Abstract
Engraftment failure following allogeneic bone marrow (BM) transplantation is of clinical concern particularly involving T-cell–depleted inoculum and transplantations for aplastic anemia. Immune resistance by lymphoid and natural killer (NK) populations with “barrier” function is well established. Major histocompatibility complex (MHC)–identical marrow allografts were examined to investigate effector pathways in non-NK–mediated resistance. Barrier function was examined in cytotoxic normal and deficient B6 (H-2b) recipients primed to donor minor histocompatibility antigen (MiHA) prior to BM transplantation. Host resistance was sensitively evaluated by colony-forming unit (CFU) assays to directly assess for donor progenitor cell (PC) and peripheral chimerism. B6 host CD8+ T cells but not CD4+ or NK1.1+ cells effected rejection of primitive (CFU-HPP [high-proliferative potential]) and lineage-committed (CFU-IL3/GM [interleukin 3/granulocyte macrophage]) allogeneic donor progenitors. To address complementation by the cytotoxic pathways existing in singly deficient (perforin or FasL) recipients, cytotoxically double (perforin plus FasL) deficient (cdd) recipients were used. Resistance in B6-cdd recipients was comparable to that of wild-type B6 recipients and was also dependent on CD8+ T cells. A “triple” cytotoxic deficient model, involving transplantation of TNFR1−/− (tumor necrosis factor receptor 1) progenitor grafts did not diminish the ability of B6-cdd recipients to reject allografts. Finally, injection of anti-TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) monoclonal antibody (mAb) in B6-cdd recipients also failed to inhibit rejection of TNFR1−/− marrow grafts. In total, these studies demonstrate that CD8+ host T cells can effectively resist MHC-matched MiHA-mismatched donor PCs via alternative effector pathway(s) independent of perforin-, FasL-, TNFR-1–, and TRAIL-dependent cytotoxicity. Therefore, inhibition of these effector pathways in sensitized recipients is unlikely to result in stem cell engraftment following PC allografts.
Introduction
In the setting of bone marrow (BM) transplantation between genetically disparate individuals, allogeneic immune responses pose complex obstacles to successful outcome. For example, host-versus-graft (HVG) responses can effect resistance against the allograft. Such immune resistance must be overcome for the successful engraftment of donor progenitor cells (PCs).1-3 Host resistance, or “barrier” activity, has necessitated use of conditioning regimens to diminish recipient immune function and, more recently, stimulated attempts to infuse high doses of marrow/PCs to overcome host resistance.4-10 Higher incidences of graft failure both (1) following BM transplantation with T-cell–depleted (TCD) marrow and (2) in multiply transfused (eg, aplastic anemic) individuals continue to engender clinical concern regarding marrow (progenitor cell) graft resistance.1,2 11
Studies by Martin and colleagues12,13 demonstrated the importance of cytotoxic function in donor T cells to overcome resistance and establish successful long-term engraftment. Findings by Murphy et al14 and others15-18 have demonstrated that acute rejection in unsensitized mice can involve natural killer (NK), CD8+ T cells and other populations. Although significant advances have been made in understanding the nature of host resistance against bone marrow allografts, particularly in mouse models of transplantation, a crucial question concerns specifically which molecules in T/NK cell populations mediate the barrier response.
This laboratory has been investigating the involvement of cytotoxic effector function in marrow graft rejection.19-21Recipients singularly lacking the ability to mediate perforin or FasL-dependent killing maintained the capacity to effect some level of resistance against major histocompatibility complex (MHC)–mismatched allografts and strong resistance against MHC-matched allografts.20,21 Studies investigating granzyme B–defective recipients also failed to detect diminished barrier responses following MHC-mismatched allogeneic stem cell transplantations.22 However, NK-dependent resistance can exhibit deficits in some strains with perforin deficiency.23 24 Thus, the contributions of various host effector pathways remain unclear and, importantly, may differ, depending on the effector cell populations involved.
The present studies investigated host resistance in recipients sensitized to donor antigens to specifically investigate effector pathways in non-NK (ie, T-cell–mediated) marrow graft resistance. To exclude any potential involvement of the intact cytotoxic pathway functioning in single cytotoxic deficient mice, resistance was examined in perforin and FasL cytotoxically double deficient (cdd) recipients.21 22 A triple cytotoxic deficient model was used to examine if the absence of TNFR1−/− (tumor necrosis factor receptor 1) or R2 signaling would inhibit this T-cell–mediated resistance. Furthermore, to prevent contributions by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)–mediated cytotoxicity, anti-TRAIL antibody was administered into B6-cdd recipients receiving allogeneic TNFR1−/−marrow PCs. Effective barrier responses resulting in resistance remained intact. Thus, the present studies have found that a radioresistant, antigen-specific effector pathway mediated by CD8+ T cells can prevent allogeneic marrow engraftment in the simultaneous absence of perforin-, CD95L-, TNFR1-, and TRAIL-dependent killing. Therefore, strategies targeted to inhibit those pathways may not successfully facilitate engraftment when transplant recipients contain sensitized antidonor reactive T cells.
Materials and methods
Mice
C57BL/6J (B6, H-2b), B6.SJL-Cd45aPepb/boyJ (H-2b, Ly5.1), BALB.B (H-2b), C3H.SW (H-2b), B6.Smn C3H-gld (B6-gld/gld, H-2b), and BALB/c (H-2d) mice were obtained from the Jackson Laboratories (Bar Harbor, ME). Mice 8 to 16 weeks of age were sex-matched for transplantations involving only healthy (ie, wild-type [wt]) mice. B6-perforin−/− (H-2b) mice were maintained in pathogen-free conditions at the University of Miami School of Medicine. Animal care was provided in accordance with the procedures outlined in the “Guide for the Care and Use of Laboratory Animals” (NIH Publication No. 86-23, 1985). C57BL/6 cytotoxic double (perforin and FasL)–deficient mice (B6-cdd) were generated from B6 Pfp+/− Faslgld/gld pairings or B6 Pfp−/− Faslgld/gld × B6 Pfp+/− Faslgld/gld pairings, as described.25 Each offspring was carefully screened for homozygosity for perforin deficiency.26 Briefly, genomic DNA was isolated from the tails of each offspring to be given transplants using DNeasy Tissue Kit (Qiagen, Hilden, Germany). Three primers, PKO1 (5′CAGGTCAGGCCAGCATAAGAGTAG 3′), PKO2 (5′ AAGGTCATCATCCCAGCCGTAGTC 3′), and PKO3 (5′ AGCCGGATCAAGCGTATGCAG 3′) were used to amplify wild-type and disrupted perforin gene from genomic DNA. PKO1 and PKO2 primers were 5′- and 3′-primers specific to the mouse perforin sequence, respectively. PKO3 primer sequence is contained within the neomycin cassette that disrupts synthesis of functional perforin mRNA The perforin−/− homozygous genotype was identified by detecting 2 distinct bands of 597 base pair (bp) and 1636 bp, whereas perforin+/+ yielded a single 336-bp band.
The gld point mutation of B6-cdd mice was identified by polymerase chain reaction (PCR) with primers designed with the terminal nucleotide pairing to either wild-type FasL or to the gld mutation.27 To prevent possible mispairing to the templates, a mismatch was introduced at the second nucleotide from the 3′-terminal of the FasL-wt or FasL-gld primers (C6A) to destabilize the 3′-end binding. Each of these primers together with an antisense primer (FasL-as) was used in PCR to identify the gld mutation of B6-cdd mice. A 563-bp fragment was amplified from samples with wild-type FasL gene or gld mutation when FasL-wt or FasL-gld primers were used, respectively. All random mice examined in the colony contained homozygous gld mutation.
