P-selectin binds to the N-terminal region of human P-selectin glycoprotein ligand-1 (PSGL-1). For optimal binding, this region requires sulfation on 3 tyrosines and specific core-2O-glycosylation on a threonine. P-selectin is also thought to bind to the N terminus of murine PSGL-1, although it has a very different amino acid sequence than human PSGL-1. Murine PSGL-1 has potential sites for sulfation at Tyr13 and Tyr15 and for O-glycosylation at Thr14 and Thr17. We expressed murine PSGL-1 or constructs with substitutions of these residues in transfected Chinese hamster ovary cells that coexpressed the glycosyltransferases required for binding to P-selectin. The cells were assayed for binding to fluid-phase P-selectin and for tethering and rolling on P-selectin under flow. In both assays, substitution of Tyr13 or Thr17 markedly diminished, but did not eliminate, binding to P-selectin. In contrast, substitution of Tyr15 or Thr14 did not affect binding. Substitution of all 4 residues eliminated binding. Treatment of cells with chlorate, an inhibitor of sulfation, markedly reduced binding of wild-type PSGL-1 to P-selectin but did not further decrease binding of PSGL-1 with substitutions of both tyrosines. These data suggest that sulfation of Tyr13 andO-glycosylation of Thr17 are necessary for murine PSGL-1 to bind optimally to P-selectin. Because it uses only one tyrosine, murine PSGL-1 may rely more on other peptide components andO-glycosylation to bind to P-selectin than does human PSGL-1.
Introduction
Leukocyte recruitment into lymphoid tissues or sites of inflammation begins with circulating leukocytes tethering to and then rolling on the vessel wall. Interactions of selectin adhesion receptors with cell surface glycoconjugates mediate this first critical event, which precedes firm adhesion and then emigration of leukocytes into underlying tissues.1,2 L-selectin, expressed on leukocytes, binds to ligands on some endothelial cells and on other leukocytes. E-selectin, expressed on cytokine-activated endothelial cells, binds to ligands on leukocytes. P-selectin, expressed on activated platelets and endothelial cells, binds to ligands on leukocytes, platelets, and some endothelial cells. Adhesion requires binding of the C-type carbohydrate recognition domain at the N terminus of each selectin to glycans capped with α2-3-sialylated and α1-3-fucosylated structures such as sialyl Lewis x (sLex).3 However, selectins bind with higher affinity or avidity to a small subset of glycoproteins on blood or vascular cells.4
The best characterized glycoprotein ligand for selectins is P-selectin glycoprotein ligand-1 (PSGL-1), an extended homodimeric mucin on leukocytes that binds to all 3 selectins.2,5 Interactions with PSGL-1 are required for leukocytes to roll optimally on P- and L-selectin under flow. The structural requirements for binding of PSGL-1 to P- and L-selectin have been most extensively studied in human cells. Monoclonal antibodies (mAbs) that block leukocyte rolling on P- and L-selectin bind to short protein epitopes near the N terminus of mature PSGL-1.6-12 The binding site for P- and L-selectin, which encompasses the epitopes, includes 3 tyrosines in a consensus motif favoring tyrosine sulfation that are located 6 to 11 residues from a threonine residue.13-15 Both tyrosine sulfation and specific O-glycosylation of the threonine are required for binding to P-selectin. Recombinant human PSGL-1 expressed in transfected Chinese hamster ovary (CHO) cells binds P-selectin if it is expressed with a core-2 β1-6-N-acetylglucosaminyltransferase (eg, Core2GlcNAcT-I) and an α1-3 fucosyltransferase (eg, FTVII), first suggesting that PSGL-1 must be modified with a core-2O-glycan(s) capped with sLex.16Mutational analysis revealed that each tyrosine contributes to binding17,18; substitution of all 3 tyrosines with phenylalanines eliminates binding in biochemical and cell adhesion assays.14,15,17 Similarly, substitution of the threonine with alanine eliminates binding. Glycosulfopeptides modeled after this region of human PSGL-1 have more specifically elucidated the structural requirements for binding to P-selectin.19,20 Sulfation of each tyrosine enhances binding affinity, and the O-glycan requires a core-2 branched structure capped with sLex. The fucose makes a major contribution to binding affinity, whereas the sialic acid residue makes a lesser contribution. Remarkably, a trisulfated glycosulfopeptide with an isomeric extended core-1O-glycan capped with sLex does not bind to P-selectin. These data reveal specific stereochemical requirements for binding. This conclusion is supported by a cocrystal structure of human P-selectin complexed with the N-terminal region of human PSGL-1.21 The C-type carbohydrate-recognition domain of P-selectin makes contacts with fucose, sialic acid, 2 of the 3 tyrosine sulfates, and certain other amino acids in the N-terminal region of PSGL-1. The structure suggests that these various components of PSGL-1 must be presented in an optimal configuration to bind productively to P-selectin.
