Abstract
A small proportion of patients with human immunodeficiency virus type 1 (HIV-1) remains asymptomatic for a long period after infection. It is thought that a vigorous immune response may contribute to long-term nonprogression, though studies are confounded by heterogeneity among patients. We studied the levels of HIV-1 receptors, costimulatory T-cell molecules, and dendritic cell (DC) numbers in 18 patients with long-term infection, CD4 counts greater than 400 cells/mm3, and HIV-1 viral loads lower than 50 copies/mL. These patients were further differentiated through the presence or absence of 2-LTR DNA circles, a possible marker for residual ongoing HIV-1 replication. A statistically significant increase in levels of CD91, the heat-shock protein (HSP) receptor, was observed in therapy-naive patients who had no evidence of ongoing viral replication (P = .01). This difference was most notable on their monocytes. High levels of CD91 may be a host factor that contributes to the maintenance of long-term nonprogression. The ability of CD91 to internalize α-defensins and to cross-present exogenous antigen to cytotoxic T lymphocytes through major histocompatibility complex (MHC) class 1 may maintain CD8+ responses in these patients.
Introduction
The pathogenic mechanisms that underlie HIV-1 infection are highly variable and depend on the interplay between numerous host and viral factors that are likely to determine the rate of clinical progression.1-3 In untreated patients, the median time from infection to the development of AIDS is approximately 10 years, though it can develop in 3 to 6 months.4,5 A minority of HIV-infected patients termed long-term nonprogressors (LTNPs) remains healthy for more than 10 years with no clinical evidence of progression to disease.6,7 These patients are characterized by stable or even increasing CD4+ T-cell counts and by stronger CD8+ cytotoxic T-lymphocyte responses against HIV and other viruses than progressors.8-11
Numerous virologic characteristics appear to play a role in the nonprogression of HIV disease. These include deletions or defects in the HIV-1 nef gene8,12 and other regulatory genes (vif, vpu,vpr,13,14rev,15tat, env,16,17 and the long terminal repeat18). Host factors reported thus far to be important include dominant type 1 cytokine production,19 a single copy of the CCR-5 Δ32 receptor mutation,20,21 a high neutralizing antibody titer,22 raised levels of γδ T cells,23 certain HLA alleles,24 and resistance to factors that induce accentuated spontaneous but not mitogen-induced cell death.25 Recently, LTNPs have been found to maintain the expression of high levels of perforin on their CD8+ T cells,11 and these cells secrete α-defensins in response to stimulation.26
In these studies, however, the proportion of patients defined as LTNPs varies.27 Those termed LTNPs were not virologically and immunologically homogenous, and many had biologic signs of progressive disease.28 We wanted to compare HIV receptor levels, T-cell costimulatory molecules, and the proportion of DC subsets in 3 different groups of patients with long-term HIV-1 infection, an HIV-1 RNA viral load exceeding 50 copies/mL, and a CD4 count greater than 400 cells/mm3. This study also used the recent ability to detect 2-LTR episomal DNA circles that may represent a marker of new cycles of infection in those patients with plasma viral levels below the standard limit of detection,29,30 thus enhancing our ability to differentiate between these apparently homogenous groups of patients. In several studies, the presence of 2-LTR episomal DNAs has been used as a marker of ongoing or recent infection.31-33It should be noted that others have suggested that circular forms of the viral genome are not unstable and, therefore, may not represent recent reverse transcription events.34
Expression of the critical costimulatory molecule CD28 and the cytotoxic T-lymphocyte antigen 4 (CTLA-4 or CD152), which determines the early outcome of stimulation through the T-cell antigen receptor, was analyzed. Because it has been suggested that heat-shock proteins (HSPs) play a major role in the cross-presentation of antigens in vivo,35 we also investigated the surface expression of the HSP receptor, CD91. Definition of a host phenotype that maintains heightened immune responses to viruses or is associated with a lack of progression to disease could provide insights into the mechanisms underlying host viral defenses, with implications for protective and therapeutic strategies.