The gld mutation was further confirmed by real-time PCR and melting point analysis using LightCycler (Roche Diagnostics, Indianapolis, IN). DNA samples were amplified with fluorescent-labeled primers (5′-TGA GGA TCT GGT GCT AAT GG-3′ and 5′-AAT ATT CCT GGT GCC CAT GA-3′). The melting temperatures (Tm) of the PCR products were tested by binding to a 6-carboxy-fluorescein–labeled anchor probe and a detection probe. Fragments containing wild-type FasL gene sequence exhibited a Tm of 52°C versus Tm of fragments containing gld mutation of 58°C. DNA samples from B6-cdd mice in the colony tested at random all contained homozygous gld mutation.
B6-cdd mice develop spontaneous “cdd syndrome,” resulting in early death by 15 weeks.25 Therefore, all cytotoxic double deficient mice were given transplants between 49 and 56 days (7-8 weeks) of age. B6-FasLgld/gld mice do not exhibit such early death; therefore, B6-cdd phenotype is also monitored by life span of genotyped mice not used as bone marrow transplant (BMT) donors. Colony life span is 97.1 ± 9.8 days, consistent with published results.25
TNFR1- and TNFR2-deficient mice on C3H and BALB/c backgrounds were generated from breeding pairs B6.129-Tnfrsflatm1Mak and B6.129S2-Tnfrsflbtm1Mwm (Jackson Laboratories). R1+/− and R2+/− F1 mice were backcrossed to either C3H.SW or BALB/c. Following the initial BALB/c backcrosses, R1+/− and R2+/− mice were selected for H-2d homozygosity (MHC phenotype). Offspring from subsequent backcrosses were screened by PCR (see “Priming against MiHA or MHC alloantigens”). Mice used as a source of donor marrow were obtained from intercross breedings of R1+/− or R2+/− mice following screening for homozygosity for TNFR1 or TNFR2 deficiency by identifying the disrupted genes using PCR following instructions provided by Jackson Laboratories.26Briefly, genomic DNA was isolated from the tails of each offspring used as a donor for priming or transplanted to MHC-matched (H-2b) B6-wt or B6-cdd recipients using DNeasy Tissue Kit. For screening TNFR1 knock-out (KO) mice, PCR was performed using TNFR1 upstream primer (primer 1A, 5′-GGCTGCAGTCCACGCACTGG-3′), downstream primer (primer 1B, 5′-TGTGAAAAGGGCACCTTTACGGC-3′), or HSV-TK insert primer (primer 1C, 5′-AATCGCCAATGACAAGACGCT-3′). A 470-bp fragment was amplified from wild-type allele using primers 1A and 1B. A 300-bp fragment was amplified from disrupted TNFR1 alleles using primers 1A and 1C. TNFR2 KO mice were screened similarly by PCR. A 200-bp fragment was amplified from wt allele using TNFR2 upstream primer (primer 2A, 5′-CCT CTC ATG CTG TCC CGG-3′) and downstream primer (primer 2B, 5′-AGC TCC AGG CAC AAG GGC GGG-3′). A 400-bp fragment was amplified from disrupted allele using neo insert primers (primer 2C, 5′-CGG TCC TTT TTG TCA AGA C-3′; and primer 2D, 5′-ATC CTC GCC GTC GGG CAT GC-3′).
Priming against minor histocompatibility antigen (MiHA) or MHC alloantigens
Single cell suspensions were prepared from spleen, lymph node (LN), and thymus tissue of C3H.SW, BALB.B, or BALB/c donor mice. Cells were adjusted to a concentration of 6 × 107/mL, and 0.5 mL was administered by intraperitoneal and intradermal (multiple) injection 3 to 4 weeks prior to BM transplantation. Where indicated, some groups were primed more than one time.
Bone marrow transplantation
Femurs and tibias were harvested from appropriate female donors as previously described.21 T cells were removed by incubation with anti-Thy1.2 monoclonal antibody (mAb; HO13.4 ascites diluted 1:200) followed by 10% vol/vol Low-Tox M rabbit complement (Accurate Chemical & Scientific, Westbury, NY) at 37°C for 45 minutes. T cells present were reduced from approximately 2.0% to below the level (flow cytometry) of detection (< 0.2%). Recipient mice were exposed to 9.0 Gy lethal total-body irradiation (TBI) from an open beam 60Co gamma (50 cGy/minute). Twenty-four hours later, 2.0 × 106 TCD bone marrow cells (BMCs) were infused intravenously, and mice were maintained on acidified/antibiotic water (pH < 2.2, 100 mg/L neomycin sulfate, 10 mg/L polymyxin B sulfate).
Adoptive transfer of allograft resistance to unprimed recipients
B6 mice were primed with BALB.B cells twice prior to BM transplantation. Spleen and LN cells from these primed mice were then fractionated to deplete lymphocyte subsets as previously described.21 NK cells were removed by in vivo depletion of B6 donors using antibody against NK1.1 (PK136 ascites, 100 μL) injected intraperitoneally 48 hours prior to removing spleen and LNs. Aliquots of cells from these donors were treated with anti-NK1.1 mAb plus C′ in vitro prior to transfer. NK cell–depleted populations contained less than 1.0% (fluorescein isothiocyanate [FITC]–conjugated antihamster immunoglobulin) staining cells. To delete T-cell subsets, spleen and LN cells from primed or naive animals were treated with anti-CD8 or anti-CD4 mAbs and C′ (1-2 times). Alternatively, lymphocytes from primed B6 mice were used to produce CD8-enriched and CD8-depleted fractions by immunomagnetically labeling with anti-CD8 MACS beads followed by column separation (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were than analyzed to assess enrichment/depletion (≥ 87%/≤ 2.1%). Desired numbers of these cells or unfractionated populations from primed or unprimed (control) B6 mice were infused intravenously into unprimed B6 mice 48 hours before BM transplantation. One day later, these mice were administered 9.0 Gy TBI and received 2 × 106 BMT-TCD BALB.B marrow 24 hours later.
In vitro CFU-GM and CFU-IL3 assays
Recipients were killed 5 days after BM transplantation. Pooled spleen cells (1 × 105) were cultured in 1 mL of a mixture containing α-modification of Eagle medium with nucleosides (α-MEM), 0.86% methyl cellulose (Methocult; StemCell Technologies, Vancouver, BC), 30% fetal calf serum (FCS), 250 μM 2-mercaptoethanol, 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 U/mL recombinant murine granulocyte-macrophage colony stimulating factor (GM-CSF) or interleukin 3 (IL-3) (R&D Systems, Minneapolis, MN).28Triplicate cultures were maintained at 37°C, 5% CO2 for 4 days. Cell aggregates containing 25 cells or more were scored as individual colonies (colony-forming unit [CFU]) on day 4. Results are presented as average CFU-GM or -IL3 ± SD/group as previously described.21
For CFU-HPP (high-proliferative potential) assay, BM cells were harvested from femurs and tibias of recipient animals 12 days after BM transplantation. Cells were pooled and washed, and 1 × 105 cells were seeded in 1.5 mL 0.3% agarose (low melting point agarose; GIBCO BRL) containing semisolid culture media in Iscoves modified Dulbecco medium (IMDM) supplemented with 20% fetal calf serum, 100 ng/mL stem cell factor (PEPROTECH, Rocky Hill, NJ), 50 U/mL IL-3, and 2700 U/mL IL-1α (PEPROTECH) for 7 to 14 days. HPP colonies in duplicate wells were counted, and results are presented as average of CFU-HPP ± SD/group.