Like human PSGL-1, murine PSGL-1 is a homodimeric sialomucin.22,23 The mAbs to N-terminal peptide epitopes of PSGL-1 block rolling of murine leukocytes on murine P-selectin in vitro and in vivo,24-26 and studies with PSGL-1–deficient mice confirm that PSGL-1 is the dominant ligand for P-selectin in murine leukocytes.27-29 Studies with glycosidases, expression of PSGL-1 and glycosyltransferases in transfected cells, and analysis of leukocytes from mice deficient in FTVII, FTIV, or Core2GlcNAcT-I suggest that murine PSGL-1 requires modification with one or more core-2 O-glycans capped with sLex.22,30-33 Glycosulfopeptides modeled after the N-terminal region of human PSGL-1 inhibit rolling of murine leukocytes on P-selectin in vivo.34 All these data suggest that murine PSGL-1 binds to P-selectin much like human PSGL-1. However, the N-terminal sequence of murine PSGL-1 shares little similarity with that of human PSGL-1.23 35 Murine PSGL-1 has only 2 tyrosines in a consensus sequence favoring tyrosine sulfation. There are also 2 nearby threonines that are candidates for attachingO-glycans required for binding. However, the spacing of these residues in the primary sequence is considerably closer than the corresponding residues in the human sequence. Given the apparent stereochemical requirements for binding of human PSGL-1 to P-selectin, the different sequence in murine PSGL-1 makes it likely that it uses a different configuration of residues to bind to P-selectin. To address this issue, we expressed wild-type murine PSGL-1 and murine PSGL-1 constructs with substitutions in various N-terminal residues in transfected CHO cells that coexpressed Core2GlcNAcT-I and FTVII. Binding of PSGL-1 to P-selectin was measured by flow cytometry and by cell adhesion assays under flow.
Materials and methods
Materials
Dulbecco modified Eagle medium (DMEM), G418 sulfate, hypoxanthine-thymidine, and nonessential amino acids were purchased from Gibco BRL (Rockville, MD). Glutamine, penicillin, and streptomycin were purchased from Irvine Scientific (Santa Ana, CA). Bovine serum and 25% human serum albumin were purchased from Summit Biotechnology (Fort Collins, CO) and Aventis Behring (King of Prussia, PA), respectively. NuSerum was purchased from Collaborative Research (Waltham, MA). Restriction enzymes were from Promega (Madison, WI). Dihydrofolate reductase-deficient (DHFR−) CHO cells and COS-7 cells were obtained from American Type Culture Collection (Rockville, MD). TRIzol was obtained from Life Technologies (Rockville, MD). Hygromycin B and FuGENE 6 were purchased from Roche (Indianapolis, IN). O-Sialoglycoprotein endopeptidase from Mannheimia haemolytica was obtained from Accurate Chemical and Scientific Corporation (Westbury, NY).
Antibodies
Rat antimurine PSGL-1 mAb 2PH1 (IgG1) and fluorescein isothiocyanate (FITC)–conjugated mouse antirat IgG1 were purchased from BD Biosciences (San Jose, CA). Murine mAb MH15-1 against human IgM (Fc)5 fragments was from Accurate Chemical and Scientific Corporation. FITC-conjugated goat antihuman IgM polyclonal antibody was purchased from Chemicon (Temecula, CA). Rat antimurine PSGL-1 functional blocking mAb 4RA10 (IgG1) was a kind gift from Dietmar Vestweber (University of Muenster, Germany).36 Mouse antihuman IgM mAb was obtained from Accurate Chemical and Scientific Corporation. Anti–T antigen mAb was a kind gift from the late George F. Springer (Chicago Medical School, Chicago, IL). The anti-sLex mAb CSLEX-1 (IgM) was prepared as described.17 37 FITC-conjugated goat antimouse IgG plus IgM (H+L) F(ab′)2 fragments were purchased from Caltag Laboratories (Burlingame, CA).
Proteins
Murine P-selectin–human IgM and murine CD45–human IgM chimeric proteins were expressed in COS-7 cells transfected, respectively, with pCDM8 vectors encoding each molecule (generous gifts from John B. Lowe, University of Michigan Medical Center, Ann Arbor).30 The transfection reagent was FuGENE 6. The transfected cells were maintained in low-glucose DMEM containing 2% NuSerum (Collaborative Research, Bedford, MA). Seven days after transfection the supernatant was harvested and concentrated using 10 000 molecular weight (MW) exclusion spin columns from Millipore.
Constructs
The murine PSGL-1 cDNA with BamHI andXhoI sites incorporated at the 5′ end and 3′ ends, respectively, was amplified by the reverse transcriptase–polymerase chain reaction (RT-PCR) from RNA extracted from mouse leukocytes. The cDNA was then cloned into pBluescript SK (+) (Stratagene, La Jolla, CA) with the BamHI and XhoI enzymes, and the sequence was confirmed by automated dideoxynucleotide sequencing. All mutations in murine PSGL-1 were made in pBluescript SK (+) using the QuickChange kit (Stratagene) according to the manufacturer's instructions. The wild-type and mutant murine PSGL-1 cDNA constructs were excised with BamHI and XhoI and ligated into the mammalian expression vector pZeoSV2(+) (Invitrogen, Carlsbad, CA), which allows selection for cells resistant to zeocin (Invitrogen). All the constructs were confirmed by DNA sequencing.
