHR, response was categorized by the degree of Ph suppression: complete cytogenetic response, Ph 0%; partial cytogenetic response, Ph 1% to 34%; minor cytogenetic response, Ph 35% to 90%. A major cytogenetic response included complete and partial cytogenetic responses (ie, Ph 0% to 34%). Cytogenetic clonal evolution was defined as the presence of any abnormality other than a single Ph; complex Ph abnormalities or loss of chromosome Y were not considered to be cytogenetic clonal evolution.
Suppression of clonal evolution was described according to the percentage of suppression. If it paralleled the degree of Ph cytogenetic response, it was described as part of the response. If there was a nonparallel response (eg, Ph suppression but persistence of clonal evolution or the reverse), the cytogenetic response reflected the higher (worse) percentage of either abnormality.
Statistical consideration
Survival was measured from the start of therapy until death from any cause. Prognostic factor analysis for response to therapy and survival and multivariate analyses studies to define the independent significance of prognostic factors, including cytogenetic clonal evolution, in chronic and accelerated phases, were conducted as previously described.25,26,28 29
Results
A total of 498 patients were analyzed. These included 261 patients on the pivotal trial of chronic-phase CML (Novartis study 110, n = 149) or on the expanded access study for chronic-phase CML (Novartis study 113, n = 112) and 237 patients on the pivotal trial of accelerated-phase CML (Novartis study 109, n = 58) or on the expanded access study for accelerated-phase CML (Novartis 114, n = 179). Seventy patients had cytogenetic clonal evolution as the only criterion for accelerated-phase disease, whereas 51 patients had clonal evolution in addition to other accelerated-phase criteria. For the purpose of this study, the 70 patients with cytogenetic clonal evolution only, whether treated on the chronic-phase studies or on the accelerated-phase studies, were analyzed as one group. The characteristics of the study groups of interest, chronic phase, cytogenetic clonal evolution only, and accelerated phase with or without cytogenetic clonal evolution, are shown in Table2.
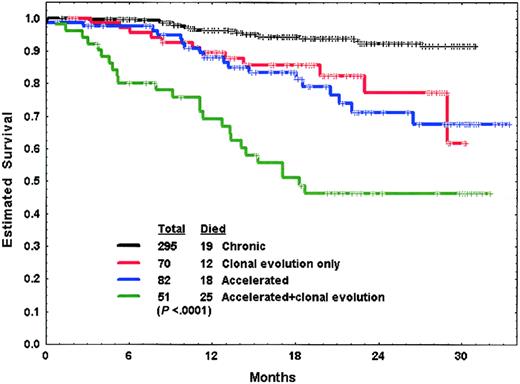
The median follow-up time for patients on these studies was 20 months (range, 4-33 months). Response in each of the study groups is shown in Table 3. Survival of patients in the 4 groups is shown in Figure 1.
Survival of patients in chronic phase, with cytogenetic clonal evolution only, and in accelerated phase with or without cytogenetic clonal evolution.
Survival of patients in chronic phase, with cytogenetic clonal evolution only, and in accelerated phase with or without cytogenetic clonal evolution.
Because cytogenetic clonal evolution may have different prognostic implications by whether or not there are other accelerated-phase criteria, we conducted subsequent analyses in 2 subsets: (1) the subset of patients in chronic phase CML with or without cytogenetic clonal evolution (groups 1 and 2) and (2) the subset of patients with accelerated phase CML with or without cytogenetic clonal evolution (groups 3 and 4).
Significance of cytogenetic clonal evolution in chronic phase CML
In this analysis, 365 patients were reviewed, including 70 patients with cytogenetic clonal evolution. Among 224 patients with active disease (ie, not in CHR at the time of study entry), the CHR rate was 90%. Of the 365 patients treated, 251 (69%) achieved a cytogenetic response, including 189 (52%) with a complete response and 42 (12%) with a partial cytogenetic response. Significant poor prognostic factors for achieving a major cytogenetic response are shown in Table 4 and did not include the presence of cytogenetic clonal evolution. A multivariate analysis identified the following to have independent poor prognostic factors for achieving a major cytogenetic response: higher marrow blast percentage (P = .004), longer time from diagnosis to therapy (P = .02), higher percentage of Ph-positive cells at start of therapy (P = .0008), and prior IFN-α response (hematologic resistance) (P = .05). Patients with none or 1, 2, 3, or all 4 factors had expected major cytogenetic response rates of 92%, 60%, 33%, and 7%, respectively (Table5). A repeat multivariate analysis of factors predictive of complete instead of major cytogenetic response yielded similar results (data not shown).