Detection of genetic markers for primitive progenitors
HPP colonies were isolated at day 6 of culture. Cells from the pooled colonies were lysed, and total RNA was prepared using RNeasy kit (Qiagen). Reverse transcription (RT)–PCR was performed using the total HPP RNA to detect expression of progenitor markers. PCR primers used were (1) stem cell antigen-1 (Sca-1), 5′CAATGTAGCAGTTCCCAATG3′ and 5′ CAGGGGCTATAAAGGCAAAA3′; (2) IL-3Rα, 5′ GAACAGATTCCACCATGGCCTCCTTG 3′ and 5′GTCTCCACTACGGACACTTCTGTC3′; and (3) glyceraldehyde phosphate dehydrogenase (GAPDH), 5′ ATGACCACAGTCCATGCCAT 3′ and 5′ GCCTGCTTCACCACCTTCTT 3′.
Chimerism
To assess peripheral blood and other tissues for chimerism following BM transplantation of MHC (H-2b)–matched C3H.SW donor inoculum into B6 recipients, Ly9.1 mAb staining was performed. Ly9.1 is expressed on most hematopoietic cells of C3H (Ly9.1+) but not BL/6 (Ly9.1−) background mice. Results, therefore, represent a conservative estimate of donor cell chimerism in the compartments examined.
BM transplantation in presence of anti-TRAIL antibody
Monoclonal anti-TRAIL antibody N2B229 or an isotype-matched control rat immunoglobulin G2a(IgG2a) antibody was injected intraperitoneally on days 0 and 1 (200 μg/injection) after BM transplantation.
Statistics
For each transplantation experiment, BM cells (BMCs) were harvested from a minimum of 2 donor mice, pooled, and transplanted into a minimum of 2 recipient mice for every experimental group. CFU values are presented as an average of triplicate (duplicate for HPP-CFU cultures) wells with SD. Statistical significance for the difference between each experimental group was examined by 2-tailed ttest and reported as P values where appropriate as previously reported.20 In all experiments presented, the difference between the CFU values obtained from primed mice compared with the numbers obtained from naive mice of the same strain were highly significant (P < .05) and, therefore, were not included in the tables or figures. Each experiment was performed independently a minimum of 2 times; most were performed more than 3 times.
Results
High-proliferative potential colony forming cells (HPP-CFCs) are targets of MiHA-disparate host resistance
Previously, we examined host resistance against hematopoietic PCs in 2 MHC (H-2b)–matched MiHA-mismatched transplant models: C3H.SW or BALB.B marrow → B6 recipients.21 These studies demonstrated that resistance was dependent on priming (Figure1) against the donor MiHA prior to transplantation and a host CD3+ population.21However, CFU-IL3 and CFU-GM are lineage-committed progenitors and do not represent pluripotent hematopoietic stem cell (HSC) populations responsible for establishing longer term chimerism and renewing multilineage cell populations.
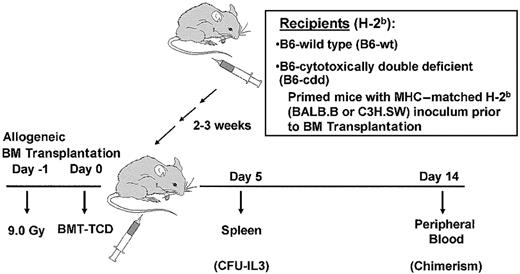
Experimental model to assess resistance against MiHA-mismatched allogeneic bone marrow grafts.
Normal or cytotoxically deficient mice were primed with donor cells (“Materials and methods”) at least 3 weeks prior to irradiation (day −1) and BM transplantation (day 0). Recipients received varying doses of donor or syngeneic BM-TCD and resistance was analyzed by CFU activity in recipient spleens (day 5) and donor (ie, C3H.SW) chimerism by staining with Ly9.1 mAb.
Experimental model to assess resistance against MiHA-mismatched allogeneic bone marrow grafts.
Normal or cytotoxically deficient mice were primed with donor cells (“Materials and methods”) at least 3 weeks prior to irradiation (day −1) and BM transplantation (day 0). Recipients received varying doses of donor or syngeneic BM-TCD and resistance was analyzed by CFU activity in recipient spleens (day 5) and donor (ie, C3H.SW) chimerism by staining with Ly9.1 mAb.
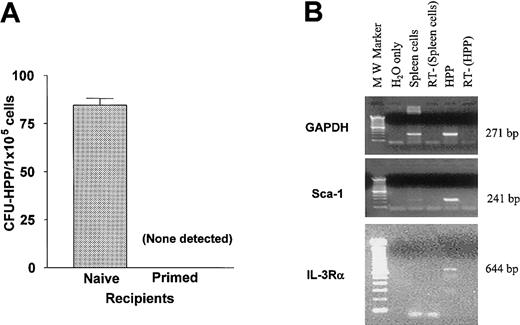
HPP-CFCs are pluripotent cells that share many features with HSCs.30 To determine whether these pluripotent progenitor populations were also subject to rejection, host resistance was examined in the MiHA-disparate BALB.B → B6 model using in vitro CFU-HPP assay. Following 9.0 Gy TBI, B6 mice received a BMT-TCD (2 × 106 cells) from MHC-matched BALB.B donors. At day 12 virtually no CFU-HPPs were identified in the marrow of recipient mice previously primed to donor BALB.B spleen cells (Figure2A). In contrast, significant numbers were always detected in cultures from unprimed (naive) B6 recipients. HPP colony forming cells are known to express a number of markers characteristic of stem cell populations.30 RT-PCR analysis of total RNA prepared from HPP-CFCs after 6 days of culture (Figure 2B) demonstrated Sca-1, IL-3Rα (Figure 2B), and c-kit (data not shown) expression by the cells. These results indicated that priming of host animals against donor minor H antigens resulted in resistance against HPP-CFCs and demonstrate that donor progenitors are not present in the marrow compartment 2 weeks after transplantation.
Primitive progenitors are targets for host resistance.
(A) Recipient B6 mice, either naive or primed to BALB.B MiHA, received 9.0 Gy TBI 1 day prior to transplantation of 2 × 106BALB.B BM-TCD. Average number ± SD of CFU-HPP per 1 × 105 marrow cells in duplicate culture from each recipient group is indicated. (B) Cells from HPP-CFU were collected on day 6 of culture for the isolation of RNA and analyzed for the expression of genetic markers by RT-PCR. RT-PCR of GAPDH message is shown as internal control. Primers used were described in “Adoptive transfer of allograft resistance to unprimed recipients.” RT−, no reverse transcription control. H2O only, negative control for PCR.
Primitive progenitors are targets for host resistance.
(A) Recipient B6 mice, either naive or primed to BALB.B MiHA, received 9.0 Gy TBI 1 day prior to transplantation of 2 × 106BALB.B BM-TCD. Average number ± SD of CFU-HPP per 1 × 105 marrow cells in duplicate culture from each recipient group is indicated. (B) Cells from HPP-CFU were collected on day 6 of culture for the isolation of RNA and analyzed for the expression of genetic markers by RT-PCR. RT-PCR of GAPDH message is shown as internal control. Primers used were described in “Adoptive transfer of allograft resistance to unprimed recipients.” RT−, no reverse transcription control. H2O only, negative control for PCR.
