Abstract
Rapamycin (RAPA) is a potent immunosuppressive macrolide hitherto believed to mediate its action primarily via suppression of lymphocyte responses to interleukin 2 (IL-2) and other growth factors. We show here that this view is incomplete and provide evidence that RAPA suppresses the functional activation of dendritic cells (DCs) both in vitro and in vivo. In vitro, RAPA inhibits IL-4—dependent maturation and T-cell stimulatory activity of murine bone marrow—derived DCs. These effects are associated with posttranscriptional down-regulation of both subunits of the IL-4 receptor complex (CD124, CD132) and are mediated via binding of RAPA to its intracellular receptor FK506-binding protein 12 (FKBP12). In vivo, RAPA impairs steady-state DC generation and fms-like tyrosine 3 kinase ligand (Flt3L)—induced DC mobilization. In addition, in vivo administration of RAPA impairs DC costimulatory molecule up-regulation, production of proinflammatory cytokines, and T-cell allostimulatory capacity. These novel findings have implications for RAPA-based therapy of chronic DC-triggered autoimmune diseases, transplant rejection, and hematologic malignancies with activating Flt3 mutations.
Introduction
Dendritic cells (DCs) are uniquely well-equipped professional antigen-presenting cells that play critical roles in the initiation and regulation of immune responses.1 They are distributed widely in peripheral tissues where they capture foreign or self antigens. DCs rapidly process and convey these antigens to secondary lymphoid organs2,3 where they prime antigen-specific T cells. The ability of DCs to initiate an immune response depends on their transition from antigen-processing to antigen-presenting cells, during which they up-regulate cell surface class II major histocompatibility complex (MHC) and T-cell costimulatory molecules (CMs; CD40, CD80, CD86), a process referred to as DC maturation.1,4 This transition constitutes an important checkpoint in mounting an immune response because immature DCs not only fail to prime T cells effectively,5,6 but also serve to promote tolerance induction.7, 8, 9, 10
Rapamycin (RAPA) is a bacterial macrolide antibiotic with potent immunosuppressive action introduced in recent years as antirejection therapy in organ transplantation.11,12 RAPA forms a complex with the intracellular immunophilin FK506-binding protein 12 (FKBP12). This complex inhibits the function of the serine/threonine kinase target of RAPA (mammalian; mTOR), a common effector protein shared by several signal transduction pathways.13 Inhibition of mTOR results in suppression of cytokine-driven cell proliferation, ribosomal protein synthesis, translation initiation, and cell cycle arrest at the G1 phase.13,14 T cells have been considered the principal therapeutic targets of RAPA, as well as other immunosuppressive agents.15 A much less studied aspect is the possible impact of RAPA on DCs and their ability to present antigen to T cells prior to antigen-specific lymphocyte activation and proliferation.
In this study, we have systematically investigated the influence of RAPA on DC function, both in vitro and in vivo. Our results reveal that RAPA interferes with DC function at various levels, impairing immune reactivity at the earliest stages. We also show that RAPA blocks the in vivo effects of fms-like tyrosine 3 kinase ligand (Flt3L), a potent endogenous DC growth factor that also regulates the proliferation of hematopoietic precursor/stem cells and monocytic precursors.16,17 These novel insights into the action of RAPA are likely to aid the development of this and other agents that impact on DC function for the treatment of transplant rejection, autoimmune diseases, and possibly certain types of hematologic malignancies.
Materials and methods
Animals
Eight- to 12-week-old C57BL/10 (B10; H2Kb) and C3H/HeJ (C3H; H2Kk) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained in the pathogen-free Central Animal Facility of the University of Pittsburgh Medical Center. Interleukin 4 (IL-4) receptor α-deficient mice (BALB/c background)18 were obtained from the Institute for Clinical Microbiology and Immunology at the University of Erlangen (Erlangen, Germany).
Generation of BM-derived DCs
Bone marrow (BM) DCs were generated as described.19 Briefly, B10 BM cells were cultured for 7 days in RPMI 1640 with 10% heat-inactivated fetal calf serum (FCS), l-glutamine, nonessential amino acids, sodium pyruvate, penicillin-streptomycin, HEPES (N-2-hydroxyethylpiperazine-N′-2-ethane-sulfonic acid), 2-mercaptoethanol (2-ME; all from Life Technologies, Gaithersburg, MD), 1000 U/mL murine granulocyte-macrophage colony-stimulating factor (GM-CSF; Schering-Plough, Kenilworth, NJ) with or without 1000 U/mL murine IL-4 (R & D Systems, Minneapolis, MN). At day 2, 1 to 100 ng/mL RAPA (Sigma, St Louis, MO) with or without 10 to 100 ng/mL FK506 (Prograf for intravenous use, Fujisawa Healthcare, Deerfield, IL) was added. Every 2 days, 75% supernatant was replaced with fresh cytokine-containing medium (with or without RAPA or FK506). On day 4, nonadherent cells were removed; on day 7, 50% or more of the nonadherent cells expressed CD11c.