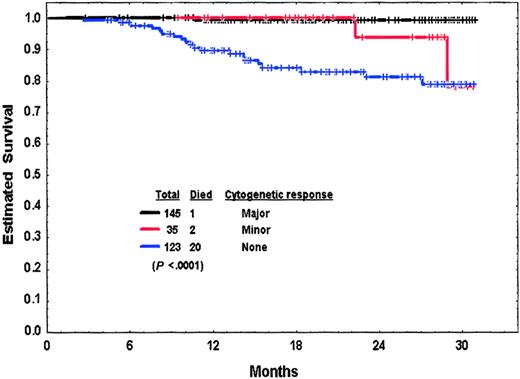
Significant poor prognostic factors for worse survival are also shown in Table 4. A multivariate analysis identified the following to remain as independent poor prognostic factors for survival: any peripheral blasts present (P = .02), cytogenetic clonal evolution (P = .03), and leukocytosis (P = .04). Survival by cytogenetic response at 3 months is shown in Figure2. We then conducted a multivariate analysis of independent factors for survival among patients on study at 3 months and included response to imatinib mesylate at 3 months as a variable. This analysis showed the following factors to be independently associated with worse survival (P<.05): (1) no major cytogenetic response at 3 months, (2) anemia (hemoglobin < 10 g/dL), (3) high percentage of Ph-positive cells at diagnosis, (4) any peripheral blasts, (5) thrombocytosis, (6) leukocytosis, and (7) prior IFN-α response. This analysis suggested that the 3-month response to imatinib mesylate therapy was a stronger independent predictor for survival than clonal evolution at diagnosis.
Survival from 3 months into therapy by cytogenetic response at 3 months of patients in chronic-phase CML with or without cytogenetic clonal evolution.
Survival from 3 months into therapy by cytogenetic response at 3 months of patients in chronic-phase CML with or without cytogenetic clonal evolution.
Significance of cytogenetic clonal evolution in accelerated phase CML
The 133 patients with accelerated phase CML were evaluated; 51 had clonal evolution in addition to other accelerated phase CML features. Significant poor prognostic factors for achieving major cytogenetic response are shown in Table 6 and did not include clonal evolution. A multivariate analysis selected the following as independent adverse prognostic features: splenomegaly 10 cm or greater below the costal margin (P = .01), and longer time from diagnosis to therapy (> 3 years;P = .06). The major cytogenetic response rates by whether patients had none, 1, or 2 of these factors were 62%, 28%, and 5%, respectively (Table 5). A repeated multivariate analysis of prognostic factors predictive of complete rather than major cytogenetic response showed splenomegaly (P = .02) and presence of peripheral blasts (P = .02) (instead of diagnosis to therapy time 3 years or longer) to be independent predictors for achieving complete cytogenetic response. The inclusion of the daily dose of imatinib (400 mg versus 600 mg) did not identify it as an independent poor prognostic factor for cytogenetic response.