Marrow allograft resistance is mediated by host CD8+NK1.1− lymphocytes
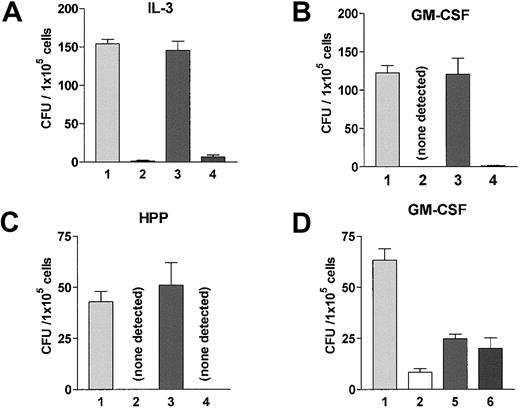
To identify the precise subset of cells responsible for barrier function, spleen and LN cells were isolated from B6 mice primed against BALB.B MiHA. Aliquots containing 2 × 107CD3+ cells were depleted of CD8+ or NK1.1+ cells (“Materials and methods”). Unfractionated and depleted populations were transferred into naive B6 recipients. One day later, these animals were prepared for BM transplantation with the same dose of TBI (9.0 Gy) used in the experiments described earlier. Resistance was examined following transplantation of 2 × 106 BALB.B-TCD marrow 1 day after irradiation. Recipients of unfractionated spleen plus LN cells from primed B6 mice efficiently rejected BALB.B donor progenitors, indicating that the resisting elements were contained in this transferred population (Figure 3). However, transfer of CD8-depleted populations failed to confer the resistance to naive recipients, (Figure 3A-C). In contrast, depletion of NK1.1+cells did not alter the resistance capacity of the transferred population (Figure 3A-C). To corroborate that CD8+ T cells effected barrier function, CD4+ T cells from B6 mice primed against donor MiHA were rigorously depleted by 2 rounds of anti-CD4 mAb depletion. Flow cytometric analysis of the remaining cells indicated less than 0.3% CD4+CD8− cells remaining in the primed spleen plus LN cell population that was transferred into naive B6 recipients. Following BM transplantation there was no diminution in the resistance transferred by this CD4-depleted versus C′-only treated population (Figure 3D). CD4−/−-deficient mice failed to exhibit resistance against BALB.B BM transplantation following the standard donor MiHA-priming protocol (Figure4). However, resistance in these recipients could be reconstituted following transfer of a syngeneic CD4+ population prior to donor MiHA priming (Figure 4). Together, these data demonstrate that CD8+CD4−NK1.1− populations from primed mice function as barrier cells in MHC-matched marrow allograft resistance and require a host CD4+ T-cell response for generation of this effector population.
Host CD8+CD4−NK1.1− T-cell populations effect barrier function.
(A-C) B6 mice were primed to BALB.B MiHA twice, at 6 weeks and 3 weeks prior to BM transplantation. Spleen and LN cells containing 2 × 107 CD3+ cells were isolated from these mice. Unfractionated or fractionated cells were then transferred into naive B6 animals, following depletion of CD8+ or NK1.1+ cells as described in “Materials and methods.” Resistance in these adoptively transferred mice was examined by transplanting 2 × 106 BALB.B BM-TCD. Unfractionated cells from naive B6 mice were transferred as a control. Naive unfractionated versus primed CD8-depleted: P = .5536 for CFU-IL3, P = .935 for CFU-GM, andP = .576 for CFU-HPP. (D) B6 mice were primed to C3H.SW MiHA twice, at 6 weeks and 4 weeks prior to BM transplantation. Spleen and LN cells (total of 6 × 107) unfractionated or fractionated by depleting CD4+ or CD4+ and CD8+ cells were adoptively transferred into naive B6 recipients as described in “Materials and methods.” Resistance in these mice were examined after transfer of 2 × 106BM-TCD. Naive unfractionated: versus primed CD4 depleted,P = .0006, versus primed complement treated,P = .0004. Transferred populations are (1) unfractionated from naive B6, (2) unfractionated from primed B6, (3) CD8 depleted from primed B6, (4) NK1.1+ depleted from primed B6, (5) complement treated from primed B6, and (6) CD4 depleted from primed B6. Values represent average number of CFU ± SD.
Host CD8+CD4−NK1.1− T-cell populations effect barrier function.
(A-C) B6 mice were primed to BALB.B MiHA twice, at 6 weeks and 3 weeks prior to BM transplantation. Spleen and LN cells containing 2 × 107 CD3+ cells were isolated from these mice. Unfractionated or fractionated cells were then transferred into naive B6 animals, following depletion of CD8+ or NK1.1+ cells as described in “Materials and methods.” Resistance in these adoptively transferred mice was examined by transplanting 2 × 106 BALB.B BM-TCD. Unfractionated cells from naive B6 mice were transferred as a control. Naive unfractionated versus primed CD8-depleted: P = .5536 for CFU-IL3, P = .935 for CFU-GM, andP = .576 for CFU-HPP. (D) B6 mice were primed to C3H.SW MiHA twice, at 6 weeks and 4 weeks prior to BM transplantation. Spleen and LN cells (total of 6 × 107) unfractionated or fractionated by depleting CD4+ or CD4+ and CD8+ cells were adoptively transferred into naive B6 recipients as described in “Materials and methods.” Resistance in these mice were examined after transfer of 2 × 106BM-TCD. Naive unfractionated: versus primed CD4 depleted,P = .0006, versus primed complement treated,P = .0004. Transferred populations are (1) unfractionated from naive B6, (2) unfractionated from primed B6, (3) CD8 depleted from primed B6, (4) NK1.1+ depleted from primed B6, (5) complement treated from primed B6, and (6) CD4 depleted from primed B6. Values represent average number of CFU ± SD.
CD4+ cells are required for the generation of barrier activity in recipients.
(Left panel) B6-CD4−/− mice were primed against donor BALB.B MiHA 4 weeks prior to BM-TCD transplantation. Resistance in these CD4 knock-out animals was examined by CFU-IL3 analyses. (Right panel) Spleen + LN cells containing desired number of CD4+ cells were transferred from naive B6-wt mice into naive B6-CD4−/− recipient mice. Groups of B6-CD4−/− recipients received (1) no lymphocyte transfer prior to priming, (2) a lymphocyte transfer inoculum containing 5.7 × 106 wild-type CD4+cells, (3) a lymphocyte transfer inoculum containing 1.6 × 106 wild-type CD4+ cells, or (4) a lymphocyte transfer inoculum depleted of CD4+ cells (< 4 × 104 CD4+ cells) as described in “Materials and methods.” One day following transfer, the CD4−/− recipient mice were immunized against donor BALB.B cells. Three weeks after priming, the allograft resistance in these adoptively transferred B6-CD4−/− recipient groups were examined by CFU-IL3 assay following transplantation of 2 × 106 BALB.B BM-TCD.
CD4+ cells are required for the generation of barrier activity in recipients.
(Left panel) B6-CD4−/− mice were primed against donor BALB.B MiHA 4 weeks prior to BM-TCD transplantation. Resistance in these CD4 knock-out animals was examined by CFU-IL3 analyses. (Right panel) Spleen + LN cells containing desired number of CD4+ cells were transferred from naive B6-wt mice into naive B6-CD4−/− recipient mice. Groups of B6-CD4−/− recipients received (1) no lymphocyte transfer prior to priming, (2) a lymphocyte transfer inoculum containing 5.7 × 106 wild-type CD4+cells, (3) a lymphocyte transfer inoculum containing 1.6 × 106 wild-type CD4+ cells, or (4) a lymphocyte transfer inoculum depleted of CD4+ cells (< 4 × 104 CD4+ cells) as described in “Materials and methods.” One day following transfer, the CD4−/− recipient mice were immunized against donor BALB.B cells. Three weeks after priming, the allograft resistance in these adoptively transferred B6-CD4−/− recipient groups were examined by CFU-IL3 assay following transplantation of 2 × 106 BALB.B BM-TCD.
