Abstract
Deficiency of von Willebrand factor (VWF) cleaving protease ADAMTS13 is associated with the development of thrombotic thrombocytopenic purpura (TTP). A case of congenital TTP that was previously reported to have normal ADAMTS13 activity was analyzed at the molecular level. Reanalysis of plasma VWF cleaving protease activity using a different assay revealed that the patient had less than 0.1 U/L ADAMTS13 protease activity, while the parents were both partially deficient. Sequence analysis of DNA amplified by polymerase chain reaction showed that the patient was homozygous for a novel TT deletion in exon 15 of the ADAMTS13 gene resulting in a frameshift, while both parents were heterozygous for the same mutation. Taken together with other recent reports, all the cases of hereditary TTP studied by DNA sequence analysis to date appear to be due to mutations within the ADAMTS13 gene.
Introduction
Thrombotic thrombocytopenic purpura (TTP), a potentially fatal disease, is characterized by thrombocytopenia, microangiopathic hemolysis, and, in the advanced cases, neurologic deficits. The presence of von Willebrand factor (VWF) on the platelet surface1 and in thrombi2 suggests that VWF-platelet binding causes the development of platelet-rich thrombi in TTP.
VWF, secreted from endothelial cells as a disulfide-bonded polymeric form, undergoes conformational unfolding when exposed to high levels of shear stress such as those in the arterioles and capillaries.3 The unfolded, elongated forms of VWF exhibit a higher capacity for supporting platelet aggregation4 but are also more susceptible to cleavage by the plasma metalloprotease ADAMTS13.5 The responsiveness of large VWF multimers to shear stress may explain why VWF is uniquely capable of supporting platelet adhesion under high shear conditions and why the size of VWF is a determinant of its hemostatic effectiveness. On the other hand, cleavage of unfolded VWF by ADAMTS13 prevents the accumulation of superactive forms of VWF, a step believed to be critical for preventing VWF-platelet binding in the circulation.
A lack of ADAMTS13 activity, caused by autoimmune inhibitors or genetic mutations, is detected in patients with TTP.5, 6, 7 Deficiency of ADAMTS13 explains why patients with TTP respond to plasma infusion. In patients with acquired TTP, plasma exchange is more effective because it allows the patients to receive a large amount of fresh-frozen plasma to overcome the inhibitors without imposing the risk of fluid overload. Removal of the inhibitors may also contribute to the greater efficacy of plasma exchange. Patients with congenital TTP typically respond to small amounts of plasma infusion because they do not have inhibitors of the protease.
Some investigators describe the presence of very low ADAMTS13 activity in patients without TTP.8, 9, 10, 11, 12, 13 A single previous study reported normal ADAMTS13 activity in a patient who had thrombocytopenia and microangiopathic hemolysis since early infancy that was responsive to plasma infusion.14 The reasons for the discrepancy among these various studies have not been adequately addressed.
In this report, we reinvestigate the case noted above,14 previously reported to have normal levels of VWF cleaving protease activity despite the presence of features that were characteristic of congenital TTP.
Study design
Case history
The course of this case has been described previously.14 Briefly, the patient, born to parents who were first cousins of Yemenite background, developed thrombocytopenia and microangiopathic hemolysis soon after birth. She required plasma infusion (10 mL/kg) every 2 weeks to maintain normal platelet counts and hemoglobin levels. Analysis of VWF cleaving protease activity using the technique of sodium dodecyl sulfate (SDS)–agarose gel electrophoresis and immunoblotting detected approximately 50% protease activity in the patient, but normal levels in her parents.15
Assay of ADAMTS13 activity
Plasma samples obtained from the patient prior to plasma transfusion and from her parents were used for analysis of VWF cleaving protease activity by using the technique of SDS–polyacrylamide gel electrophoresis and immunoblotting as previously described.6
Sequence analysis
All ADAMTS13 exons and intron-exon boundaries (except for exons 3, 4, 7, 8, 16, 19, and 25) were amplified by PCR from peripheral blood leukocyte DNA prepared from the patient and both parents as previously described.7 Sequencing reactions were performed by the University of Michigan DNA Sequencing Core. Approval was obtained from the Montefiore Medical Center and University of Michigan institutional review boards for these studies. Informed consent was provided according to the Declaration of Helsinki.