Transfections
CHO DHFR− cells were maintained in DMEM containing 10% fetal calf serum, 1% hypoxanthine-thymidine, 0.1 mM nonessential amino acids, 1% penicillin/streptomycin, and 2 mM glutamine. Transfected cells were also maintained in selection antibiotics as described.17 18 A clone of transfected CHO cells stably expressing Core2GlcNAcT-I and FTVII was transfected with empty pZeoSV2(+) or with pZeoSV2(+) containing wild-type or mutant murine PSGL-1 constructs, using electroporation. Parental CHO cells or transfected CHO cells expressing only FTVII were transfected with vectors expressing wild-type PSGL-1 or PSGL-1 constructs Tyr13/15Phe or Thr14/17Ala. To confirm that each cell line expressed the correct construct, total RNA was extracted using TRIzol reagent according to the manufacturer's protocol. RT-PCR was then carried out using primers flanking the mutations. The amplified PCR products were purified and sequenced.
Sodium chlorate treatment
Flow cytometry
Expression levels of murine PSGL-1 in transfected CHO cells were measured by flow cytometry using a saturating concentration of mAb 2PH1 (20 μg/mL). Bound antibody was detected with FITC-conjugated rat antimouse IgG1 mAb. All cell incubations were for 30 minutes on ice, followed by washing. After the final wash, cells were fixed in 0.1% paraformaldehyde and then analyzed by flow cytometry on a FACscan (Becton Dickinson, San Jose, CA). Cell lines expressing matched levels of each construct were isolated by sorting with a MoStar cell sorter (Cytomation, San Jose, CA and Becton Dickinson) using the mAb 2PH1. Matched activities of Core2GlcNAcT-I and FTVII in these cell lines were established using flow cytometry with anti–T antigen mAb (measured with desialylated cells) or anti-sLex mAb CSLEX -1 as described.17
To measure binding of fluid-phase P-selectin, 106transfected CHO cells were incubated with saturating concentrations of murine P-selectin/IgM or, as a control, with buffer only or with murine CD45/IgM in 100 μL Hanks balanced salt solution containing 0.1% human serum albumin (HBSS/HSA). Bound P-selectin/IgM was detected with FITC-conjugated goat antihuman IgM polyclonal antibody. Incubations with IgM chimeras or antibody were for 30 minutes on ice, followed by washing. Specificity of binding was assessed by incubations in the presence of 10 mM EDTA (ethylenediaminetetraacetic acid) or 10 μg/mL anti–PSGL-1 mAb 4RA10 or by pretreating cells with 200 μg/mL O-sialoglycoprotein endopeptidase for 30 minutes at 37°C. After the final wash, cells were fixed in 0.1% paraformaldehyde and then analyzed by flow cytometry.
Cell accumulation, shear resistance, and tethering under flow
Murine P-selectin/IgM or control CD45/IgM was captured on antihuman IgM/Fc mAb MH15-1 immobilized on a 35-mm dish mounted in a parallel-plate flow chamber. Site densities of captured P-selectin were measured by binding of saturating concentrations of125I-labeled antimurine P-selectin mAb RB40.34.40 In some experiments, human recombinant soluble P-selectin41 was immobilized on the dish. Transfected CHO cells (106/mL in HBSS/HSA) were perfused over the substrates at varying wall shear stresses.18 42 The accumulated number of rolling cells was measured after 4 minutes of perfusion by using a videomicroscopy system coupled to a digitized image analysis system (Inovision, Cleveland, OH) on a Silicon Graphics workstation (SGI, Mountain View, CA). For each experiment, adherent cells in 10 to 12 × 20 fields were counted. To measure resistance to detachment under flow, cells were allowed to accumulate at 0.5 dyn/cm2, and cell-free buffer was then introduced. Wall shear stress was increased every 30 seconds, and the percentage of remaining adherent cells was determined. The rate that cells tethered to P-selectin was measured during the first 60 seconds of perfusion. Cells that detached in fewer than 30 frames were defined as transient tethers. Cells that remained attached for at least 30 frames were defined as rolling tethers.
Results
Expression of wild-type or mutant murine PSGL-1 in CHO cells expressing Core2GlcNAcT-I and FTVII
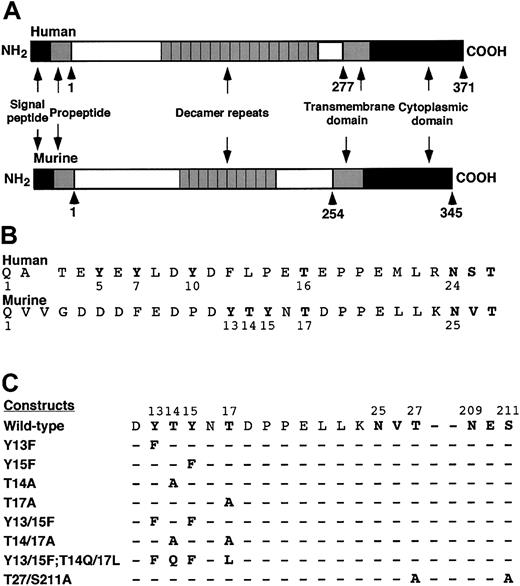
Both human and murine PSGL-1 are extended sialomucins that are expressed as homodimers. Each has a signal peptide and a propeptide that are thought to be cleaved during synthesis (Figure1A). In this study, amino acids were numbered beginning with the first residue of the putative mature protein. Alignment of the mature N-terminal sequences of human and murine PSGL-1 revealed similarities but also important differences (Figure 1B). Each protein has tyrosine residues within a region rich in acidic amino acids that favors tyrosine sulfation. Human PSGL-1 has 3 tyrosines at residues 5, 7, and 10, all of which are sulfated and contribute to binding to P-selectin.17,20 A 5-residue segment containing a proline separates the tyrosines from a threonine at residue 16; the latter residue is modified with a core-2O-glycan capped with sLex that is also required for binding to P-selectin.17 19 By contrast, murine PSGL-1 has only 2 tyrosines at residues 13 and 15. These are very close to threonines 14 and 17, the only residues in the N-terminal region that might be O-glycosylated.
