Rituximab, a chimeric monoclonal anti-CD20 antibody, has been successfully used to in vivo purge CD20+ tumor cells during mobilization for high-dose therapy with autologous stem cell transplantation.1-6 Posttransplantation neutropenia has been observed in as many as 25% of patients, although, to date, all reported patients have responded to growth-factor administration. In this report, we describe a case of agranulocytosis refractory to high-dose stem cell growth factors and ultimately responsive to cyclosporine following autologous stem cell transplantation for non-Hodgkin lymphoma (NHL) purged in vivo with rituximab.
A 46-year-old man who presented with stage IV lymphocyte-predominant Hodgkin disease was treated with standard chemotherapy and achieved a brief partial response. Review of the pathology resulted in modification of the diagnosis to diffuse large B-cell lymphoma, T cell and histiocyte rich. Standard CHOP chemotherapy resulted in another brief partial response. At the time of disease progression, in preparation for an autologous stem cell transplantation, the patient received and responded to 2 cycles of ifosphamide, mesna, carboplatin, and etoposide (Figure1) . He underwent successful chemomobilization therapy that included 2 doses of 375 mg/m2 rituximab, 7 days apart. He received 3.3 × 106 CD34 cells/kg following a myeloablative conditioning regimen. Trilineage engraftment occurred by day 9 after transplantation. Rituximab was administered on days 30 and 37 without complication. On day 77, his total white blood cell (WBC) count was 5000 mm3 with 86% neutrophils, hemoglobin level 12.0 g/dL, platelet count 132 mm3. On day 122, the patient had a WBC of 1000/mm3 with 0.0% neutrophils, hemoglobin level 13.0 g/dL, platelet count 185 000 mm3. A bone marrow biopsy performed on day 128 showed hypocellular bone marrow with little maturation of the myeloid series and no evidence of disease relapse. Dysplastic features were not observed despite a new cytogenetic abnormality, del(20)(q11.2). An extensive evaluation for an infectious etiology was negative. Autoantibodies against granulocytes were not detected.
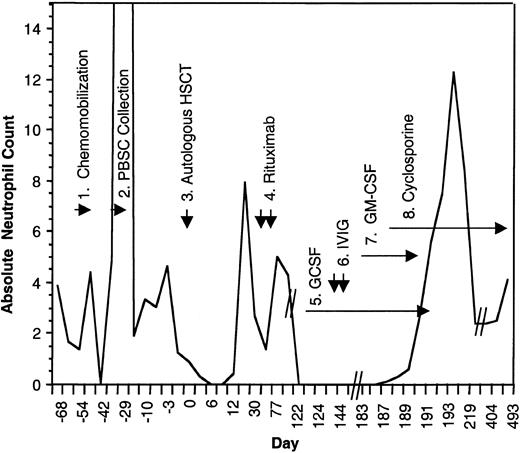
Absolute neutrophil count during the course of treatment.
(1) The patient received chemomobilization therapy with rituximab 375 mg/m2, days −54 and −47, cyclophosphamide 4 g/m2 intravenously, day −52, mesna 1.33 g/m2, thrice a day on day −52, etoposide 200 mg/m2 intravenously on days −52 to −50, and dexa-methasone 10 mg orally four times a day, day −52 to −49. (2) Peripheral blood stem cell collection. (3) Autologous transplant following 12 Gy of linear accelerator radiation, etoposide 60 mg/kg, and cyclophosphamide 100 mg/kg. (4) Posttransplantation rituximab (375 mg/m2 ) was given on days +30 and +37. (5) GCSF 480 μg/day beginning on day +130 and doubled on day +134. (6) Two doses of intravenous immunoglobulin (IVIG). (7) GM-CSF, 250 μg/m2/day. (8) Cyclosporine 200 mg orally twice a day, tapered ultimately to 50 mg orally twice a day.
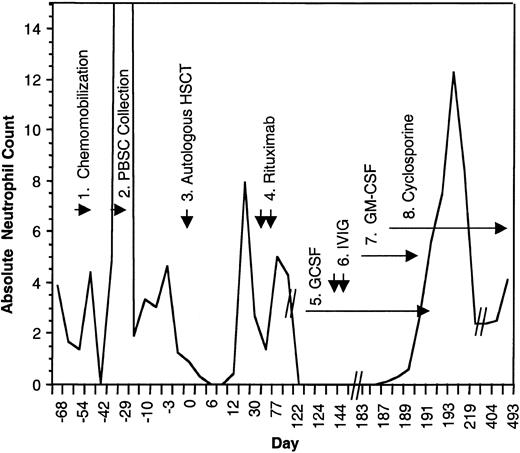
Absolute neutrophil count during the course of treatment.
(1) The patient received chemomobilization therapy with rituximab 375 mg/m2, days −54 and −47, cyclophosphamide 4 g/m2 intravenously, day −52, mesna 1.33 g/m2, thrice a day on day −52, etoposide 200 mg/m2 intravenously on days −52 to −50, and dexa-methasone 10 mg orally four times a day, day −52 to −49. (2) Peripheral blood stem cell collection. (3) Autologous transplant following 12 Gy of linear accelerator radiation, etoposide 60 mg/kg, and cyclophosphamide 100 mg/kg. (4) Posttransplantation rituximab (375 mg/m2 ) was given on days +30 and +37. (5) GCSF 480 μg/day beginning on day +130 and doubled on day +134. (6) Two doses of intravenous immunoglobulin (IVIG). (7) GM-CSF, 250 μg/m2/day. (8) Cyclosporine 200 mg orally twice a day, tapered ultimately to 50 mg orally twice a day.
Possible offending agents were discontinued. Granulocyte colony–stimulating factor (GCSF) began followed by 2 doses of intravenous immunoglobulin and granulocyte-macrophage colony–stimulating factor (GM-CSF). Despite these maneuvers, absolute neutropenia persisted for longer than 65 days. Within 5 days of initiating therapy with cyclosporine (200 mg orally twice a day), the neutrophil count returned to normal levels. GM-CSF and GCSF were tapered with no decrement in neutrophil count. The patient continues on cyclosporine with stable neutrophil counts 14 months later. Attempts at dose reduction to 25 mg orally twice a day have persistently resulted in relapsed neutropenia suggesting ongoing autoimmune destruction of myeloid precursor cells.
This is the first reported case of agranulocytosis associated with rituximab therapy that was refractory to growth-factor support but responsive to cyclosporine. The efficacy of cyclosporine suggests a T-lymphocyte–specific cellular response against autologous myeloid precursors. Although cyclosporine has been used in the post–autologous stem cell transplantation setting to induce autologous graft-versus-host disease (GVHD), there are no reports of its use in the post–autologous transplantation setting for the treatment of cytopenias. Del(20)(q11.2) has been reported with myelodysplastic syndromes (MDSs) related to high-dose therapy and autologous transplantation for patients with non-Hodgkin lymphoma, and successful immunosuppressive therapy with cyclosporine for MDS has been reported.7-9 This patient, however, had no other features suggestive of MDS. This single case experience suggests that cyclosporine be considered in patients with rituximab-associated neutropenia not responsive to GCSF.