Phenotypic analysis of DCs
DC surface antigen expression was analyzed by flow cytometry on day 7 of BM culture and in freshly and stimulated BM/spleen cells isolated from injected (with or without RAPA, with or without vehicle, with or without Flt3L) mice. Stimulation was performed with lipopolysaccharide (LPS; 50 ng/mL, Escherichia coli serotype 026:B6; Sigma) in the presence of low-dose GM-CSF (50 U/mL RPMI culture medium) for 16 hours at 37°C. Fluorescein isothiocyanate (FITC)—, phycoerythrin (PE)—, CyChrome-conjugated or biotinylated monoclonal antibodies (mAbs) used to detect expression of CD11c (HL3), CD40 (HM40-3), CD54 (intercellular adhesion molecule 1 [ICAM-1]; 3E2), CD80 (16-10A1), CD86 (GL1), IAb β chain (25-9-17), H2Kb (AF6-88.5), CD124 (Institute for Clinical Microbiology and Immunology, University of Erlangen, Germany) or CD132 (TUGm2), as well as isotype-matched control mAbs and streptavidin-CyChrome, were purchased from BD Pharmingen (San Diego, CA), unless otherwise noted. Cells (5 × 105) were blocked with 10% vol/vol normal goat serum (10 minutes; 4°C) then stained with mAb (30 minutes; 4°C). Appropriate isotype-matched immunoglobulins were used as negative controls. The cells were analyzed using an EPICS Elite flow cytometer (Beckman Coulter, Hialeah, FL).
RNase protection assay
The procedure adopted for RNase protection assay was performed as described in detail.20 Briefly, RNA was isolated from 5 × 106 snap-frozen, magnetic bead—sorted DCs using a total RNA isolation kit (BD Pharmingen). RNase protection assay was performed using the RiboQuant Multi-Probe RPA system (BD Pharmingen) with 32P-uridine triphosphate (UTP)— labeled antisense RNA probes specific for CD124, CD132, and the housekeeping genes L32 and glyceraldehyde-3-phosphatedehydrogenase (GAPDH) according to the manufacturer's instructions. Mouse RNA and RNA degradation controls were included. Yeast tRNA served as negative control.
Analysis of apoptosis
DCs were stimulated with LPS (1 μg/mL RPMI culture medium) or left without any stimuli and apoptosis was analyzed over time by staining of phosphatidylserine translocation with FITC-annexin V in combination with the vital dye 7-amino-actinomycin D (7-AAD; BD Pharmingen) according to the manufacturer's instructions. Cells were costained for CD11c to allow specific analysis of DC by flow cytometry.
In vivo DC expansion and RAPA administration
The in vivo effects of RAPA were investigated in healthy animals and in mice in which DCs were expanded by administration of rhuFlt3L (CHO cell derived; 10 μg/d, intraperitoneally, days 1-10; Immunex, Seattle, WA). RAPA (Wyeth-Ayerst, Princeton, NJ) was dissolved in 51% PEG300, 5% polysorbate 80, 5% ethanol (vehicle, all reagents from Sigma). Mice were injected with RAPA (0.5 mg/kg/d, intraperitoneally) or vehicle for 7 or 10 days (days 3-10; days 1-10). Due to the long elimination half-life, mice received a loading dose on day 1 (1.5 mg/kg), according to the recommendation of Mahalati and Kahan.21
DC isolation and purification
Spleens were injected with 100 U/mL type IV collagenase (Sigma) in RPMI, and disrupted and chopped with fine scissors, and the resulting cell suspension kept at 4°C. The remaining tissue fragments were digested in 400 U/mL collagenase/RPMI solution for 45 minutes at 37°C. Finally, the cells were pooled, passed through a strainer, and washed in sterile, ice-cold, Ca+-free phosphate-buffered saline (PBS). DCs were further enriched by density gradient centrifugation using 16% wt/vol metrizamide (Sigma) in PBS at 1200g for 20 minutes at 4°C. BM cells were isolated from femurs and tibias and subjected to density gradient centrifugation. To obtain highly purified DC populations for analysis of allostimulatory activity, cytokine production, or their adoptive transfer, the cells were labeled with magnetic bead—conjugated anti-CD11c mAb (Miltenyi Biotec, Auburn, CA) followed by positive selection through paramagnetic columns (LS columns, Miltenyi Biotec) according to the manufacturer's instructions. DC purity of 90% to 95% was consistently achieved.
Cytokine quantitation and allostimulatory activity
Production of IL-12p70 and tumor necrosis factor α (TNF-α) was measured in 24-hour supernatants of LPS-stimulated (2 μg/mL), immunomagnetic bead—purified DCs (106/mL) using enzyme-linked immunosorbent assay (ELISA) kits (Quantikine, BD Pharmingen). LPS stimulation was performed in the presence of low-dose GM-CSF (50 U/mL RPMI culture medium). IL-2, IL-4, IL-10, and interferon γ (IFN-γ) were quantified in 72-hour supernatants of mixed leukocyte reaction (MLR) cultures using reagents and procedures recommended by the manufacturer (BD Pharmingen). Graded numbers of γ-irradiated (20 Gy), magnetic bead—sorted B10 DCs were used as stimulators in 72-hour MLRs with nylon-wool column-purified allogeneic (C3H) splenic T cells as responders (2 × 105/mL) as described.19
Statistical analysis
Statistical analysis was performed using Student t test or the Wilcoxon rank sum test. All tests were performed 2-tailed; P < .05 was considered significant. Normal distribution of values, a prerequisite for using the Student t test, was established by using the Kolmogorov-Smirnov test.