Perforin/FasL B6-cdd mice resist MiHA-mismatched marrow allografts
Granzyme B-defective recipients effectively rejected C3H.SW progenitors following priming against the donor MiHA prior to transplantation (data not shown). This observation is consistent with and extends the previous finding that perforin-deficient recipients are also capable of efficient resistance against MiHA-mismatched allografts.21 A mixed-marrow inoculum containing allogeneic (BALB.B) and syngeneic, congenic (B6-Ly5.1) marrow transplanted into granzyme B−/− recipients, paralleled previous findings using cytotoxically healthy recipients21and indicated that in the absence of granule-dependent killing (see “Marrow allograft resistance in cytotoxic double deficient mice is CD8+ T-cell dependent”), the efficiency as well as specificity of the resistance was not altered (data not shown).
Because either the granule or Fas-mediated pathways can induce rapid and potent apoptotic signaling, a cytotoxic deficiency in one effector pathway could be compensated for by the concurrently functioning alternative pathway. If true, simultaneous impairment of both would result in the loss of barrier function. To address this possibility, B6-cdd (perforin and FasL = cdd) mice were used as recipients. All B6-cdd mice used in these studies were screened for homozygous perforin deficiency by PCR prior to transplantation. Because FasLgld/gldB6Pfp+/− mice were used for breeding the colony, random screening for gld/gld was also performed (see “Materials and methods”).25 Normal B6 and B6-cdd mice 5 to 6 weeks of age were primed against C3H.SW (H-2b) MiHA and given transplants of a standard dose of 2 × 106 C3H.SW TCD marrow. Spleen cell cultures from unprimed naive B6-cdd (and B6 wild-type) recipients contained CFU-GM as expected (Table 1, lines 1 and 5). However, B6-cddC3H.SW as well as B6C3H.SWrecipients demonstrated effective resistance; the former when twice the number of BMCs was transplanted (Table 1, lines 6 and 7). These findings showed that allograft resistance in the cdd mice also required priming, indicative of a T-cell–mediated response (see “Marrow allograft resistance in cytotoxic double-deficient mice is CD8+ T-cell dependent”). To extend these findings, a second donor MiHA-mismatched strain was used (BALB.B) to prime naive B6-cdd mice and to examine their resistance to increasing doses (6-12 × 106) of donor marrow (data not shown). B6-cddBALB.B recipients exhibited no difference from healthy B6BALB.B recipients in their ability to resist each of these marrow doses. In summary, the simultaneous absence of granule (perforin dependent) and FasL-mediated cytotoxicity did not detectably impair the B6-cdd recipient's capacity to effect this marrow allograft resistance.
Marrow allograft resistance in cytotoxic double-deficient mice is CD8+ T-cell dependent
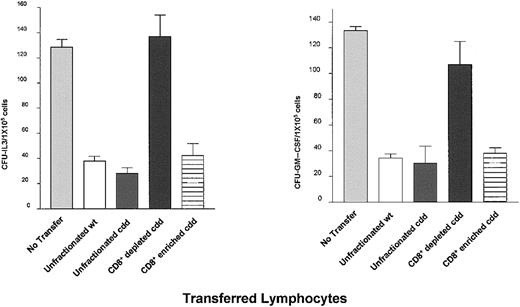
To determine the cell population required for resistance in B6-cdd recipients, adoptive transfer experiments were performed using cells from B6-cdd donors primed against BALB.B MiHA (Figure5). Unfractionated spleen cells were prepared from B6-wt and B6-cdd mice primed against BALB.B antigens. Aliquots containing 1 × 107 CD8+ cells were transferred into syngeneic unprimed B6 recipients prior to irradiation and BM transplantation using the model (Figure 1) described earlier. Recipients receiving either B6-wt or B6-cdd populations demonstrated effective allograft resistance compared with control (ie, untransferred) naive mice (P < .05). The same total number of B6-cddBALB.B cells transferred following depletion of CD8+ T cells failed to demonstrate resistance after BALB.B marrow transplantation (Figure 5). In contrast, transplantation of CD8+ T-cell–enriched fractions (containing 7.1 × 106 CD8+ T cells, ie 71% versus the number contained in unfractionated populations) again demonstrated efficient resistance. These findings demonstrated that CD8+ T cells generated after MiHA priming in B6-cdd mice can efficiently transfer resistance against donor PCs.
Cytotoxically double deficient CD8+populations effect barrier function in host resistance to donor progenitors.
Cytotoxically normal/wild-type B6 (wt) or B6-cdd mice were primed to BALB.B MiHA twice at 3 weeks and 1 week prior to BM transplantation. Following isolation of spleen and LN cells from these animals, unfractionated or fractionated cells (CD8+-depleted/enriched fractions prepared by negative and positive selections using MACS; “Materials and methods”) were transferred into naive B6 mice. Unfractionated lymphocyte transfer inoculum (for both wt and cdd) contained 1 × 107CD8+ cells. CD8-depleted and -enriched B6-cdd lymphocyte transfer inoculum contained 1.7 × 107 CD8+cells and 7.1 × 107 CD8+ cells, respectively. Resistance in the adoptively transferred mice was examined by transplanting 2 × 106 BALB.B marrow cells. Untransferred naive B6 recipients were provided as control. B6 recipients transferred with B6-wt or B6-cdd unfractionated cells versus no transfer: P = .0002 (CFU-IL3, left panel),P = .0017 (CFU-GM–CSF, right panel).
Cytotoxically double deficient CD8+populations effect barrier function in host resistance to donor progenitors.
Cytotoxically normal/wild-type B6 (wt) or B6-cdd mice were primed to BALB.B MiHA twice at 3 weeks and 1 week prior to BM transplantation. Following isolation of spleen and LN cells from these animals, unfractionated or fractionated cells (CD8+-depleted/enriched fractions prepared by negative and positive selections using MACS; “Materials and methods”) were transferred into naive B6 mice. Unfractionated lymphocyte transfer inoculum (for both wt and cdd) contained 1 × 107CD8+ cells. CD8-depleted and -enriched B6-cdd lymphocyte transfer inoculum contained 1.7 × 107 CD8+cells and 7.1 × 107 CD8+ cells, respectively. Resistance in the adoptively transferred mice was examined by transplanting 2 × 106 BALB.B marrow cells. Untransferred naive B6 recipients were provided as control. B6 recipients transferred with B6-wt or B6-cdd unfractionated cells versus no transfer: P = .0002 (CFU-IL3, left panel),P = .0017 (CFU-GM–CSF, right panel).
Resistance against TNFR1−/− and R2−/−progenitor cell allografts
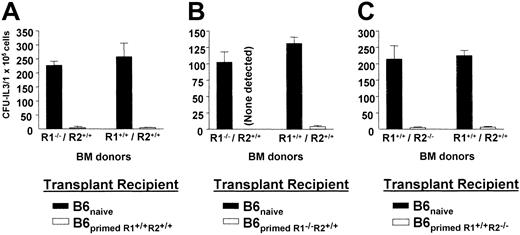
To determine if TNF signaling involving R1 or R2 was required for resistance, marrow from TNFR1−/− or TNFR2−/− donors was transplanted into healthy B6 recipients previously primed to donor C3H.SW MiHA (Figure6). B6-wt recipient mice primed by administration of healthy C3H.SW cells exhibited efficient resistance to marrow allografts containing PCs from healthy C3H.SW donors or donors lacking TNFR1 (Figure 6A). These findings were verified when B6-wt recipients were primed prior to BM transplantation using cells from R1−/− (6B) or R2−/− (6C) mice and found to strongly resist transplantations containing progenitors from these TNFR1−/− and R2−/− marrow donors, respectively.
Primed host resists MHC-matched MiHA-mismatched bone marrow allograft in the absence of TNFR1- or R2-dependent cytotoxic pathways.