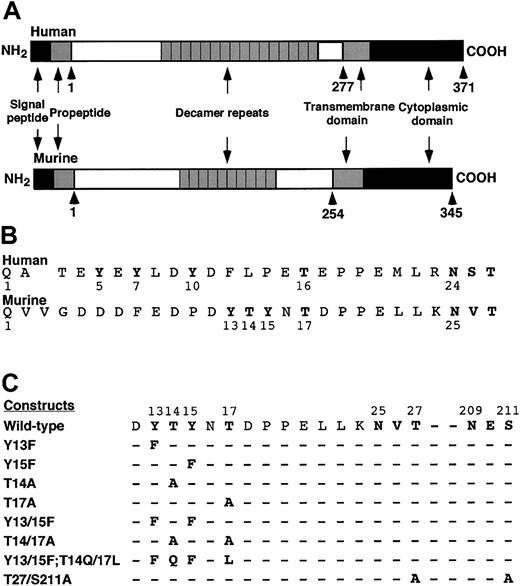
Schematic diagram of murine PSGL-1 constructs.
(A) Diagram of the domains in human and murine PSGL-1. (B) Alignment of the mature N-terminal sequences of human and murine PSGL-1. A one-residue gap in the human sequence was introduced to align the putative O-glycosylated threonine and the conserved N-glycosylation site with those in the murine sequence. (C) Sequences of the amino acid substitutions in the full-length murine PSGL-1 constructs.
Schematic diagram of murine PSGL-1 constructs.
(A) Diagram of the domains in human and murine PSGL-1. (B) Alignment of the mature N-terminal sequences of human and murine PSGL-1. A one-residue gap in the human sequence was introduced to align the putative O-glycosylated threonine and the conserved N-glycosylation site with those in the murine sequence. (C) Sequences of the amino acid substitutions in the full-length murine PSGL-1 constructs.
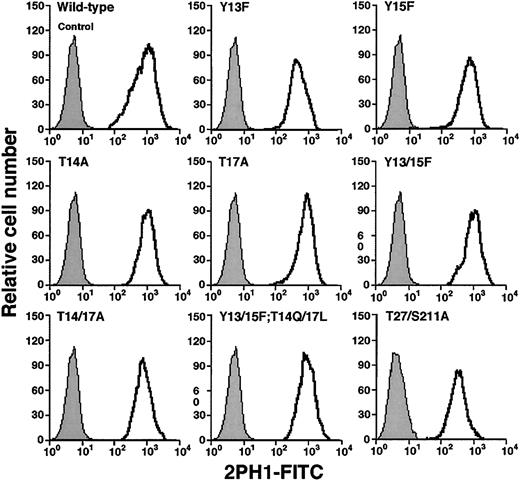
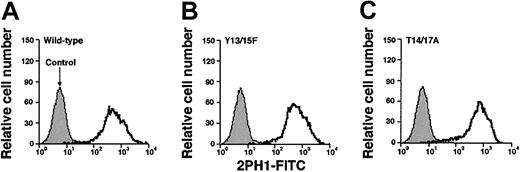
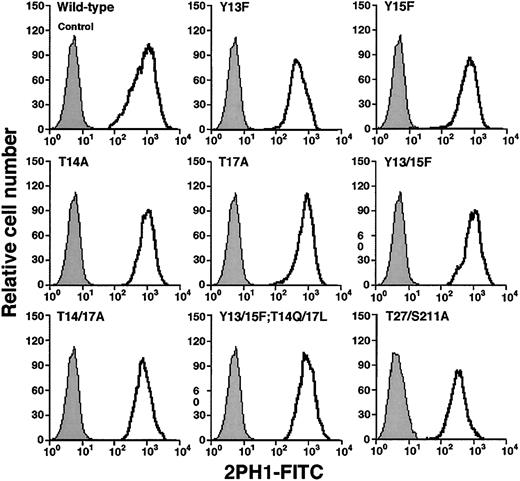
To determine whether the tyrosine and threonine residues contributed to binding of murine PSGL-1 to P-selectin, we made a series of constructs in which we altered these residues either individually or in combination (Figure 1C). We also prepared a construct with mutations in the third residue of each sequon for the 2 potential N-glycosylation sites at residues 25 and 209. The cDNA encoding each construct was transfected into CHO cells that were previously transfected with expression vectors encoding Core2GlcNAcT-I and FTVII. Fluorescence-activated cell sorting was used to isolate clones that expressed matched levels of wild-type murine PSGL-1 or each mutant PSGL-1 construct (Figure 2). Each clone expressed similar levels of Core2GlcNAcT-I and FTVII, as measured by binding of mAbs to the T antigen and to sLex, respectively (Table 1).
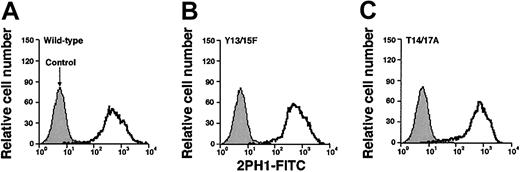
Expression of wild-type or mutated murine PSGL-1 on transfected CHO cells coexpressing Core2GlcNAcT-I and FTVII.