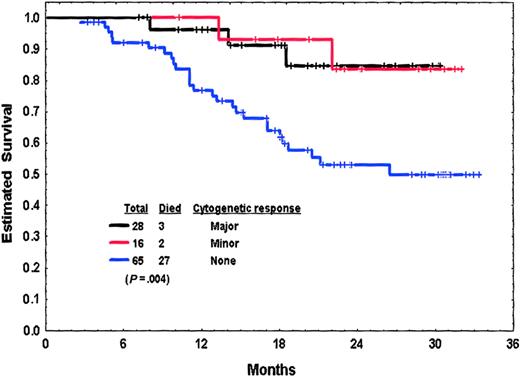
Poor prognostic factors for survival are also listed in Table 6. A multivariate analysis identified the following as independent poor prognostic factors for survival: older age (P = .01), higher percentage of marrow basophils (P = .03), and cytogenetic clonal evolution (P = .03). Survival by response at 3 months is shown in Figure3. A multivariate analysis of factors independently associated with survival among patients on study at 3 months, including 3-month response as a variable identified the following adverse prognostic factors: lack of any cytogenetic response at 3 months (P = .002), older age (P = .02), high percentage of Ph-positive cells at diagnosis (P = .04), clonal evolution (P = .06), and lower imatinib mesylate dose (400 versus 600 mg daily;P = .07). As with patients in chronic phase, this analysis suggested that the 3-month response to imatinib mesylate therapy was a stronger independent predictor for survival than clonal evolution at diagnosis of accelerated phase.
Survival from 3 months into therapy by cytogenetic response at 3 months of patients in accelerated-phase CML with or without cytogenetic clonal evolution.
Survival from 3 months into therapy by cytogenetic response at 3 months of patients in accelerated-phase CML with or without cytogenetic clonal evolution.
Thus, cytogenetic clonal evolution was not an independent significant factor for achieving a major or complete cytogenetic response, but it remained a poor independent prognostic factor for survival in the setting of both chronic and accelerated phases of CML. However, response to imatinib mesylate after 3 months of therapy was also a strong predictor for survival in both chronic and accelerated phase, and it was more important than clonal evolution as an independent prognostic factor.
Response to imatinib therapy by characteristics of clonal evolution
In a previous study, the characteristics of the cytogenetic clonal evolution (chromosome 17 abnormality, associated accelerated-phase criteria, time of clonal evolution, frequency in metaphase analysis)38 39 were associated with different outcomes. We thus analyzed whether these previously identified clonal evolution characteristics were associated with different cytogenetic response profiles (Table 7). As shown, the presence of isochromosome 17, the presence of other accelerated-phase criteria, the later development of clonal evolution, and its predominance in metaphase analysis were all associated with lower rates of complete and major cytogenetic responses. This was statistically significant for the first 2 characteristics. Interestingly, the presence of trisomy 8 was associated with a relatively high cytogenetic response rate.
Development of new chromosomal abnormalities on therapy
In our study, 32 patients of a total of 377 patients without clonal evolution at the start of therapy developed later additional chromosomal abnormalities, including trisomy 8 (n = 16), chromosome 5 or 7 abnormalities (n = 8), and others (n = 8). Fifteen of the 32 cases occurred in patients with cytogenetic response, in the Ph-positive cells (n = 12), or in the diploid cells (n = 3). Six of the 15 cases were transient clonal evolutions, whereas none of the 17 cases occurring in patients without cytogenetic response was transient. Among 44 patients with clonal evolution at the start of therapy who later achieved complete cytogenetic response, 9 developed clonal evolution. Only 1 of the 9 cases was a transient clone, whereas the other 8 have so far persisted (Table8).
The development of new chromosomal abnormalities in these patients has so far not predicted for a worse outcome, but the follow-up period is short (data not shown).
Discussion
In this analysis, we identified cytogenetic clonal evolution to be an independent prognostic factor for survival in both chronic and accelerated phases of CML on imatinib mesylate therapy, even though it did not affect independently the achievement of major or complete cytogenetic response. This finding suggested that clonal evolution as a single feature of CML progression resulted in a worse outcome with imatinib mesylate therapy, perhaps because of its relative independence from the Ph-related molecular events, despite it not affecting the rates of major and complete cytogenetic response. An interesting question is whether the initial response to imatinib mesylate therapy becomes a more important predictor for survival than clonal evolution at diagnosis. By analyzing patients evaluable at 3 months of therapy, it appeared that lack of a cytogenetic response (major cytogenetic response versus other in chronic phase; any cytogenetic response versus other in accelerated phase) was a stronger independent poor prognostic factor for survival than cytogenetic clonal evolution at the start of therapy.