B6 host resistance in the absence of TNF-α pathway was examined by transplanting BMCs from either TNFR1 or TNFR2 knock-out H-2b MiHA-disparate donors. (A) Recipients were primed as described in “Materials and methods” with wild-type C3H.SW cells. Three weeks after priming, mice received 9.0 Gy TBI and the following day transplantation of wild-type or TNFR1-deficient marrow (2 × 106 BMT-TCD). (B) Recipients were primed with spleen cells from splenectomized TNFR1-deficient H-2b donor mice and 3 weeks after priming received 2 × 106 BMT-TCD from either wild-type or the same splenectomized TNFR1-deficient donors. (C) Recipients were primed with spleen cells from splenectomized TNFR2-deficient H-2b donor mouse and received 2 × 106 BMT-TCD from either wild-type or the same splenectomized TNFR2-deficient donors. Host resistance was examined 5 days after BM-TCD transplantation by CFU-IL3 assay.
Primed host resists MHC-matched MiHA-mismatched bone marrow allograft in the absence of TNFR1- or R2-dependent cytotoxic pathways.
B6 host resistance in the absence of TNF-α pathway was examined by transplanting BMCs from either TNFR1 or TNFR2 knock-out H-2b MiHA-disparate donors. (A) Recipients were primed as described in “Materials and methods” with wild-type C3H.SW cells. Three weeks after priming, mice received 9.0 Gy TBI and the following day transplantation of wild-type or TNFR1-deficient marrow (2 × 106 BMT-TCD). (B) Recipients were primed with spleen cells from splenectomized TNFR1-deficient H-2b donor mice and 3 weeks after priming received 2 × 106 BMT-TCD from either wild-type or the same splenectomized TNFR1-deficient donors. (C) Recipients were primed with spleen cells from splenectomized TNFR2-deficient H-2b donor mouse and received 2 × 106 BMT-TCD from either wild-type or the same splenectomized TNFR2-deficient donors. Host resistance was examined 5 days after BM-TCD transplantation by CFU-IL3 assay.
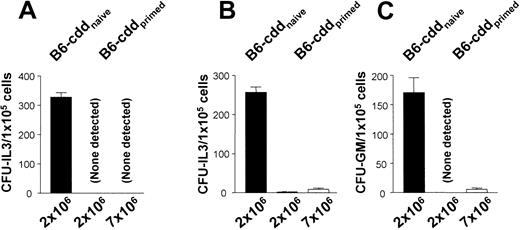
Next, the ability of B6-cdd recipients to resist marrow allografts containing PCs lacking the ability to signal via TNFR1 or R2 was examined. TNFR1−/− or TNFR2−/− mice were partly splenectomized, and the cells were used to prime B6-cdd recipients. Three weeks later, naive and primed B6-cdd mice were given transplants of marrow from the respective donors or healthy C3H.SW mice. B6-cdd primed, but not naive, recipients demonstrated intact resistance against transplants containing 2 × 106 TCD MiHA-disparate marrow grafts (Figure 7). Moreover, a more than 3 time increase in marrow dosage failed to reveal any deficiency in the resistance effected involving both CFU-GM and CFU-IL3 R1−/− or R2−/− progenitors by the perforin- and FasL-defective recipients (Figure 7). To further assess this resistance, chimerism was examined 2 weeks after transplantation for the presence of circulating donor C3H.SW TNFR1−/−cells (Figure 8). B6-cdd unprimed recipients contained circulating donor (ie, Ly9.1+), cells in the periphery as well as central lymphoid compartments, as expected. In contrast, consistent with resistance, no Ly9.1+ donor cells were detected in B6-cdd recipients sensitized against donor C3H MiHA (Figure 8).
Effective host barrier response against donor progenitors in the simultaneous absence of perforin-, FasL-, and TNFR1- or R2-dependent cytotoxic pathways.
(A) Cytotoxic double (perforin and FasL)–deficient B6-cdd recipients were either naive or primed with H-2bTNFR1−/− donor cells. Three weeks after priming, recipients received increasing doses of bone marrow (2 and 7 × 106 BMT-TCD) from the same splenectomized H-2b TNFR1−/− donors. The resistance in this triple knock-out model was examined by standard CFU-IL3 assay. (B-C) B6-cdd recipients were either naive or primed with H-2b TNFR2−/− donor cells. Three weeks after priming, recipients received 2.0 or 7.0 × 106 BMT-TCD from the same splectomized H-2b TNFR2−/−donors. Resistance was examined by CFU-IL3 (B) or CFU–GM-CSF assay (C).
Effective host barrier response against donor progenitors in the simultaneous absence of perforin-, FasL-, and TNFR1- or R2-dependent cytotoxic pathways.
(A) Cytotoxic double (perforin and FasL)–deficient B6-cdd recipients were either naive or primed with H-2bTNFR1−/− donor cells. Three weeks after priming, recipients received increasing doses of bone marrow (2 and 7 × 106 BMT-TCD) from the same splenectomized H-2b TNFR1−/− donors. The resistance in this triple knock-out model was examined by standard CFU-IL3 assay. (B-C) B6-cdd recipients were either naive or primed with H-2b TNFR2−/− donor cells. Three weeks after priming, recipients received 2.0 or 7.0 × 106 BMT-TCD from the same splectomized H-2b TNFR2−/−donors. Resistance was examined by CFU-IL3 (B) or CFU–GM-CSF assay (C).
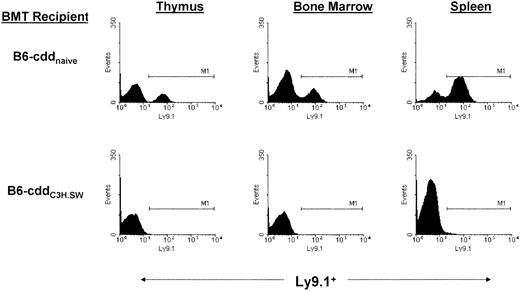
Absence of donor chimerism in central and peripheral lymphoid compartments of B6-cdd recipients.
TNFR1−/− marrow was transplanted into B6-cdd (Ly9.1−) naive and primed recipients 1 day after 9.0 Gy TBI. Thymus, bone marrow, and spleen cells were analyzed 14 days later. Ly9.1 (clone 30C7) staining identifies expression (Ly9.1) on most thymocytes, peripheral T and B cells, bone marrow, lymphoid cells, and hematopoietic progenitors of the C3H.SW strain but not C57BL/6 (Ly9.1−). B6-cdd naive recipients contained 24.2%, 23.3%, and 68.9% donor positive in the thymus, bone marrow, and spleen, respectively, whereas primed B6-cdd recipients demonstrated an absence of donor cells in all compartments tested. M1 indicates channel marker.
Absence of donor chimerism in central and peripheral lymphoid compartments of B6-cdd recipients.
TNFR1−/− marrow was transplanted into B6-cdd (Ly9.1−) naive and primed recipients 1 day after 9.0 Gy TBI. Thymus, bone marrow, and spleen cells were analyzed 14 days later. Ly9.1 (clone 30C7) staining identifies expression (Ly9.1) on most thymocytes, peripheral T and B cells, bone marrow, lymphoid cells, and hematopoietic progenitors of the C3H.SW strain but not C57BL/6 (Ly9.1−). B6-cdd naive recipients contained 24.2%, 23.3%, and 68.9% donor positive in the thymus, bone marrow, and spleen, respectively, whereas primed B6-cdd recipients demonstrated an absence of donor cells in all compartments tested. M1 indicates channel marker.