Patients, materials, and methods
Patients and blood samples
We studied 18 HIV-1–positive male patients who had been infected with HIV-1 for more than 10 years. HIV-1 RNA viral loads were lower than 50 copies/mL (bDNA; Chiron, Emeryville, CA) in all patients, CD4 counts were greater than 400 cells/mm3, and CD8 counts were greater than 800 cells/mm3. There were no statistically significant differences between these parameters, the median age of the patients in each group, or time since HIV-1 diagnosis. Group 1 (n = 6) consisted of patients without evidence (at any time) of 2-LTR circles and were called true LTNP patients (the only such patients in our cohort, one of the largest in Europe). These patients had never received anti-HIV therapy. Group 2 (n = 6) consisted of patients on antiretroviral treatment in whom 2-LTR circles were undetectable—that is, they were chronically suppressed patients on highly active antiretroviral therapy (HAART) without active viral replication. Group 3 (n = 6) consisted of patients on antiretroviral treatment with demonstrable 2-LTR circles (chronically suppressed patients with viral replication). Patients in group 1 had no history of antiretroviral use, and those in groups 1 and 2 lacked 2-LTR circles at more than one time point preceding the study sample. We also investigated levels of CD91 on a group of HIV-1–negative controls (n = 6). Blood was collected into heparinized vacutainers and was separated using Ficoll-Histopaque (Sigma Aldrich, Poole, United Kingdom) density-gradient centrifugation. Peripheral blood mononuclear cells (PBMCs) were cryopreserved in fetal calf serum (FCS)/10% dimethyl sulfoxide (DMSO) medium (Sigma).
Measurement of 2-LTR circles
HIV-1 2-LTR circles were detected as previously described32 by amplifying HIV-1 cDNA using real-time LCqPCR (LightCycler; Roche, Mannheim, Germany). Because of the low copy number of target DNA in the circulating lymphocyte population of patients on HAART, a modified proteinase K digest (Qiagen, Hilden, Germany) plasmid extraction protocol was used.33 Episomal DNA was further digested with plasmid-safe DNase and 1 mM adenosine triphosphate (ATP) in Platinum Taq polymerase chain reaction (PCR) buffer (Invitrogen, Paisley, United Kingdom). Purified HIV-1 2-LTR circles were linearized within the LightCycler capillary tubes by the introduction of the endonuclease Msp 1 (5 minutes at 37°C).
Primers M669 (5′-GGAACCCACTGCTTAAGCCTCAA-3′) and M847 (5′-GTGTAGTTCTGCCAATCAGGGAA-3′) were used under modified Platinum Taq buffer conditions: linearization, 37°C for 5 minutes; activation, 95°C for 5 minutes; preamplification, 5 cycles of denaturation at 95°C for 10 seconds; annealing, 62°C for 5 seconds; extension, 72°C for 10 seconds; fluorescence acquisition, 83°C for 5 seconds; amplification, 35 cycles of denaturation at 89°C for 10 seconds; annealing, 60°C for 5 seconds; extension, 72°C for 10 seconds; and fluorescence acquisition, 83°C for 5 seconds. Melting-curve analysis was performed over the temperature range 65°C to 95°C, with sequence analysis on randomly selected samples as a product control.
Standards were prepared using serial dilutions of extracted, linearized (EcoR1) DNA from an HIV-1 2-LTR–containing plasmid. Primary concentrations were determined spectroscopically, and the copy number was calculated. Standards were run in duplicate with the patient sample extracts and an equal number of controls. After amplification and acquisition, a standard curve was generated using LightCycler software, and these values were used to quantify the target sample product. The median number of 2-LTR circles in the 6 patients in whom these were positive was 45 (range, 16-141 circles).