The cells were permanently transfected with an empty expression vector (control) or with an expression vector encoding wild-type murine PSGL-1 or the indicated mutant PSGL-1 construct. The cells were incubated with antimurine PSGL-1 mAb 2PH1. Bound mAb was detected with FITC-conjugated rat antimouse IgG1 mAb. Incubation of PSGL-1–transfected cells with FITC-conjugated rat antimouse IgG1 mAb alone gave the same low background staining as observed with the cells transfected with the empty expression vector. The data are representative of at least 5 experiments.
Expression of wild-type or mutated murine PSGL-1 on transfected CHO cells coexpressing Core2GlcNAcT-I and FTVII.
The cells were permanently transfected with an empty expression vector (control) or with an expression vector encoding wild-type murine PSGL-1 or the indicated mutant PSGL-1 construct. The cells were incubated with antimurine PSGL-1 mAb 2PH1. Bound mAb was detected with FITC-conjugated rat antimouse IgG1 mAb. Incubation of PSGL-1–transfected cells with FITC-conjugated rat antimouse IgG1 mAb alone gave the same low background staining as observed with the cells transfected with the empty expression vector. The data are representative of at least 5 experiments.
Tyr13 and Thr17 contribute to binding of murine PSGL-1 to P-selectin
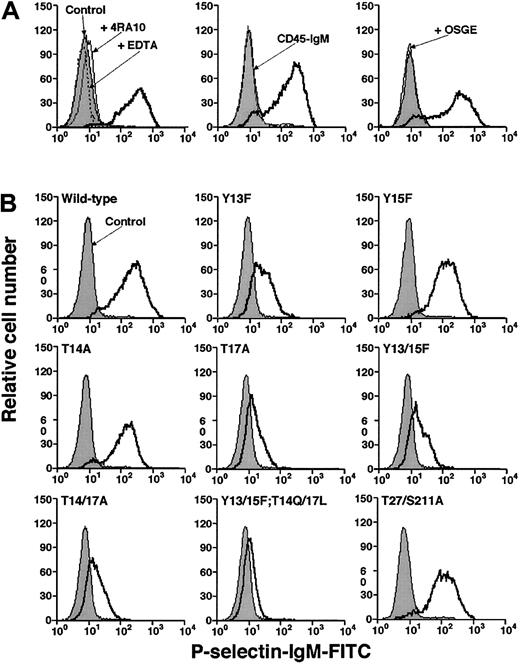
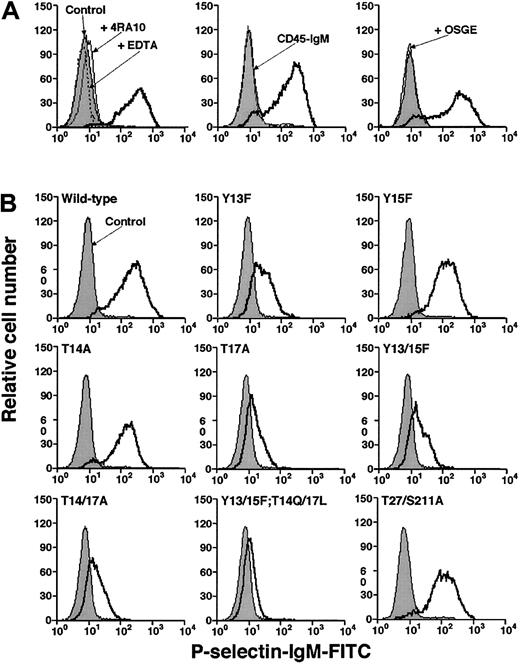
We used flow cytometry to compare binding of a murine P-selectin/IgM chimera to CHO cells expressing wild-type or mutant murine PSGL-1. P-selectin binding was specific because it was Ca2+ dependent and was blocked by 4RA10, a mAb that identifies an N-terminal epitope on murine PSGL-1, or by pretreatment of cells with O-sialoglycoprotein endopeptidase, which cleaves the N-terminal P-selectin–binding site of PSGL-143 (Figure3A). P-selectin/IgM bound equivalently to wild-type PSGL-1 and to the Tyr15Phe and Thr14Ala constructs, demonstrating that neither Tyr15 nor Thr14 is required for binding to P-selectin in this assay (Figure 3B). P-selectin/IgM also bound normally to the Thr27/Ser211Ala construct, indicating that the N-glycosylation sites are not required for binding. However, P-selectin/IgM binding to the Tyr13Phe and Tyr17Ala constructs was substantially decreased. The residual binding was specific because it was blocked by EDTA or by mAb 4RA10 (data not shown). Mutation of both tyrosines (Tyr13/15Phe) or both threonines (Thr14/17Ala) did not further reduce binding, whereas mutation of all 4 residues eliminated binding (Figure 3B). Eightfold higher concentrations of P-selectin/IgM did not detectably increase binding to cells expressing either wild-type or mutant PSGL-1 (data not shown). These results demonstrate that Tyr13 and Thr17 make major contributions to binding, whereas the adjacent Tyr15 and Thr14 do not.
Binding of fluid-phase murine P-selectin to transfected CHO cells.