Results and discussion
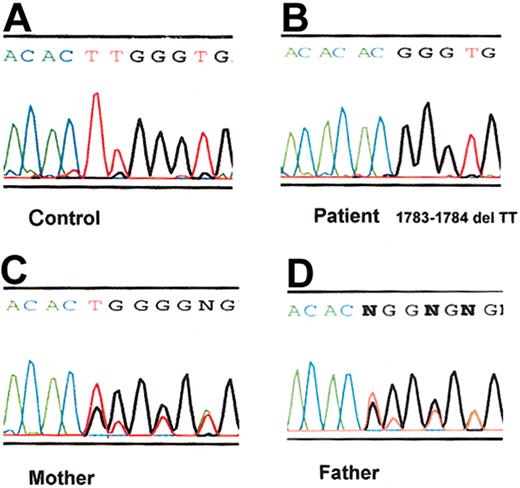
The VWF cleaving protease activity level was less than 0.1 U/mL in the patient and partially deficient in both parents (Figure 1). When a heat-treated plasma sample of the patient was incubated with normal control plasma, no inhibitors of the VWF cleaving activity were detected. Sequence analysis of PCR-amplified DNA products revealed that the patient was homozygous for a TT deletion near the end of exon 15. The deletion resulted in a shift in the reading frame and truncation of the predicted protein product. The parents were heterozygous for the same mutation (Figure 2).
ADAMTS13 activity levels in the plasma samples of the patient and of her parents. (A) An immunoblot depicts the generation of specific VWF fragments, the homodimers of the 176-kDa ([176 kDa]2) and the 140-kDa ([140 kDa]2) fragments,15 from the VWF substrate by ADAMTS13. NP indicates normal control plasma; I-1, the father; I-2, the mother; and II-1, the patient. Each sample was analyzed in the absence (–) or presence (+) of EDTA (ethylenediaminetetraacetic acid). (B) The pedigree depicts the ADAMTS13 activity levels of the father (I-1), the mother (I-2), and the patient (II-1). The values, expressed in U/mL (1 U/mL being the activity in the normal control plasma), are the means of 3 separate assays. The normal range of the assay is 0.79 to 1.27 U/mL.
ADAMTS13 activity levels in the plasma samples of the patient and of her parents. (A) An immunoblot depicts the generation of specific VWF fragments, the homodimers of the 176-kDa ([176 kDa]2) and the 140-kDa ([140 kDa]2) fragments,15 from the VWF substrate by ADAMTS13. NP indicates normal control plasma; I-1, the father; I-2, the mother; and II-1, the patient. Each sample was analyzed in the absence (–) or presence (+) of EDTA (ethylenediaminetetraacetic acid). (B) The pedigree depicts the ADAMTS13 activity levels of the father (I-1), the mother (I-2), and the patient (II-1). The values, expressed in U/mL (1 U/mL being the activity in the normal control plasma), are the means of 3 separate assays. The normal range of the assay is 0.79 to 1.27 U/mL.
ADAMTS13 mutation in the patient with congenital TTP. DNA sequence chromatograms corresponding to ADAMTS13 exon 15 are shown here for PCR-amplified genomic DNA from a healthy control (A), the patient (B), the patient's mother (C), and the patient's father (D). The patient DNA exhibits a homozygous 2-nucleotide (TT) deletion at the end of exon 15 (corresponding to position 1783-1784 of the ADAMTS13 cDNA), resulting in a frameshift after His594, followed by a predicted 18–amino acid extension and premature termination. The DNA chromatograms from both parents demonstrate superimposed normal and deletion allele sequences, indicating that they are heterozygous for this mutation.
ADAMTS13 mutation in the patient with congenital TTP. DNA sequence chromatograms corresponding to ADAMTS13 exon 15 are shown here for PCR-amplified genomic DNA from a healthy control (A), the patient (B), the patient's mother (C), and the patient's father (D). The patient DNA exhibits a homozygous 2-nucleotide (TT) deletion at the end of exon 15 (corresponding to position 1783-1784 of the ADAMTS13 cDNA), resulting in a frameshift after His594, followed by a predicted 18–amino acid extension and premature termination. The DNA chromatograms from both parents demonstrate superimposed normal and deletion allele sequences, indicating that they are heterozygous for this mutation.
In a case with characteristic features of hereditary TTP, or Shulman-Upshaw syndrome, we detected very low VWF cleaving protease activity (< 0.1 U/mL) in the patient and partial deficiency in her parents. DNA sequence analysis detected a novel 2–base pair deletion in the ADAMTS13 gene, confirming the genetic nature of the deficiency. The homozygous state of the patient is consistent with the known consanguinity between the parents (first cousins).