Surface marker expression
Three- or 4-color flow cytometric analysis was performed on 5 × 105 thawed and washed PBMCs, previously stored in liquid nitrogen. To analyze DC subsets, they were labeled with phycoerythrin (PE)–conjugated anti-CD3, -CD14, -CD16 (PharMingen, Oxford, United Kingdom), and -CD19, anti–HLA-DR (BD Biosciences, Oxford, United Kingdom), and fluorescein isothiocyanate (FITC)–conjugated anti-CD11c (DAKO, Ely, United Kingdom). DCs were defined by the absence of labeling with the PE-conjugated lineage marker cocktail and the expression of HLA-DR. They were further subdivided into myeloid (mDC) and plasmacytoid (pDC) subsets based on expression of the β-integrin CD11c36 and were counted as previously described.37 HIV cell surface receptors were analyzed with FITC-conjugated anti-CD4 and allophycocyanin (APC)–conjugated anti-CXCR4 or anti-CCR5 (PharMingen). T-cell costimulatory markers were analyzed using PE-conjugated anti-CD28 or PercP-conjugated anti–CTLA-4 (CD152). CD91 (the HSP receptor) surface expression was detected using FITC-conjugated anti–α2-macroglobulin α-chain (BioMac, Leipzig, Germany). In some experiments, PBMCs were labeled after incubation for 30 minutes at room temperature with 100 μL culture supernatant from PM1 cells infected with HIV-1 Bal, a CCR5-utlizing virus. At least 100 000 cells were acquired in the live gate and analyzed on a Becton Dickinson FACScalibur using CellQuest software. Positive staining for each marker was determined by comparison with appropriate isotype-matched controls, and identical settings were used on each sample.
Statistical analysis
The significance of the differences between groups was analyzed using the Kruskal-Wallis nonparametric (one-way analysis of variance) test using SAS software version 8.0 (Cary, NC). This determined whether any of the 3 groups showed a statistically significant difference with the other 2 (Table 1). Differences before and after the addition of HIV-1 were determined by comparing median differences in all 3 groups together.
The main hypothesis in our protocol was to look for changes in mean fluorescence staining (or percentage of population stained) between group 1 with group 2 and group 1 with group 3. We also observed differences between group 1 and groups 2 and 3. Because of the small study size, we used the nonparametric Kruskal-Wallis test to determine whether there was a difference overall between any of the 3 study groups. Where this was significant, we investigated the study groups further to determine which 2 groups showed a difference. For this analysis, we did not adjust the P values using the Bonferroni correction because this comparison was predetermined. The Pearson correlation coefficient was used to investigate correlation (P calculated after regression analysis).
Results and discussion
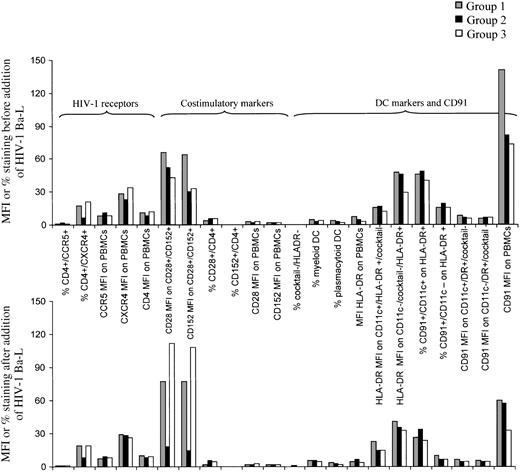
We studied phenotypic markers on PBMCs in the untreated LTNP (group 1) and in patients with chronically suppressed HIV-1 on HAART, with and without evidence of ongoing viral replication as ascertained by the presence or absence of 2-LTR episomal DNA (groups 2 and 3). There were no statistically significant differences among the 3 groups in levels of HIV-1 receptors (CD4, CXCR4, CCR5), costimulatory T-cell molecules (CD28, CTLA-4), or percentage of DCs before or after the addition of HIV-1 Bal-1. Expression of CD28 and CTLA-4 decreased in chronically suppressed patients on HAART without 2-LTR circles (group 2) and increased in those on HAART with circles (group 3), but these changes were not statistically significant (Figure 1; Table 1). In addition, there were no differences in numbers of myeloid (CD11c+) and plasmacytoid (CD11c−) DCs between the groups. We did, however, observe a statistically significant increase in CD91 expression in LTNP (group 1; P = .008) compared with other treated patients (groups 2 and 3) with HIV-1 viral loads below the conventional limit of detection (CD91 MFI on PBMCs). Interestingly, the addition of HIV-1 Bal for a short amount of time inhibited binding of the anti CD91 antibody (Figure 1, lower graph).