(A) Specificity of binding. Left panel: CHO cells expressing Core2GlcNAcT-I, FTVII, and wild-type PSGL-1 were incubated with murine P-selectin/IgM in the presence or absence of EDTA or anti–PSGL-1 mAb 4RA10. Middle panel: The cells were incubated with P-selectin/IgM or murine CD45-IgM. Right panel: The cells were preincubated in the presence or absence of O-sialoglycoprotein endopeptidase (OSGE) and then incubated with P-selectin/IgM. Bound P-selectin/IgM was detected with FITC-conjugated goat antihuman IgM. Control cells were incubated with FITC-conjugated goat ant-human IgM alone. (B) CHO cells expressing Core2GlcNAcT-I, FTVII, and either wild-type or the indicated mutant murine PSGL-1 construct were incubated with murine P-selectin/IgM. Bound P-selectin/IgM was detected with FITC-conjugated goat antihuman IgM. Control cells were incubated with FITC-conjugated goat antihuman IgM alone. The data are representative of at least 5 experiments.
Binding of fluid-phase murine P-selectin to transfected CHO cells.
(A) Specificity of binding. Left panel: CHO cells expressing Core2GlcNAcT-I, FTVII, and wild-type PSGL-1 were incubated with murine P-selectin/IgM in the presence or absence of EDTA or anti–PSGL-1 mAb 4RA10. Middle panel: The cells were incubated with P-selectin/IgM or murine CD45-IgM. Right panel: The cells were preincubated in the presence or absence of O-sialoglycoprotein endopeptidase (OSGE) and then incubated with P-selectin/IgM. Bound P-selectin/IgM was detected with FITC-conjugated goat antihuman IgM. Control cells were incubated with FITC-conjugated goat ant-human IgM alone. (B) CHO cells expressing Core2GlcNAcT-I, FTVII, and either wild-type or the indicated mutant murine PSGL-1 construct were incubated with murine P-selectin/IgM. Bound P-selectin/IgM was detected with FITC-conjugated goat antihuman IgM. Control cells were incubated with FITC-conjugated goat antihuman IgM alone. The data are representative of at least 5 experiments.
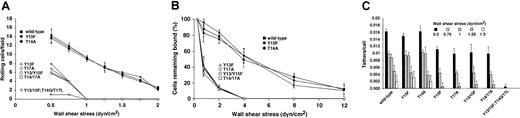
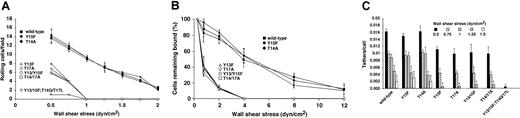
As an independent assay for interactions of murine PSGL-1 with P-selectin, we measured tethering and rolling of transfected CHO cells on immobilized P-selectin under flow. Equivalent numbers of CHO cells expressing wild-type PSGL-1 or the Tyr15Phe or Thr14Ala constructs rolled on P-selectin/IgM at all wall shear stresses examined (Figure4A). Frame-by-frame analysis revealed that the cells expressing these constructs rolled with similar mean velocities and variances of velocity (data not shown) and resisted detachment similarly in response to increasing wall shear stress (Figure 4B). Furthermore, flowing cells tethered to P-selectin at similar rates (Figure 4C). Thus, neither Tyr15 nor Thr14 is required for cells to interact with P-selectin under the flow conditions examined. In contrast, substantially fewer cells expressing Tyr13Phe, Thr17Ala, or the double-tyrosine or double-threonine mutations rolled on P-selectin, and this rolling was observed only at low wall shear stresses (Figure 4A). The residual rolling was specific because mAb 4RA10 eliminated rolling (data not shown). However, the residual cells rolled irregularly and detached much more readily in response to increasing wall shear stress (Figure 4B). Furthermore, fewer flowing cells expressing Tyr13Phe, Thr17Ala, or the double-tyrosine or double-threonine mutations tethered to P-selectin (Figure 4C). Cells expressing the construct in which all tyrosines and threonines were mutated exhibited no PSGL-1–dependent tethering to or rolling on P-selectin (Figure 4A,C). Similar results were observed when tethering and rolling of cells on immobilized human P-selectin was measured (data not shown). These results demonstrate that Tyr13 and Thr17 make major contributions to tethering and rolling of cells expressing murine PSGL-1 on P-selectin under flow, whereas Tyr15 and Thr14 do not.
Tethering and rolling of transfected CHO cells on murine P-selectin.
(A) CHO cells expressing Core2GlcNAcT-I, FTVII, and either wild-type PSGL-1 or the indicated mutant murine PSGL-1 construct were perfused over captured murine P-selectin/IgM at the indicated wall shear stress. After 4 minutes, the number of rolling cells was quantified. (B) Transfected CHO cells were allowed to accumulate on murine P-selectin/IgM at 0.5 dyn/cm2 and cell-free buffer was then introduced. Wall shear stress was increased every 30 seconds, and the percentage of remaining adherent cells was determined. (C) The number of cells that tethered to murine P-selectin/IgM during the first 60 seconds was quantified and normalized by dividing by the number of cells delivered across the field of view in the focal plane of the substrate. The percentage of tethers that were transient or that were converted to rolling adhesion is also indicated. The P-selectin/IgM density was 31 sites/μm2. The data represent the mean ± SEM of at least 4 independent experiments. For some points, the error bars are smaller than the data symbols.