Results
RAPA inhibits maturation of BM-derived DCs in an IL-4—dependent manner
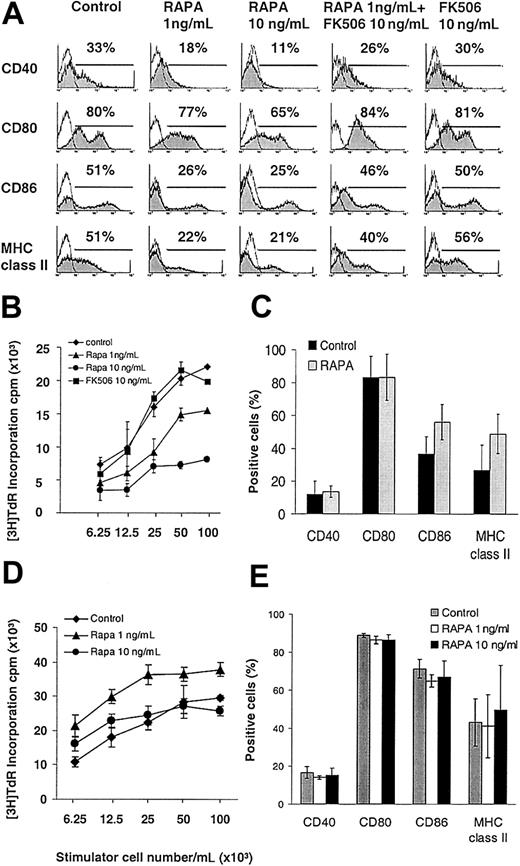
RAPA concentrations within the human whole blood trough therapeutic range22 decreased surface expression of CD40, CD80, CD86, and MHC class II molecules on GM-CSF plus IL-4— expanded DCs harvested on day 7 of culture (Figure 1A). The T-cell allostimulatory activity of purified DCs was also markedly impaired by RAPA in a dose-related manner, whereas the structurally related immunosuppressant FK506 had no effect on DC phenotype or function (Figure 1A-B). RAPA, when added late at day 5 of culture, inhibited DC maturation similarly as compared to day 2, though to a slightly lesser extent (eg, 57% of control versus 30% of RAPA group expressing MHC class II, 50% versus 32% expressing CD86, and 47% versus 16% expressing CD40), ruling out the possibility that it affects the generation of a certain DC subtype in cultures.
The inhibitory effect of RAPA on DC maturation is IL-4 dependent and mediated via FKBP-12 binding. BM-derived DCs were generated with GM-CSF with or without IL-4 and analyzed on day 7. (A-B) In the presence of IL-4, RAPA inhibited the cell surface expression of CD40, CD80, CD86, and MHC class II molecules and the allostimulatory activity of purified CD11c+ DCs, whereas FK506 exhibited no effect. Competition for RAPA's intracellular receptor FKBP12 by a molar excess of FK506 (panel A, second column from right) antagonized the inhibitory effects of RAPA on DC maturation. (C-E) In the absence of IL-4 (C-D), or in IL-4Rα—deficient mice (E), RAPA exerted no inhibitory effect on DC surface expression of CD40, CD80, CD86, MHC class II molecules, or T-cell allostimulatory acitivity. (A,C,E) Cells were gated on CD11c. The incidence of CD11c+ cells expressing the antigen of interest is indicated. Results show representative data from 10 (A), 3 (B,E), 5 (C), and 2 (D) similar experiments.
The inhibitory effect of RAPA on DC maturation is IL-4 dependent and mediated via FKBP-12 binding. BM-derived DCs were generated with GM-CSF with or without IL-4 and analyzed on day 7. (A-B) In the presence of IL-4, RAPA inhibited the cell surface expression of CD40, CD80, CD86, and MHC class II molecules and the allostimulatory activity of purified CD11c+ DCs, whereas FK506 exhibited no effect. Competition for RAPA's intracellular receptor FKBP12 by a molar excess of FK506 (panel A, second column from right) antagonized the inhibitory effects of RAPA on DC maturation. (C-E) In the absence of IL-4 (C-D), or in IL-4Rα—deficient mice (E), RAPA exerted no inhibitory effect on DC surface expression of CD40, CD80, CD86, MHC class II molecules, or T-cell allostimulatory acitivity. (A,C,E) Cells were gated on CD11c. The incidence of CD11c+ cells expressing the antigen of interest is indicated. Results show representative data from 10 (A), 3 (B,E), 5 (C), and 2 (D) similar experiments.
Next we analyzed whether the inhibition of DC maturation by RAPA was IL-4 dependent because IL-4 promotes DC maturation6,23 and performed similar experiments in the absence of IL-4. The results (Figure 1C) demonstrated that the suppressive effect of RAPA on DC maturation was IL-4 dependent because no significant inhibition of up-regulation of MHC class II or CMs was observed in the absence of the cytokine. Additionally, the allostimulatory capacity of RAPA DCs generated without IL-4 was also not suppressed (Figure 1D). DC maturation was also not impaired when BM-derived DCs from IL-4 receptor α (IL-4Rα)—deficient animals were expanded with GM-CSF plus IL-4 in the presence of RAPA (Figure 1E). These data indicate that RAPA inhibited IL-4—mediated DC maturation. Importantly, in all experiments cells were gated over the DC-specific marker CD11c to analyze costimulatory molecule expression specifically on DCs and to rule out confounding effects due to different total numbers of DCs.
