González-Conejero and coworkers describe how the −1C>T mutation, located in the Kozak sequence of the annexin A5gene (formerly named annexin V), reduced the risk of myocardial infarction in young patients.1 They postulated and showed supporting evidence that −1C>T in the Kozak sequence of theannexin A5 gene increased translation, resulting in higher plasma levels of annexin A5 in T allele carriers. These findings do not support the Kozak context rules as reported by Marilyn Kozak.2,3 She furthermore had some practical comments about the reliability of the in vitro translation assay used in the study.2
Our concerns are related to the determination of the plasma annexin A5 levels in citrated plasma. González-Conejero and coworkers used the enzyme-linked immunosorbent assay (ELISA) technique of Diagnostica Stago.1 This ELISA allows only the measurement of free nonbound annexin A5. As annexin A5 binds with high affinity to negatively charged phospholipids in the presence of calcium,4 addition of citrate will not disturb the binding efficiently and thus, the levels of annexin A5 measured in citrated plasma will be underestimated. The annexin A5 levels are significantly higher in EDTA (ethylenediaminetetraacetic acid) plasma.5 In all our studies performed so far, we measured only increased annexin A5 levels in EDTA plasma. This might indicate that the annexin A5 levels in citrated plasma are not biologically active or are released out of the residual platelets due to freeze-thawing the plasma.
To determine whether T allele carriers indeed have increased levels of circulating annexin A5, we measured the annexin A5 levels in citrated and EDTA plasma of 40 healthy volunteers and linked the results to the −1C>T mutation. All plasma samples were obtained by centrifugation of citrated and EDTA blood for 10 minutes at 4000g. The plasma samples were stored at −80°C.
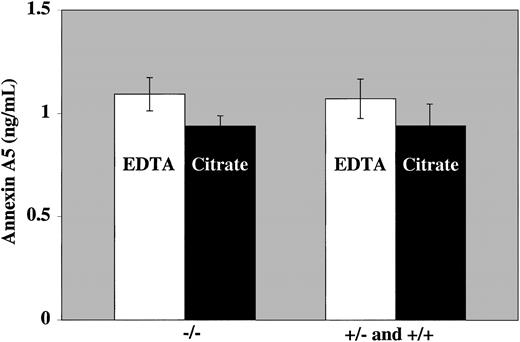
Our results show significantly higher annexin A5 levels in EDTA plasma compared with citrated plasma (1.15 ± 0.56 vs 0.95 ± 0.28 ng/mL). Furthermore, among these 40 healthy volunteers, no differences in annexin A5 levels (both in citrated and EDTA plasma) were observed between T allele and C allele carriers (Figure1). Our measurements are in contrast to the results of González-Conejero et al, who showed increased levels of annexin A5 in plasma of T allele carriers.1 The prevalence of the mutation in our control group was comparable to the prevalence in the Mediterranean population (26%).
Annexin A5 levels in C allele carriers and carriers of the −1C>T mutation.
Annexin A5 levels were measured with the Zymutest Annexin A5 ELISA (Hyphen Biomed, Andresy, France), and the polymorphism was determined by NcoI restriction fragment-length polymorphisms (RFLP). The mean annexin A5 levels are expressed (± SEM).
Annexin A5 levels in C allele carriers and carriers of the −1C>T mutation.
Annexin A5 levels were measured with the Zymutest Annexin A5 ELISA (Hyphen Biomed, Andresy, France), and the polymorphism was determined by NcoI restriction fragment-length polymorphisms (RFLP). The mean annexin A5 levels are expressed (± SEM).
We have shown that the −1C>T mutation does not result in elevated plasma annexin A5 levels. More studies have to be performed to understand the reduced risk of myocardial infarction in young patients carrying the T allele in the Kozak sequence of the annexin A5 gene.
Annexin V polymorphisms, plasma levels, and myocardial infarction
Studies about common polymorphisms give conflicting results when analyzing both their functional role and their clinical relevance. For every study that finds a positive association with a particular polymorphism, there are several reporting no effect or the opposite.1-1-1-4 As expected for genetic changes present in more than 1% of the population, common polymorphisms would unlikely have a strong effect. Thus, even minor modifications in selection of samples, design of the study, environmental factors, or genetic background of different populations might modify the functional or clinical consequences associated with a single polymorphism. In a recent study, we found the −1T allele of the annexin V (ANV; highly prevalent in the white Mediterranean population [23%]) associated with increased circulating levels of this protein in citrated samples. This allele also related to increased translation of this protein when using in vitro systems containing either whole ANV cDNA or mini-cDNA generated by genomic polymerase chain reaction. In agreement with these results, we found that the −1T allele conferred protection against premature myocardial infarction.1-5 van Heerde and coworkers have argued against some points of our findings. First, they are concerned about the appropriateness of ANV analysis in citrated samples. Certainly it is necessary to use EDTA (ethylenediaminetetraacetic acid) samples to determine total circulating ANV. However, we consider that citrated values could give more clinical information. As indicated by van Heerde et al, citrated samples allow the measurement of free nonbound ANV, the molecule with potential antithrombotic role, by binding to negatively charged phospholipids exposed in procoagulant surfaces during pathologic prothrombotic states. Moreover, although ANV levels in samples anticoagulated with EDTA are higher than those obtained using citrated samples, we observed a correlation between these samples in all tested individuals.
Second, these authors found no difference in the plasma level of ANV associated with the −1C>T polymorphism in a Dutch population. The association between the −1C>T polymorphism and circulating ANV levels in both studies were established with data from few C>T individuals (7 Dutch and 10 Spanish subjects). Moreover, circulating ANV values displayed a wide interindividual variation independently of the −1C>T genotype, especially in the Dutch study. Thus, further studies including more individuals with either genotype (C>C, C>T, and T>T) would give definitive insight on whether the −1C>T polymorphism influences the plasma levels of ANV.
Our in vivo, in vitro, and clinical data consistently support that the ANV −1 T allele plays a minor but significant role influencing the levels of ANV in our population. The relevance of the Kozak sequence in the control of translation led us to suggest that the −1C>T polymorphism could effect its influence by affecting translation. However, Dr Kozak recently has suggested that increase in plasma levels of ANV associated with the −1T allele could reflect an effect on mRNA stability or splicing, rather than an effect on translation.1-6 Noteworthy, we also identified a second polymorphism in the ANV gene: ISV2 + 18 C>G. This polymorphism is located near the splicing signal of exon 1 and interestingly, is in linkage disequilibrium with the −1 C>T polymorphism. The −1T allele always associated with the ISV2 + 18G allele in the Spanish population. It seems also reasonable to test whether this linkage is also observed in other populations. Moreover, additional experiments are required to clarify the mechanism controlling the levels of ANV associated with the −1 C>T and ISV2 + 18 C>G polymorphisms. Finally, it would also be necessary to perform further studies to confirm the clinical relevance of this polymorphism, as we stated in our original manuscript.