A recent report identified an inactivating mutation in the ADAMTS13 gene in all studied patients with hereditary TTP.7 Subsequent studies also described the presence of genetic defi-ciency in the VWF cleaving activity among patients with congenital TTP.16,17 This patient and her parents were previously reported to have normal levels of VWF cleaving activity.14 The presence of ultra-large multimers in the patient would have suggested the possibility of resistant VWF. Since mutations of VWF that increase its susceptibility to ADAMTS13 cause type 2A von Willebrand disease,18 it is conceivable that mutations decreasing the proteolytic susceptibility of the VWF may result in the presence of ultra-large multimers. According to the shear-stress model,4 such mutations could cause accumulation of unfolded, superactive forms of VWF in the circulation, resulting in VWF-platelet binding and platelet thrombosis such as that observed in patients with ADAMTS13 deficiency. However, patients with such mutations are unlikely to respond to a small amount of normal plasma. Thus, the plasma responsiveness of this patient makes such an explanation unlikely. In addition, no other protease regulating the size of VWF in the normal circulation has yet been identified as a potential second cause of TTP.
The assay used in this study differs from the assay used in the previous report of this case14 in that the VWF substrate was incubated directly with the plasma sample in the presence of 0.15 M guanidine hydrochloride, not dialyzed against 1.5 M urea; the incubation lasted for one hour instead of overnight; no barium chloride was added; and cleavage of VWF was detected by measuring the dimer of the 176-kDa fragment generated from cleavage of the VWF substrate by ADAMTS13 at the bond of Tyr842 and Met843 of the mature VWF instead of SDS–agarose gel electrophoresis and immunoblotting of VWF multimers. How and whether a difference in the experimental procedures contributed to the results previously obtained in this family remain to be explored. SDS–agarose gel electrophoresis could have detected a decrease in the size of VWF multimers caused by nonspecific adsorption of the large multimers on to surface in contact with the sample mixtures or by plasmin or other proteases that were generated during the long incubation. Also, the potential contribution of barium chloride to the assay variability is unknown.
In summary, this study confirms that hereditary TTP results from genetic deficiency of ADAMTS13, with gene mutations identified in all patients studied to date at the molecular level. Furthermore, the experience in this family highlights the importance of future research to establish a standardized, validated procedure for the measurement of ADAMTS13 enzymatic activity that can be readily adapted to the clinical laboratory.
Prepublished online as Blood First Edition Paper, February 6, 2003; DOI 10.1182/blood-2002-12-3796.
Supported in part by grants from the National Heart Lung and Blood Institute of the National Institutes of Health (R01 HL62136 to H.-M.T.; R01 HL39693 and P01 HL57346 to D.G.; and F32 HL71473 to S.-K.L.). D.G. is an investigator of the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr M. Furlan for advice and comments on the manuscript and S. Chang for technical assistance.

![Figure 1. ADAMTS13 activity levels in the plasma samples of the patient and of her parents. (A) An immunoblot depicts the generation of specific VWF fragments, the homodimers of the 176-kDa ([176 kDa]2) and the 140-kDa ([140 kDa]2) fragments,15 from the VWF substrate by ADAMTS13. NP indicates normal control plasma; I-1, the father; I-2, the mother; and II-1, the patient. Each sample was analyzed in the absence (–) or presence (+) of EDTA (ethylenediaminetetraacetic acid). (B) The pedigree depicts the ADAMTS13 activity levels of the father (I-1), the mother (I-2), and the patient (II-1). The values, expressed in U/mL (1 U/mL being the activity in the normal control plasma), are the means of 3 separate assays. The normal range of the assay is 0.79 to 1.27 U/mL.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/11/10.1182_blood-2002-12-3796/5/m_h81134420001.jpeg?Expires=1769205431&Signature=Wpp2P1wQxY~qvBBzN2KkXCtT1hirjWyTPEswUK3ZZvyII911vXwQyP8OnHK6Wr5XN98gW5q146mb27AL0DaeYk8v3XH72-kwycdvs86Bf~C95zMdnxAmTx~ku~50GAGFyfhhPEJh-SrqlNgH8SLjtecOPxOIzKFsIoUCjF5tPtjj3ZOdHTAesvBg7Z1HOFJJgrarL9etzvckov929zOwVciKYO3p6LZ~49ZnWcnTcCzB0OLZ7woy0Ocpr9BBxSBLjYX~TrYEic4U0XS5sZdeuVbC6QvNV7jxzRs3pTvNvg67FSA3lhD~rJpSlaEhxC9stCBr3AQtNJmMvxoV7y7DEA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)