RAPA down-regulates posttranscriptional expression of the functional IL-4R complex on DCs
To elucidate the underlying mechanism of IL-4—mediated DC maturation inhibition in the presence of RAPA, we investigated expression of both chains of the functional IL-4R complex on purified DCs at both the transcriptional and posttranscriptional levels by RNase protection assay and flow cytometry, respectively. The high-affinity functional IL-4R is a heterodimer composed of the IL-4Rα chain (IL-4Rα; CD124) and the common cytokine receptor γ chain (CD132). The results revealed that while RAPA suppressed cell surface expression of both CD124 and CD132 subunits, their mRNA expression was not significantly altered (Figure 2). These data suggest that RAPA targets IL-4—mediated myeloid DC maturation via posttranscriptional inhibition of both chains of the functional IL-4R complex and is in agreement with RAPA's potent inhibitory effects on protein translation.13,14
RAPA suppresses DC high-affinity IL-4R complex expression at the posttranscriptional level. BM-derived DCs were generated with GM-CSF plus IL-4, purified by immunomagnetic bead sorting and subjected to RNase protection assay or directly analyzed by flow cytometry. (A) Comparative RNase protection assay analysis indicates no effect of RAPA on CD124 or CD132 mRNA expression. L32 and GAPDH represent internal controls. (B-C) Down-regulation of CD124 and CD132 cell surface expression by RAPA. Cells were gated on CD11c. The incidence of CD11c+ cells expressing the antigen of interest is indicated. Results show representative data of 3 (A-B) and 5 (C) experiments.
RAPA suppresses DC high-affinity IL-4R complex expression at the posttranscriptional level. BM-derived DCs were generated with GM-CSF plus IL-4, purified by immunomagnetic bead sorting and subjected to RNase protection assay or directly analyzed by flow cytometry. (A) Comparative RNase protection assay analysis indicates no effect of RAPA on CD124 or CD132 mRNA expression. L32 and GAPDH represent internal controls. (B-C) Down-regulation of CD124 and CD132 cell surface expression by RAPA. Cells were gated on CD11c. The incidence of CD11c+ cells expressing the antigen of interest is indicated. Results show representative data of 3 (A-B) and 5 (C) experiments.
The inhibitory effects of RAPA on DC maturation are not due to increased apoptosis
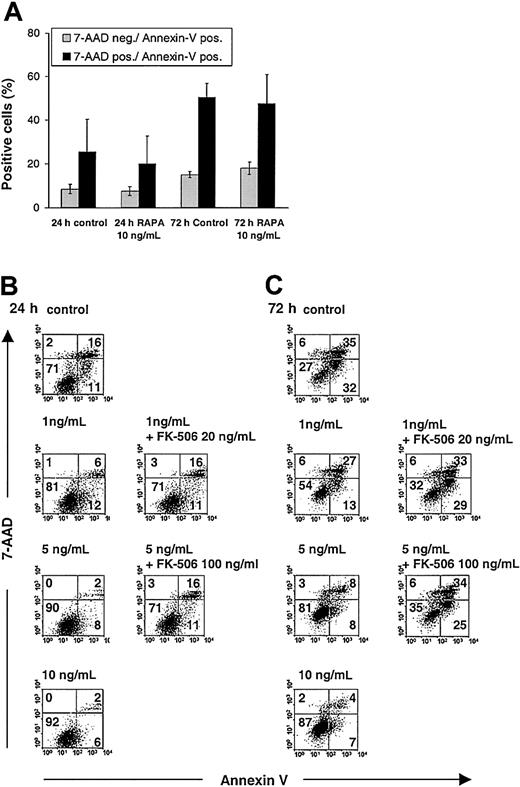
Inhibition of IL-4—mediated DC maturation does not exclude the possibility that the observed effects of RAPA were related to increased DC apoptosis.24 We have found that apoptosis of BM-derived DCs cultured in the presence of RAPA at different time points is consistently less than 10%, as evidenced by annexin V/7-AAD and TUNEL staining of CD11c+ DCs.25 To ascertain whether RAPA increases the susceptibility of DCs to apoptosis induction, we either stimulated DCs propagated in GM-CSF plus IL-4 with LPS only (in the absence of cytokines) or cultured DCs with medium alone in the absence of any stimuli (LPS, cytokines), then analyzed apoptosis in CD11c+ DCs, 24 and 72 hours later by annexin V/7-AAD staining. The results show that RAPA inhibited DC death when cells were stimulated for an extended time with LPS only (Figure 3B-C) but did not significantly affect DC death when cells were cultured for an extended time in the absence of LPS (Figure 3A). Thus, it is unlikely that in these experiments RAPA acts primarily on DCs via apoptosis induction.
Analysis of RAPA effects on apoptosis in DCs. RAPA inhibits apoptosis of LPS-stimulated DCs in an FKBP12-dependent manner but does not affect DC apoptosis when cells are cultured for an extended time in the absence of any stimuli (LPS, cytokines). BM-derived DCs were generated with GM-CSF plus IL-4 and washed, and equal numbers of cells were stimulated on day 7 with LPS only or cultured without any stimuli and analyzed after staining of phosphatidylserine translocation with FITC-annexin V in combination with the vital dye 7-AAD. (A) RAPA does not affect apoptosis and death when DCs are cultured for an extended time in the absence of any stimuli. (B-C) RAPA inhibits DC apoptosis and death in a dose-and time-dependent manner when DCs are stimulated with LPS. Competition for RAPA's intracellular receptor FKBP12 by a molar excess of FK506 blocks the inhibitory effect of RAPA on DC apoptosis and death. Doses indicate RAPA, unless stated otherwise. Cells were gated on CD11c. The incidence of cells in each quadrant is indicated. Apoptotic cells are stained annexin V+/7-AAD– and dead cells (late apoptotic or necrotic) are stained annexin V+/7-AAD+. Results show representative data from 3 (A) and 4 (B-C) experiments. Similar results were obtained with DCs generated in GM-CSF only.