Mean fluorescence intensity (MFI) or percentage staining using peripheral blood mononuclear cells from 3 groups of patients.
Each group consisted of 6 patients. Levels of HIV-1 receptors, costimulatory molecules CD28 and CTLA-4, DC markers, and CD91 are shown. The top graph shows the baseline, and the lower one follows incubation with Ba-L supernatant (R5 tropic HIV-1). DCs were defined as cocktail-negative (CD3, CD14, CD16, CD19), HLA-DR–positive cells.
Mean fluorescence intensity (MFI) or percentage staining using peripheral blood mononuclear cells from 3 groups of patients.
Each group consisted of 6 patients. Levels of HIV-1 receptors, costimulatory molecules CD28 and CTLA-4, DC markers, and CD91 are shown. The top graph shows the baseline, and the lower one follows incubation with Ba-L supernatant (R5 tropic HIV-1). DCs were defined as cocktail-negative (CD3, CD14, CD16, CD19), HLA-DR–positive cells.
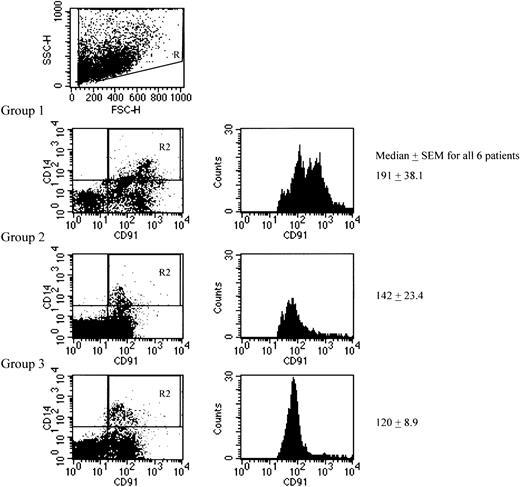
Because staining of DC subsets in PBMCs with CD91 failed to yield significant levels of expression or differences among the 3 groups and the CD91 MFI on PBMCs was significantly different (Figure 1; Table 1), we studied levels of CD91 on other peripheral blood cells. The lack of CD91 on blood DCs may reflect the fact that they are preimmature cells en route to the tissues, where they become fully competent in antigen capture. We found no evidence of CD91 expression on CD3+ T cells but did observe expression on CD14+ monocytes (one representative figure from each group is shown in Figure2). Mean fluorescence of CD91 staining on CD14+ monocytes was significantly different among the 3 groups, with the “true” LTNP having the highest values (P = .0171). In this small cohort, we also observed a statistically significant correlation between the CD91 MFI on CD14+ monocytes and the number of 2-LTR circles (r = 0.52; P < .01, in those who were 2-LTR circle positive—that is, group 3), CD91 MFI, and CD4 count (r = 0.62; P < .01). We also observed a statistically significant correlation between the number of 2-LTR circles and the CD4 count (r = 0.96;P < .001; Figure 3). There was no correlation with CD4 percentage (r = 0.17) or CD8 count (r = 0.16).
Representative dot plot and histogram from one patient in each group showing CD14+/CD91+ populations in all 3 groups.
The histograms represent the median CD91 MFI on the CD14+ cells (the gate in the dot plot is shown). The mean for the CD91 MFIs on monocytes measured 227.3 in group 1, 148.3 in group 2, and 121.5 in group 3. Differences between the means also demonstrated a significant increase in HSP receptor levels on monocytes derived from patients in group 1. Median MFI on HIV-1–negative controls measured 114.4 ± 18.9 (mean, 125.2).
Representative dot plot and histogram from one patient in each group showing CD14+/CD91+ populations in all 3 groups.
The histograms represent the median CD91 MFI on the CD14+ cells (the gate in the dot plot is shown). The mean for the CD91 MFIs on monocytes measured 227.3 in group 1, 148.3 in group 2, and 121.5 in group 3. Differences between the means also demonstrated a significant increase in HSP receptor levels on monocytes derived from patients in group 1. Median MFI on HIV-1–negative controls measured 114.4 ± 18.9 (mean, 125.2).
Number of 2-LTR circles correlated with CD4 count.