O'Dwyer et al41 analyzed the outcome of 71 patients with Ph-positive CML treated with imatinib mesylate 600 mg orally daily: 15 patients had chronic-phase CML with clonal evolution, 32 had accelerated phase without clonal evolution, 24 patients had accelerated-phase CML and clonal evolution. The major cytogenetic response rates were 73%, 31%, and 12.5%, respectively (P < .01). The complete cytogenetic response rates were 60%, 31%, and 8% respectively (P < .01). With a mean follow-up of 11 months, the 1-year survival rates were 100%, 85%, and 67.5%, respectively (P = .01). The researchers concluded that good prognosis was still possible if cytogenetic clonal evolution was the only criterion of accelerated phase, whereas clonal evolution with other accelerated features predicted for the worst outcome. Our results confirm some of the findings of O'Dwyer et al41and provide additional insights with more detailed (multivariate) analysis of a larger number of patients (n = 498 versus 71 patients) with a longer follow-up (median of 30 months versus mean of 11 months), and a comparison to patients with chronic-phase CML. Unlike the results of O'Dwyer et al,41 our study did not identify clonal evolution to be associated with a significant difference in major or complete cytogenetic response rates within specific CML phases (ie, chronic ± clonal evolution, accelerated ± clonal evolution). However, clonal evolution was still independently associated with worse survival in both chronic and accelerated phase, independent of other prognostic factors. In addition, we found initial cytogenetic response to imatinib mesylate after 3 months of therapy to be also a strong independent predictor of survival outcome in these patients.
An important question is whether, despite the worse prognosis with clonal evolution in chronic- and accelerated-phase CML, imatinib mesylate therapy still offers improved outcome compared with IFN therapy. This outcome could not be proven definitely because of the design of this single-arm study. An ongoing randomized study of imatinib mesylate versus IFN plus cytarabine in patients with CML in chronic phase has demonstrated significantly better rates of major and complete cytogenetic responses, slower time to transformation, and less toxicity with imatinib mesylate.42 Comparison of the survival results from this study to our historical experience suggested a significant reduction in the yearly mortality incidence, expected to range from 15% to 30% in chronic-phase CML after interferon failure and from 30% to 40% in accelerated-phase CML.
The development of additional chromosomal abnormalities in patients on imatinib therapy, whether they achieved a cytogenetic response or not, has been observed in both the Ph-positive clones and in the emerging diploid cells. These chromosomal abnormalities could be the same original clonal evolution or new additional chromosomal abnormalities involving chromosome 17, trisomy 8, chromosome 5 or 7 abnormalities, or others.43-45 These new chromosomal abnormalities may be transient and disappear with continued therapy, particularly in patients who had achieved a complete cytogenetic response; may persist in a small percentage of metaphases; or may become predominant in most metaphases. The significance of this finding is yet unknown, but it is reminiscent of the experience with IFN-α therapy36-39 and has not yet predicted for a worse outcome with the present follow-up.43 This finding may be a manifestation of a “fragile” primitive stem cell that is susceptible to the development of both (1) Ph-positive CML disease and (2) additional chromosomal abnormalities that are now “unmasked” by the rapid suppression of the Ph-positive cells with imatinib therapy. However, unlike the experience in other myeloid leukemias, these unmasked additional chromosomal abnormalities may or may not persist and may not have the same adverse implications as in other conditions. Their prognostic significance cannot at present be anticipated and should await maturation of the current experience with imatinib therapy.
In summary, in the era of imatinib therapy for CML, cytogenetic clonal evolution was still associated with an adverse survival in patients with chronic and accelerated phases of CML. These results should be considered in the selection of particular therapies of such patients, in relation to dose escalation of imatinib mesylate, imatinib mesylate combination regimens, investigational strategies, and the choice and timing of allogeneic SCT.
Prepublished online as Blood First Edition Paper, January 30, 2003; DOI 10.1182/ blood-2002-09-2790.
J.E.C. is a Clinical Research Scholar for The Leukemia and Lymphoma Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jorge E. Cortes, Department of Leukemia, Box 428, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail:jcortes@mdanderson.org.