Analysis of RAPA effects on apoptosis in DCs. RAPA inhibits apoptosis of LPS-stimulated DCs in an FKBP12-dependent manner but does not affect DC apoptosis when cells are cultured for an extended time in the absence of any stimuli (LPS, cytokines). BM-derived DCs were generated with GM-CSF plus IL-4 and washed, and equal numbers of cells were stimulated on day 7 with LPS only or cultured without any stimuli and analyzed after staining of phosphatidylserine translocation with FITC-annexin V in combination with the vital dye 7-AAD. (A) RAPA does not affect apoptosis and death when DCs are cultured for an extended time in the absence of any stimuli. (B-C) RAPA inhibits DC apoptosis and death in a dose-and time-dependent manner when DCs are stimulated with LPS. Competition for RAPA's intracellular receptor FKBP12 by a molar excess of FK506 blocks the inhibitory effect of RAPA on DC apoptosis and death. Doses indicate RAPA, unless stated otherwise. Cells were gated on CD11c. The incidence of cells in each quadrant is indicated. Apoptotic cells are stained annexin V+/7-AAD– and dead cells (late apoptotic or necrotic) are stained annexin V+/7-AAD+. Results show representative data from 3 (A) and 4 (B-C) experiments. Similar results were obtained with DCs generated in GM-CSF only.
Suppressive effects of RAPA on DC maturation and apoptosis are antagonized by competition for FKBP12 binding
To reveal whether the suppression of DC maturation and apoptosis were specific RAPA-related effects, we performed additional control experiments in the presence of a molar excess of the immunophilin ligand FK506. This structurally similar macrolide competes for RAPA's intracellular receptor FKBP12 and thus prevents specific interaction of the RAPA-FKBP12 complex with mTOR and subsequent inhibition of TOR signaling.13 Whereas FK506 alone did not interfere with these processes, addition of a 10-fold or more molar excess of FK506 antagonized the inhibitory action of RAPA on DC maturation (Figure 1A) and DC apoptosis (Figure 3B-C), indicating that these effects were mediated by binding of RAPA to its intracellular receptor FKBP12. However, the combination of RAPA and FK506 did not always give the same costimulatory expression profile compared with the control condition, although the percentages of positive cells were very similar (Figure 1A). In our view, this observation might indicate the presence of residual RAPA-related effects that interfere with DC maturation and are not antagonized completely by a molar excess of FK506.
RAPA suppresses DC generation in vivo
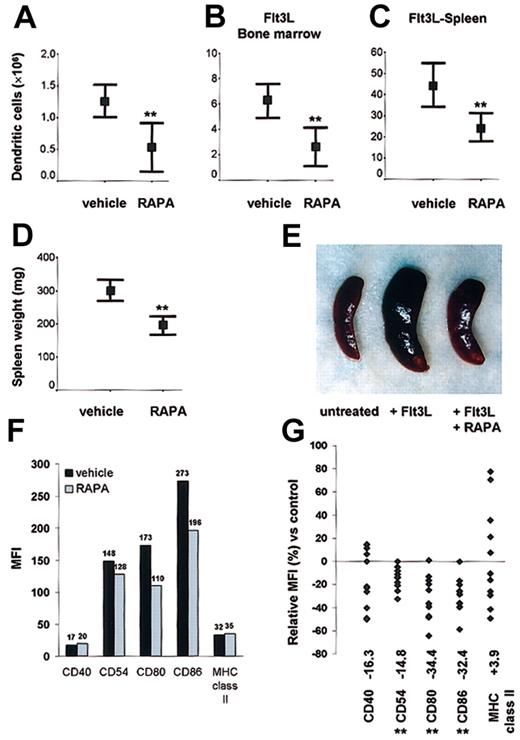
To address its in vivo effects, we first analyzed the impact of RAPA on DC generation in normal mice. Mice were injected with 0.5 mg/kg/d RAPA for 10 days; then DCs in spleen were purified and quantitated. RAPA administration significantly reduced (> 50%) the number of DCs in comparison with animals injected with the drug vehicle (Figure 4A). To explore the effects of RAPA on DC expansion in vivo under dynamic conditions, we expanded DCs, injecting the endogenous DC growth factor Flt3L for 10 days in combination with either RAPA or vehicle. Using this model, we confirmed that RAPA impaired DC expansion, as evidenced by a 40% to 50% reduction in BM and spleen DC numbers (Figure 4B-C). In Flt3L-treated animals, the inhibitory effect of RAPA on DC expansion was apparent from the appearance and significant reduction in weights of the spleens (Figure 4D-E). The rate of apoptosis in DCs freshly isolated from RAPA-treated animals was consistently low (≤ 8%, n = 3/group).
DC generation in steady-state and dynamic conditions. In vivo administration of RAPA suppresses DC generation under (A) steady-state and (B-C) dynamic conditions and inhibits up-regulation of costimulatory molecules. (A-C) Effect of RAPA or drug vehicle on the number of CD11c+ DCs/tissue on day 10 (with or without Flt3L). Results are representative of 8 to 10 animals/treatment group. **P = .005 versus vehicle (A, normal spleen), P = .003 versus vehicle (B, Flt3L bone marrow), P = .002 versus vehicle (C, Flt3L spleen); 2-tailed Student t test. Bars indicate 95% confidence interval and mean (rectangle). (D-E) Effect of RAPA or drug vehicle on spleen weight and appearance (8 animals/treatment group) in Flt3L-treated animals on day 10. **P = .0004 versus vehicle, 2-tailed Student t test. Bars indicate 95% confidence interval and mean (rectangle). (F-G) Effect of in vivo RAPA or drug vehicle administration on costimulatory and MHC class II molecule up-regulation after ex vivo LPS stimulation. Cells were gated on CD11c. The median fluorescence intensity (MFI) (F) and relative MFI (G) of CD11c+ cells expressing the antigen of interest in comparison with cells from drug vehicle-treated control animals is indicated. (F) Typical data from one representative experiment on day 10 after start of treatment. (G) Each point represents a single experiment with 3 to 6 animals (with or without Flt3L) per treatment group after in vivo administration of RAPA (7-10 days) or vehicle. **P < .01 versus vehicle (Wilcoxon test).