To corroborate and extend these observations, resistance was examined in a second T-cell–dependent model between MHC-mismatched donors and recipients (Figure 9). Previous studies have demonstrated that, although NK cells play a crucial role in the resistance against MHC-mismatched marrow grafts in naive recipients, the resistance is virtually completely T-cell dependent if the recipient is sensitized against donor antigens prior to BM transplantation.15 B6-wt and B6-cdd recipients (H-2b) were, therefore, primed to complete MHC class I and II mismatched donor MHC H-2d alloantigens prior to transplantation. As previously detected in MiHA-mismatched transplants, cytotoxically normal B6-wt mice effectively and equivalently rejected TNFR1−/− or TNFR1+/+ complete MHC-mismatched (H-2d) marrow allografts (Figure 9). B6-cdd mice given transplants of, in increasing numbers, up to 3 × 107, of H-2d TNFR1−/− marrow also exhibited equivalent resistance to that effected against TNFR1+/+healthy donor marrow (Figure 9).
Intact barrier response against MHC-mismatched progenitors in the absence of 3 cytotoxic pathways.
B6-cdd recipients were primed against H-2d by injection of BALB/c cells 3 weeks prior to BM transplantation. Naive BALB/c recipient controls were used to demonstrate the upper limit (100%) of lineage-committed progenitors in the CFU-IL3 assay for each of the donor marrow inocula. Naive BALB/c recipients of 5 × 106TCD–wild-type BM contained 98 ± 25 CFU-IL3/spleen and 414 ± 124 CFU-IL3/spleen from TCD-TNFR1−/− BM.
Intact barrier response against MHC-mismatched progenitors in the absence of 3 cytotoxic pathways.
B6-cdd recipients were primed against H-2d by injection of BALB/c cells 3 weeks prior to BM transplantation. Naive BALB/c recipient controls were used to demonstrate the upper limit (100%) of lineage-committed progenitors in the CFU-IL3 assay for each of the donor marrow inocula. Naive BALB/c recipients of 5 × 106TCD–wild-type BM contained 98 ± 25 CFU-IL3/spleen and 414 ± 124 CFU-IL3/spleen from TCD-TNFR1−/− BM.
Resistance occurs in the simultaneous absence of TRAIL, perforin, FasL, and TNFR1 signaling
The triple cytotoxic defective resistance model using TNFR1−/− marrow donors was extended to examine for TRAIL involvement. Studies using the anti-TRAIL mAb N2B2 demonstrated its in vivo ability to interfere with tumor clearance.31,32 TRAIL transfectants, but not control transfectant cells, were shown to effectively lyse L929 target cells as previously reported, and the mAb N2B2 effectively inhibited this TRAIL-mediated cytotoxic killing31 (M.M. and R.B.L., unpublished observations, April 2002). We found that this anti-TRAIL mAb (N2B2) markedly augmented metastatic carcinoma lesions over a 2-week time period in vivo.33 B6-cdd recipients primed to C3H.SW MiHA were given transplants of TNFR1−/− marrow. This same anti-TRAIL mAb administered in the tumor experiments was used to examine rejection in the triple deficient model. Groups received 2 injections of anti-TRAIL antibody or the isotype control immunoglobulin on days 0 and +1. Day 5 CFU assay indicated that efficient resistance occurred in the recipients receiving anti-TRAIL antibody (Figure10A). A second experiment was performed using the MHC-mismatched model. B6-cdd recipients injected with anti-TRAIL antibody efficiently rejected 1 × 107allogeneic H-2d-R1−/− marrow (Figure 10B). We have also found (T.J.S. et al, unpublished observations, October 2002) that treatment of BMCs with recombinant TRAIL (1000 ng/mL) overnight in vitro prior to transfer to irradiated syngeneic recipients does not affect immune reconstitution, strongly suggesting that marrow precursors are not particularly sensitive to TRAIL-mediated apoptosis. In total, these results demonstrated that in the simultaneous absence of 4 cytotoxic effector pathways used by cytologic T lymphocytes (CTLs), strong host resistance could be detected.
Strong host resistance mediated in the absence of perforin-, FasL-, TNFR1-, and TRAIL-dependent killing.
Naive and primed B6-cdd and B6-gld (littermates) recipients received 9.0 Gy TBI 1 day prior to transplantation of 2 × 106TCD-C3H.SW bone marrow lacking TNFR1 (A) or 1 × 107TCD-BALB/c bone marrow lacking TNFR1 (B). Purified TRAIL antibody (N2B2, ▪) or an isotype-matched control antibody (░) was injected (200 μg) intraperitoneally on day 0 just prior to BM transplantation and again 24 hours later. Recipients were examined by CFU-IL3 assay. (A) Naive B6-cdd, 3682 ± 294; primed B6-cdd injected with isotype control, 80 ± 25; primed B6-cdd injected with N2B2, 7 ± 12; naive B6-gld, perf+/−, 3101 ± 353; B6-gld, perf+/−, injected with isotype control, 428 ± 16; B6-gld, perf+/−, injected with N2B2, 36 ± 12.
Strong host resistance mediated in the absence of perforin-, FasL-, TNFR1-, and TRAIL-dependent killing.
Naive and primed B6-cdd and B6-gld (littermates) recipients received 9.0 Gy TBI 1 day prior to transplantation of 2 × 106TCD-C3H.SW bone marrow lacking TNFR1 (A) or 1 × 107TCD-BALB/c bone marrow lacking TNFR1 (B). Purified TRAIL antibody (N2B2, ▪) or an isotype-matched control antibody (░) was injected (200 μg) intraperitoneally on day 0 just prior to BM transplantation and again 24 hours later. Recipients were examined by CFU-IL3 assay. (A) Naive B6-cdd, 3682 ± 294; primed B6-cdd injected with isotype control, 80 ± 25; primed B6-cdd injected with N2B2, 7 ± 12; naive B6-gld, perf+/−, 3101 ± 353; B6-gld, perf+/−, injected with isotype control, 428 ± 16; B6-gld, perf+/−, injected with N2B2, 36 ± 12.
Discussion
Significant advances have been made in understanding the nature of barrier responses against bone marrow allografts, particularly in mouse transplant models. The acute rejection of allogeneic marrow can occur within several days of transplantation in irradiated recipients.34 Elegant studies by Murphy et al14 and others15-18 have demonstrated that acute rejection in unsensitized mice can involve NK, CD8+ T cells and other populations. Previous findings by several laboratories have showed that CD4+ as well as CD8+ T cells can participate in rejection of MHC and MiHA marrow allografts.35 36
CTLs have been identified in patients rejecting marrow grafts,37 and, thus, it has generally been considered that cytotoxic function by host T cells is a crucial effector pathway in marrow allograft resistance. Experimental studies have isolated and cloned antidonor alloreactive T cells from primates and mice rejecting hematopoietic grafts38,39 and used them to transfer resistance to nonresistant recipients.39 Studies by Kernan et al37 reported that the rejection of HLA nonidentical marrow grafts was associated with the presence of host antidonor-specific CTLs. Nonetheless, demonstration that transferred CTLs can mediate resistance does not conclusively constitute mea culpa of cytotoxic function in the transplantation setting. A crucial question concerns which molecules in CTL (and non-CTL) populations can mediate resistance function(s).