CD91 MFI on CD14+ cells correlated significantly with the number of 2-LTR episomal DNA circles and with the CD4 count (cells/mm3) in this small group of patients (P < .001).
Number of 2-LTR circles correlated with CD4 count.
CD91 MFI on CD14+ cells correlated significantly with the number of 2-LTR episomal DNA circles and with the CD4 count (cells/mm3) in this small group of patients (P < .001).
After incubation with HIV-1, CD91 expression decreased to similar levels in all 3 groups (Figure 1, CD91 MFI on PBMCs on far right of graphs). Recently, specific incorporation of HSPs into the coat of HIV-1 was demonstrated.38 We presume that the binding of virion coat HSPs to CD91 decreases anti-CD91 antibody binding by competition for the binding site. Alternatively, the interaction of virion hsp70 with CD91 may lead to endocytosis of the complex, reducing the availability of surface CD91 for binding the anti-CD91 antibody. The inhibition of CD91 expression following incubation with HIV-1 occurred in a short time span in our assay, suggesting a high binding affinity between HSPs in the virion coat and CD91. To confirm this, a study to identify the HIV-1 binding site on CD91 will be carried out.
High levels of CD91 on monocytes may lead to the enhanced cross-presentation of HIV antigens by these cells and to the consequent enhanced stimulation of activated anti-HIV CTLs. This observation may explain the preservation of CD8+ cytotoxic T-lymphocyte responses that have been consistently observed in LTNPs.8-11 It will be of interest to determine whether levels of CD91 in DCs of relevant tissues have a bearing on the initial priming of naive anti-HIV CTLs in LTNPs and others. Because recent data have demonstrated that CD91 mediates the internalization of α-defensins in a specific, dose-dependent manner,39 the response to secreted α-defensins from stimulated CD8+ T cells in LTNPs26 may be further enhanced by CD91 up-regulation in these patients.
This study suggests the possible importance and role of DCs in this process. Typically, DCs can present exogenous antigens on MHC class 2 molecules and endogenously synthesized antigen on MHC class 1.40 DCs can also take up exogenous peptides chaperoned by HSPs released as a consequence of cell death,41,42 and they re-present them through the classical proteosome/transporter-associated antigen processing (TAP)–dependent endogenous pathway complexed with their MHC class 1 molecules.43 The high efficiency of this process is attributed in all patients to direct binding to and internalization through the CD91 molecule (also called α2-macroglobulin receptor or low-density lipoprotein-related protein).44,45Maintenance of CD8 responses through antigen entering the class 1 pathway exogenously through CD91 may explain many features of true LTNPs. DCs have also been shown to cross-present antigens released by macrophages,46 and this may represent an alternative mechanism for the activation of naive anti-HIV CTLs in LTNPs.
Correlative evidence for the down-regulation of a CD91-mediated pathway of immune activation as a mechanism of immune escape exists. Thus, levels of a CD91 ligand, α2-macroglobulin, are elevated in animal models of cancer47 and during the recovery of animals from autoimmune encephalitis.48 Indeed, the infusion of animals with α2-macroglobulin protects them from experimental autoimmune disease. Blocking of CD91 through pharmacologic agents abrogates the cross-presentation of model antigens and cancer immunity (R.J. Binder and P.K.S., unpublished observation). Elevation of tissue/serum α2-macroglobulin levels and CD91 expression may thus be seen as 2 faces of a host–pathogen interaction. Up-regulation of CD91 may, therefore, represent a useful therapeutic, and perhaps even a preventive, strategy against HIV.
We thank Sundhiya Mandalia for help with statistical analysis, the patients and others who provided samples, and Drs John Morlese, Nad Qazi, and Julia Riley for help obtaining the samples. J.S. is a Medical Research Council PhD student.
Prepublished online as Blood First Edition Paper, January 16, 2003; DOI 10.1182/blood-2002-11-3353.
J.S. and B.G. are joint first authors. P.S. and S. Patterson are joint last authors.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Justin Stebbing, Department of Immunology, The Chelsea and Westminster Hospital, 369 Fulham Rd, London SW10 9NH, United Kingdom; e-mail: j.stebbing@ic.ac.uk.