DC generation in steady-state and dynamic conditions. In vivo administration of RAPA suppresses DC generation under (A) steady-state and (B-C) dynamic conditions and inhibits up-regulation of costimulatory molecules. (A-C) Effect of RAPA or drug vehicle on the number of CD11c+ DCs/tissue on day 10 (with or without Flt3L). Results are representative of 8 to 10 animals/treatment group. **P = .005 versus vehicle (A, normal spleen), P = .003 versus vehicle (B, Flt3L bone marrow), P = .002 versus vehicle (C, Flt3L spleen); 2-tailed Student t test. Bars indicate 95% confidence interval and mean (rectangle). (D-E) Effect of RAPA or drug vehicle on spleen weight and appearance (8 animals/treatment group) in Flt3L-treated animals on day 10. **P = .0004 versus vehicle, 2-tailed Student t test. Bars indicate 95% confidence interval and mean (rectangle). (F-G) Effect of in vivo RAPA or drug vehicle administration on costimulatory and MHC class II molecule up-regulation after ex vivo LPS stimulation. Cells were gated on CD11c. The median fluorescence intensity (MFI) (F) and relative MFI (G) of CD11c+ cells expressing the antigen of interest in comparison with cells from drug vehicle-treated control animals is indicated. (F) Typical data from one representative experiment on day 10 after start of treatment. (G) Each point represents a single experiment with 3 to 6 animals (with or without Flt3L) per treatment group after in vivo administration of RAPA (7-10 days) or vehicle. **P < .01 versus vehicle (Wilcoxon test).
In vivo administration of RAPA impairs up-regulation of costimulatory but not MHC class II molecules on DCs
Freshly isolated DCs from RAPA and vehicle-injected animals displayed an immature phenotype. To test whether in vivo administration of RAPA affected the up-regulation of CMs, we stimulated DCs from RAPA- or vehicle-treated animals with LPS (50 ng/mL). DCs from RAPA-injected animals showed significantly impaired (P < .01) up-regulation of the CM CD54, CD80, CD86, whereas the expression of MHC class II was unaffected (Figure 4F-G). This pattern of reduced CM expression but unaffected MHC class II expression in response to LPS was detected reproducibly in spleen and BM DCs from both normal and Flt3L-treated animals. The absence of a significant inhibitory effect and the broad variation of MHC class II expression after RAPA administration might be related to DC activation during ex vivo preparation of the cells that was performed in the absence of RAPA to strictly analyze only the in vivo effects of drug administration.
In vivo RAPA administration impairs DC T-cell stimulatory activity in vitro and after adoptive transfer
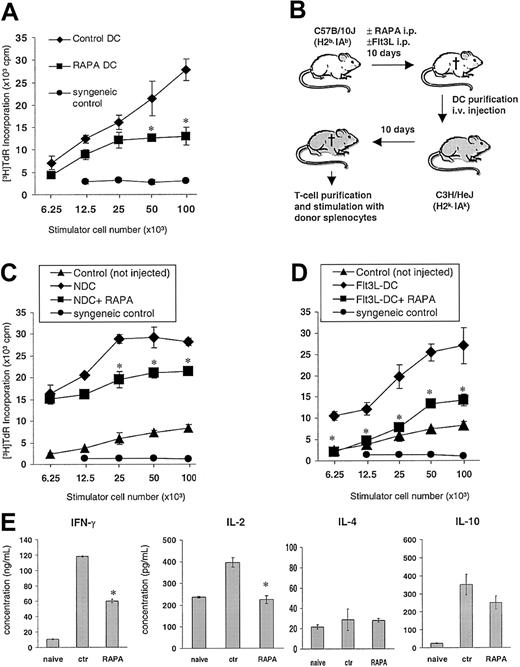
To assess the in vivo effects of RAPA on the capacity of DCs to stimulate T cells on a per cell basis, the animals were treated with 0.5 mg/kg/d RAPA or vehicle (7-10 days) and DCs purified by density gradient centrifugation and immunomagnetic-bead sorting to more than 90% purity as determined by CD11c+ staining. DCs generated in vivo in the presence of RAPA were found to be less efficient stimulators of fully allogeneic naive C3H T cells in 72-hour MLRs (Figure 5A). Next, we investigated the in vivo priming ability of DCs isolated from RAPA-injected animals by performing adoptive intravenous transfer of 5 × 105 purified DCs into naive allogeneic C3H recipients (Figure 5B). Control recipients were injected with DCs purified from age- and sex-matched control mice that had been injected with drug vehicle. Two weeks later, the animals were killed, and recipient T cells were restimulated with donor splenocytes. T cells of mice that had been injected with DCs purified from RAPA-treated donors showed markedly reduced T-cell proliferative responses compared with controls, indicating that RAPA impaired the in vivo priming efficacy of the DCs (Figure 5C-D). These T cells displayed significantly reduced IL-2 and IFN-γ production, whereas secretion of IL-4 and IL-10 was not significantly affected in response to restimulation with donor alloantigens (Figure 5E).