Tethering and rolling of transfected CHO cells on murine P-selectin.
(A) CHO cells expressing Core2GlcNAcT-I, FTVII, and either wild-type PSGL-1 or the indicated mutant murine PSGL-1 construct were perfused over captured murine P-selectin/IgM at the indicated wall shear stress. After 4 minutes, the number of rolling cells was quantified. (B) Transfected CHO cells were allowed to accumulate on murine P-selectin/IgM at 0.5 dyn/cm2 and cell-free buffer was then introduced. Wall shear stress was increased every 30 seconds, and the percentage of remaining adherent cells was determined. (C) The number of cells that tethered to murine P-selectin/IgM during the first 60 seconds was quantified and normalized by dividing by the number of cells delivered across the field of view in the focal plane of the substrate. The percentage of tethers that were transient or that were converted to rolling adhesion is also indicated. The P-selectin/IgM density was 31 sites/μm2. The data represent the mean ± SEM of at least 4 independent experiments. For some points, the error bars are smaller than the data symbols.
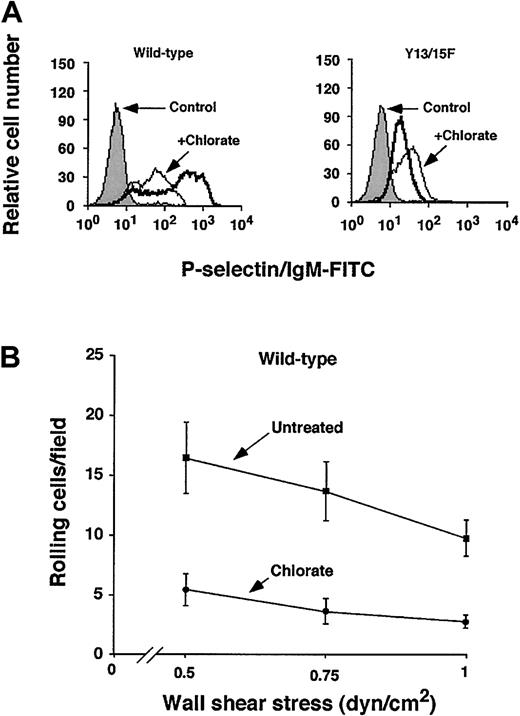
Inhibition of sulfation markedly reduces binding of murine PSGL-1 to P-selectin
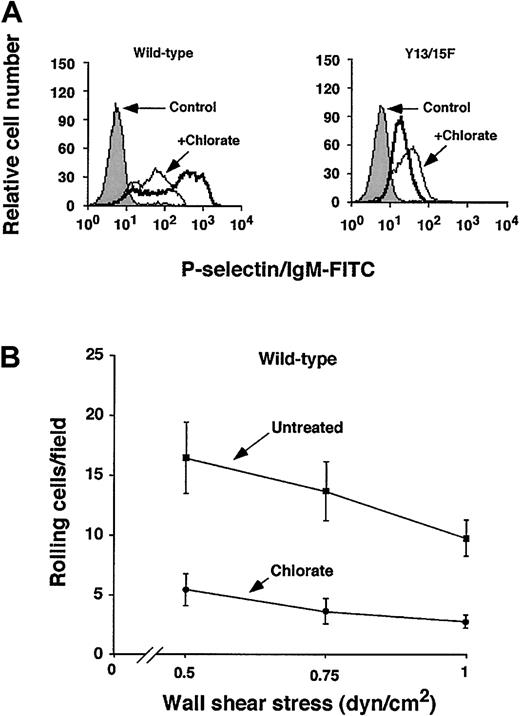
Human PSGL-1 requires sulfation of each of its 3 N-terminal tyrosines to interact optimally with P-selectin.20 To determine whether sulfation of murine PSGL-1 enhances binding to P-selectin, we cultured CHO cells expressing wild-type or Tyr13/15Phe murine PSGL-1 in the presence or absence of the sulfation inhibitor chlorate for 3 days to allow turnover of previously sulfated molecules. Chlorate is a selective inhibitor of adenosine triphosphate (ATP) sulfurylase (ATP sulfate adenylyltransferase), which is required for formation of phosphoadenosine phosphosulfate (PAPS), the donor for sulfation reactions. Chlorate-treated cells expressing wild-type PSGL-1 bound much less P-selectin/IgM, to a level similar to that observed for untreated cells expressing Tyr13/15Phe PSGL-1 (Figure5A). Chlorate treatment of cells expressing Tyr13/15Phe did not further reduce binding of P-selectin/IgM. Chlorate treatment also substantially decreased rolling of cells expressing wild-type PSGL-1 on immobilized P-selectin under flow (Figure 5B). Chlorate treatment did not further reduce rolling of CHO cells expressing Tyr13/15Phe PSGL-1 on P-selectin (data not shown). These results demonstrate that sulfation of murine PSGL-1 contributes significantly to binding to P-selectin, and further suggest that sulfation of tyrosine is required for optimal binding.
Effect of inhibiting sulfation of murine PSGL-1 on binding to P-selectin.
CHO cells expressing Core2GlcNAcT-I, FTVII, and either wild-type or Tyr13/15Phe murine PSGL-1 were cultured in the presence or absence of 100 mM sodium chlorate for 72 hours. (A) Binding of P-selectin/IgM was measured by flow cytometry as in Figure 3. The data are representative of 3 experiments. (B) The number of cells rolling on P-selectin was measured as in Figure 4A. The data represent the mean ± SEM of 3 experiments.