To carefully analyze the potential effector pathways used by T cells during the rejection of progenitor cell allografts, models of resistance involving recipients sensitized to donor antigens have been analyzed.21 The use of MHC-matched, MiHA-mismatched strains together with a priming event results in a T-cell–dependent barrier, found here to be transferrable by CD8+NK1.1− T cells. Although it is not possible to formally exclude contributions of other cell populations, the requirement for priming, antigen specificity, and resistance against MHC-matched allografts makes it unlikely to be effected by low numbers of contaminating non-CD8 T cells. A second priming model also used in the present studies involved resistance against MHC disparate allografts. Although NK cells play a crucial role in the resistance against MHC-mismatched marrow grafts in naive recipients, the resistance is predominantly T-cell dependent if the recipient is sensitized against donor antigens prior to BM transplantation.15 21 Nonetheless, the present studies have demonstrated that in sensitized BMT recipients, a quadruple cytotoxic deficit does not abrogate the capacity to effect strong marrow graft resistance. Therefore, a nonperforin, FasL-, TNFR1-, and TRAIL-dependent host T-cell pathway(s) must be capable of effecting significant barrier activity following allogeneic BM transplantation. Several possibilities could account for these findings. Although unlikely, a novel pathway presently unidentified in CD8 cells might be used by CD8 cells only during rejection (perhaps accounting for its unidentified nature to date). This pathway may be cytotoxic. Alternatively, donor PCs may not have been killed after transplantation, rather some noncytotoxic pathway may inhibit progenitor expansion. Such an inhibitory effect could be mediated by a known cytokine and accordingly enable “recovery” of functional progenitor populations from recipients.
Studies from several laboratories have failed to detect diminished host resistance in mice with severe cytotoxic deficiencies. In BMT models involving varying donor/recipient genetic disparities, perforin- or FasL-defective (MHC class I/II + MiHA; MiHA only), granzyme B KO (P6F1), and granzyme A promoter diphtheria toxin transgenic animals (MHC class I/II + MiHA; and P6F1) have failed to show defects in marrow graft resistance, respectively.19-22,40 Studies using β2μ KO class I–deficient transplanted marrow have demonstrated a decrease in this NK-dependent resistance in the 129 strain, but not B6 recipients.23 Such results are important because together with the present findings they raise the possibility that NK and T cells may not use completely overlapping effector modalities in mediating marrow graft resistance. Perforin-dependent cytotoxic function clearly can contribute to the resistance effected in naive recipients. Resistance in naive recipients in the absence of perforin and FasL indicates that, compared with wild-type recipients, lower doses of allogeneic marrow can override the resistance24(M.M. and R.B.L., unpublished data, October 2000). However, even in naive recipients, nonperforin pathways are likely contributory, because the level of donor CFU activity is clearly lower in allogeneic B6-cdd versus syngeneic BALB/c recipients of the same marrow inoculum (M.M. and R.B.L, unpublished data, October 2000).
Initial findings examining contributions by the 2 major cytotoxic pathways failed to demonstrate an obvious deficiency in the ability of perforin knock-out or FasL-deficient recipients to reject short-term CFU cells following complete MHC-mismatched allogeneic marrow transplantations into naive recipients.19,20 Additional studies using Fas-deficient donor marrow and perforin-deficient recipients suggested, but could not prove because of “leakage” in the lpr mouse, that neither pathway was contributory.20The present studies used cytotoxic double deficient recipients sensitized against donor MiHA antigens to definitively demonstrate that, in the simultaneous absence of both pathways, strong T-cell–mediated barrier responses to marrow grafts can be effected. Despite augmenting the dose 3 to 6 times and 15 times above the standard level, respectively, no differences were observed between healthy and cytotoxi-deficient recipients. Therefore, in the combined absence of functional perforin/granzymes- and FasL-dependent cytotoxicity, other effector pathways can mediate resistance to bone marrow transplantation. We have examined interferon γ (IFN-γ) KO B6 recipients primed to donor MiHA antigens and observed that these recipients readily reject MiHA disparate normal bone marrow, suggesting that host IFN-γ is not important (M.J. and R.B.L., unpublished observations, September 1997).
TNF has a number of effects on hematopoiesis and has been shown to inhibit erythroid colony-forming unit (CFU-E) formation in vitro and suppress erythropoiesis in vivo.41-46 TNFR1 can induce target cell apoptosis via caspase activation and DNA degradation,47,48 is ubiquitously expressed, and contains a death domain (DD). TNFR2 expression is more limited, lacks a DD, and can signal proliferation via nuclear factor κB (NF-κB) activation.49,50 Examination of the involvement of the R1 and R2 receptors was important because the significance of TNF signaling through R2 and induction of apoptosis remains unclear. For example, because TRAF2−/− cells demonstrate increased sensitivity to TNF-induced death, it has been proposed that the absence of R2 may remove antiapoptotic signals, thereby effectively increasing R1-mediated apoptosis.51 Notably, in both the MiHA- and MHC-mismatched models examined here, the presence of a triple cytotoxic deficiency (ie, perforin dependent, FasL-Fas dependent, and TNF-TNFR1 or R2 signaling) failed to ablate resistance against donor progenitor populations following allogeneic BM transplantation.
Several reports concerning TRAIL suggest it may affect some hematopoietic progenitor populations.52 53 A quadruple cytotoxic deficient model was generated by administering anti-TRAIL mAb to donor antigen primed B6-cdd recipients given transplants of TNFR1−/− marrow. Again, regardless of whether a MiHA or MHC difference existed between the donor and host, no diminution in the capacity of the sensitized recipients to reject donor PCs was observed. Together these studies emphasize that alternative T-cell effector pathways to those mediated by perforin, FasL, TNFR signaling, and TRAIL can resist marrow allografts. This pathway(s) is “vigorous” in that resistance against large numbers of allogeneic marrow has been routinely observed.
The findings in this study do not eliminate the possibility that other cytotoxic pathways contribute to the observed resistance. In addition to the clinical study noted earlier, at least one study failed to identify direct lysis of hematopoietic stem cell populations by NK cells.54 Alternatively, or in addition, noncytotoxic molecules (eg, transforming growth factor β [TGFβ] and macrophage inflammatory protein α [MIP1α]46) may be capable of effecting resistance by inhibition of progenitor cell differentiation/proliferation. TGFβ can inhibit c-kit expression and can inhibit proliferation by primitive stem cell populations, including HPP PCs.55,56 Such regulation may also be reversible, an effect which would also be consistent with our findings to date. The continuing “dissection” of the hematopoietic progenitor cell compartment has identified several functionally disparate pluripotential hematopoietic cell types within the Thy1loSca1+Lin− stem cell phenotype, including cells with short-term repopulating function.57-59 However, the long-term maintenance of hematopoiesis is dependent on more primitive stem cells.60 Studies have reported that Fas expressed on marrow cells can signal apoptosis in some but not all progenitor populations.61-63 It is possible that effector cells may use different pathways in rejection/inhibition of hematopoietic PC populations dependent on their receptor expression. However, in the present studies, resistance against MiHA-disparate allografts resulted in the same pattern of inhibition against both committed (CFU-IL3 or GM-CSF) and more primitive (CFU-HPP) progenitor populations. These findings may indicate that multiple progenitor populations, perhaps those contributing both to short- and longer-term engraftment, are susceptible to the nonperforin/granzyme, FasL, TNFR, and DR3/4 effector pathway.
We thank Dr Zhe Jiang for the PCR screening and typing and Emma Weaver for maintenance of the B6-cdd breeding colony used in these experiments. We also acknowledge the Sylvester Comprehensive Cancer Center for their support of the Flow Cytometry Facility for the phenotypic analysis of cell populations used in these experiments.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Prepublished online as Blood First Edition Paper, January 9, 2003; DOI 10.1182/blood-2002-09-2859.
Supported by grants 1R01 RR11576 and 5RO1 HL52461 (R.B.L.) from the National Institutes of Health. This publication has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. NO1-CO-12400.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert B. Levy, University of Miami School of Medicine, Department of Microbiology and Immunology, PO Box 016960 (R-138), Miami, FL 33101; e-mail:rlevy@med.miami.edu.