In vivo administration of RAPA impairs DC T-cell stimulatory activity. (A) Allostimulatory activity of freshly isolated, immunomagnetic bead—purified B10 (H2Kb,IAb) DCs from animals (3/group) injected with RAPA (10 days) or drug vehicle (“vehicle DC”). Mean proliferative activity of fully allogeneic C3H (H2Kk, IAk) responder T cells in 72-hour MLRs is shown (± SD). (B-E) Adoptive transfer of freshly isolated splenic B10 DCs from animals (panel C, normal; panels D and E, Flt3L-treated) that were injected with RAPA (7 days) or vehicle into fully allogeneic C3H recipients. C3H mice received 5 × 105 magnetic bead—purified B10 DCs (intravenously). Ten days later, the mice were killed and splenic T cells restimulated with graded numbers of γ-irradiated donor splenocytes. (C-D) Mean proliferation of C3H responder T cells (3 animals/group) in 72-hour MLRs is shown (± SD). T cells from nonimmunized animals (“naive”), from animals given DCs from drug vehicle-injected controls, as well as syngeneic splenocytes (“syngeneic control”) were used as controls. (E) Effect of adoptive transfer of DCs on IFN-γ, IL-2, IL-4, and IL-10 production by recipient T cells after restimulation with donor alloantigen (splenocytes). Mean cytokine production of C3H responder T cells (3 animals/group) in a 72-hour MLR is shown (± SD). *P < .05 versus vehicle (2-tailed Student t test).
In vivo administration of RAPA impairs DC T-cell stimulatory activity. (A) Allostimulatory activity of freshly isolated, immunomagnetic bead—purified B10 (H2Kb,IAb) DCs from animals (3/group) injected with RAPA (10 days) or drug vehicle (“vehicle DC”). Mean proliferative activity of fully allogeneic C3H (H2Kk, IAk) responder T cells in 72-hour MLRs is shown (± SD). (B-E) Adoptive transfer of freshly isolated splenic B10 DCs from animals (panel C, normal; panels D and E, Flt3L-treated) that were injected with RAPA (7 days) or vehicle into fully allogeneic C3H recipients. C3H mice received 5 × 105 magnetic bead—purified B10 DCs (intravenously). Ten days later, the mice were killed and splenic T cells restimulated with graded numbers of γ-irradiated donor splenocytes. (C-D) Mean proliferation of C3H responder T cells (3 animals/group) in 72-hour MLRs is shown (± SD). T cells from nonimmunized animals (“naive”), from animals given DCs from drug vehicle-injected controls, as well as syngeneic splenocytes (“syngeneic control”) were used as controls. (E) Effect of adoptive transfer of DCs on IFN-γ, IL-2, IL-4, and IL-10 production by recipient T cells after restimulation with donor alloantigen (splenocytes). Mean cytokine production of C3H responder T cells (3 animals/group) in a 72-hour MLR is shown (± SD). *P < .05 versus vehicle (2-tailed Student t test).
In vivo administration of RAPA promotes IL-4 hyporesponsiveness of DCs and dramatically impairs TNF-α secretion
Based on our in vitro finding indicating that RAPA only affected IL-4—dependent DC maturation, we hypothesized that DCs generated in vivo might be hyporesponsive to IL-4. To address this question, we took account of the finding that IL-4 is a major inducer of bioactive IL-12p70 production in DCs.26 Splenic DCs harvested from animals injected with drug vehicle then stimulated with increasing IL-4 concentrations in the presence of LPS exhibited a striking increase in IL-12p70 production, in agreement with Hochrein et al.26 By contrast, when DCs from RAPA-injected animals were stimulated in the same manner, IL-4—induced production of IL-12p70 was abrogated, in an IL-4—dependent manner (Figure 6A-B). We then analyzed production of TNF-α, a second major proinflammatory cytokine induced in DCs by LPS stimulation. In contrast to the IL-12p70 data, TNF-α production by purified DCs was dramatically impaired at all IL-4 concentrations. This finding (Figure 6C) suggested IL-4—independent suppression of TNF-α production by RAPA.
In vivo administration of RAPA promotes IL-4 hyporesponsiveness of DCs and suppresses TNF-α production. Animals were treated with RAPA (▴) or vehicle (plus Flt3L, 10 days; ▪). Splenic DCs were purified by density gradient centrifugation and immunomagnetic bead sorting and stimulated with LPS for 24 hours. (A-B) Titration of the effect of IL-4 (in the presence of LPS) on the production of bioactive IL-12p70. Mean IL-12p70 production (± 1 SD) by DCs from mice given RAPA for 7 days (A) and 10 days (B) versus drug vehicle-injected controls (3 animals/group). (C) Effect of RAPA on TNF-α production. Mean TNF-α production (± 1 SD) by DCs from animals that received RAPA for 10 days versus drug vehicle-injected controls (3 animals/group). *P < .05 versus vehicle (2-tailed Student t test).
In vivo administration of RAPA promotes IL-4 hyporesponsiveness of DCs and suppresses TNF-α production. Animals were treated with RAPA (▴) or vehicle (plus Flt3L, 10 days; ▪). Splenic DCs were purified by density gradient centrifugation and immunomagnetic bead sorting and stimulated with LPS for 24 hours. (A-B) Titration of the effect of IL-4 (in the presence of LPS) on the production of bioactive IL-12p70. Mean IL-12p70 production (± 1 SD) by DCs from mice given RAPA for 7 days (A) and 10 days (B) versus drug vehicle-injected controls (3 animals/group). (C) Effect of RAPA on TNF-α production. Mean TNF-α production (± 1 SD) by DCs from animals that received RAPA for 10 days versus drug vehicle-injected controls (3 animals/group). *P < .05 versus vehicle (2-tailed Student t test).
Discussion
Numerous studies have demonstrated RAPA's potent suppression of the effectors of immune responses, T and B lymphocytes,12,13 but its influence on DCs, the most specialized inducers of immune responses, are not well understood. Here we provide comprehensive in vitro and in vivo evidence that RAPA potently targets functional DC activation. The principal findings of this study provide novel insight into the immunopharmacology of this agent and have implications with respect to the therapeutic application of RAPA.
First, RAPA targets responsiveness to the key DC regulatory cytokine IL-4 and this effect is associated with down-regulation of the high-affinity IL-4R complex, which consists of the IL-4Rα chain and the common cytokine receptor γ chain. The inhibitory effects on DC maturation are mediated through RAPA's intracellular receptor FKBP12 and IL-4 hyporesponsiveness of DCs can be confirmed with DCs generated in vivo obtained from RAPA-injected mice. However, inhibition of IL-4—mediated DC activation is only one of several aspects by which RAPA can interfere with DC function. It is important to note that RAPA affects also IL-4—independent pathways of DC activation, such as LPS-induced TNF-α production (Figure 6C) as well as Flt3L-induced DC expansion (Figure 4B-C). Down-regulation of the common cytokine receptor γ chain, which is an indispensable component not only of the IL-4R, but also of the functional IL-2R, IL-7R, IL-9R, IL-15R, and IL-21R complexes,27, 28, 29, 30 may have important additional implications with respect to the immunosuppressive effects of RAPA on DCs and other cells.
Second, in vivo administration of RAPA inhibits the in vivo priming capacity of DCs in naive, fully allogeneic recipients, as shown by adoptive transfer experiments. These findings were obtained in 2 different in vivo models and suggest that RAPA is effective at impairing DC activation. In addition, we found markedly reduced production of the proinflammatory cytokine TNF-α by DCs isolated from animals injected with RAPA.
Third, RAPA inhibits not only the in vivo generation of DCs under steady-state conditions in normal animals but also Flt3L-induced in vivo expansion of DCs. Through reduction of the total DC pool, this action compounds the inhibitory effect of RAPA independent of its effect at the single cell level. We carefully tested the hypothesis that the observed effects might be related to apoptosis. However, in contrast to a recent report indicating that RAPA induced apoptosis specifically in DCs, and not in monocytes,24 we found the frequency of apoptotic or dead cells to be consistently less than 10% in BM-derived, in vitro-generated DCs, as well as in vivo—generated DCs obtained from animals injected with RAPA. Moreover, when DCs were stimulated with LPS, RAPA inhibited cell death in a dose-dependent, FKBP12-mediated manner. It should be noted that there have been several reports that RAPA can exert antiapoptotic effects on different cell populations.31, 32, 33, 34
Pharmacologic suppression of DC proliferation and activation by RAPA has important implications for impairment of immune responses at the level of the antigen-presenting cell. These cells are of pivotal importance in pathologic conditions where DCs perpetuate chronic inflammatory immune responses, for example, chronic graft vasculopathy,35 atherosclerosis,36 and autoimmune diseases.37 One disease that may be especially amenable to RAPA's inhibitory action on DCs is systemic lupus erythematosus (SLE). Blanco and coworkers demonstrated that serum from patients with SLE contained elevated levels of IFN-α that induced normal monocytes to differentiate into DCs.38 They were able to correlate disease activity with the capacity of patients' serum to induce DC differentiation and concluded that unabated induction of DCs by IFN-α may drive the immunopathologic process in SLE.38 Farkas et al39 reported that plasmacytoid DCs, which represent the natural IFN-α—producing cells, accumulate in cutaneous SLE lesions. In this context it is of interest that RAPA has been reported to arrest pathophysiologic changes in murine SLE.40,41 Thus, based on the pathogenetic importance of altered DC numbers and function in SLE, results from animal models, and our present findings, we propose to initiate controlled human trials to evaluate the impact of RAPA for the treatment of SLE.
Besides clinical implications for further understanding of the mechanistic basis of RAPA action in DC-triggered autoimmune diseases and allograft rejection, our findings indicate unexpectedly the potential of RAPA for the treatment of hematologic malignancies where increased Flt3 signaling is involved in disease pathogenesis. Our data indicate that RAPA inhibits Flt3L-induced DC expansion in vivo, as well as accompanying splenomegaly. With respect to this finding, it is of interest that activating Flt3 mutations are present in more than 25% of patients with acute myelogenous leukemia and the most common form, internal tandem duplications, confers a poor prognosis.42, 43, 44, 45, 46 Therefore, in addition to the recently published therapeutic effects of RAPA on solid tumor progression and metastasis,47 we propose to examine the therapeutic effects of RAPA in models of acute myelogenous leukemia.
In summary, by using different in vitro and in vivo models, we have provided evidence that RAPA potently targets functional DC activation and expansion. These findings provide new insight into the immunopharmacology of RAPA, and also have significant implications for the development of new therapeutic strategies in disease processes in which DCs may play a crucial immunopathologic role.
Prepublished online as Blood First Edition Paper, January 16, 2003; DOI 10.1182/blood-2002-11-3370.
Supported by National Institutes of Health grants R01DK 49745 and R01AI 41011 (A.W.T.) and R21HL69725 (A.E.M.). H.H. is supported by a scholarship from the “Stiftung Hämotherapie-Forschung,” Bonn, Germany. H.H. and T.T. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank the Immunex Corporation for providing Flt3L and Wyeth-Ayerst for providing RAPA for in vivo experiments. We are grateful to Ms Bridget L. Colvin for technical assistance and Dr R. Venkataramanan, University of Pittsburgh, for helpful discussion.