Effect of inhibiting sulfation of murine PSGL-1 on binding to P-selectin.
CHO cells expressing Core2GlcNAcT-I, FTVII, and either wild-type or Tyr13/15Phe murine PSGL-1 were cultured in the presence or absence of 100 mM sodium chlorate for 72 hours. (A) Binding of P-selectin/IgM was measured by flow cytometry as in Figure 3. The data are representative of 3 experiments. (B) The number of cells rolling on P-selectin was measured as in Figure 4A. The data represent the mean ± SEM of 3 experiments.
Cells expressing murine PSGL-1 must coexpress Core2GlcNAcT-I and FTVII to interact with P-selectin
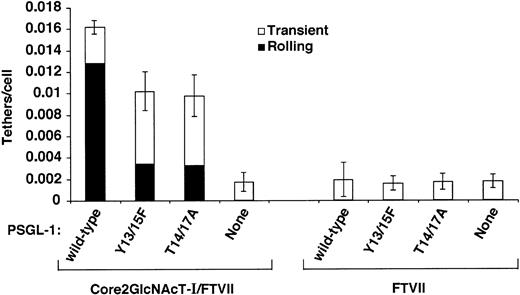
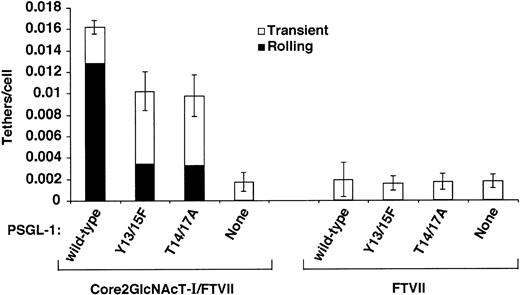
Human PSGL-1 must express a core-2 O-glycan capped with sLex at Thr16 to bind detectably to P-selectin,15-17,19 and very few leukocytes from mice lacking Core2GlcNAcT-I or FTVII roll on P-selectin.30 31To confirm that these glycosyltransferases must modify murine PSGL-1 expressed in CHO cells to confer binding to P-selectin, we expressed wild-type murine PSGL-1 or the Tyr13/15Phe or Thr14/17Ala constructs in parental CHO cells or in transfected CHO cells coexpressing FTVII but not Core2GlcNAcT-I. CHO cell clones were selected that expressed matched levels of PSGL-1 (Figure 6) or FTVII (Table 1). Virtually no parental cells or cells expressing FTVII rolled on P-selectin, regardless of whether PSGL-1 was expressed (data not shown). As a more sensitive assay, we looked for transient tethers of flowing cells to P-selectin. A very small number of cells expressing FTVII tethered transiently to P-selectin at a very low wall shear stress, but none of these tethers converted to even brief rolling adhesion (Figure 7). These transient tethers required expression of FTVII because they were not observed in parental CHO cells. They did not require PSGL-1 because the number of tethers was not increased in cells expressing wild-type or mutant PSGL-1. Significantly more cells expressing wild-type or mutant PSGL-1 tethered to P-selectin when they coexpressed both Core2GlcNAcT-I and FTVII. These tethers required PSGL-1 because anti–PSGL-1 mAb 4RA10 eliminated them (data not shown), and cells expressing both glycosyltransferases in the absence of PSGL-1 formed only a few transient tethers like those seen in cells expressing only FTVII. These data confirm that murine PSGL-1 expressed in CHO cells must be modified with both Core2GlcNAcT-I and FTVII to bind P-selectin. It is noteworthy that cells expressing the Thr14/17Ala construct tethered to and rolled on P-selectin much more readily when they coexpressed both Core2GlcNAcT-I and FTVII. This suggests that murine PSGL-1 may use a core-2 O-glycan(s) on a residue other than Thr17 to also contribute to optimal binding to P-selectin. Alternatively, CHO cells may express one or more core-2 O-glycans on proteins other than PSGL-1 that facilitate binding. The putativeO-glycan(s) is likely to cooperate with Tyr13 or perhaps Tyr15 because simultaneous mutation of Tyr13, Tyr15, Thr14, and Thr17 eliminated tethering to P-selectin even when the cells expressed both glycosyltransferases (Figure 4C).
Expression of wild-type or mutated murine PSGL-1 on transfected CHO cells coexpressing FTVII.
The cells were permanently transfected with an empty expression vector (control) or with an expression vector encoding wild-type murine PSGL-1 or the indicated mutant PSGL-1 construct. Staining with antimurine PSGL-1 mAb 2PH1 was performed as in Figure 2. The data are representative of at least 5 experiments.
Expression of wild-type or mutated murine PSGL-1 on transfected CHO cells coexpressing FTVII.
The cells were permanently transfected with an empty expression vector (control) or with an expression vector encoding wild-type murine PSGL-1 or the indicated mutant PSGL-1 construct. Staining with antimurine PSGL-1 mAb 2PH1 was performed as in Figure 2. The data are representative of at least 5 experiments.
Effect of coexpression of Core2GlcNAcT-I on tethering of cells expressing FTVII and PSGL-1 constructs to murine P-selectin.
The number of cells that tethered to murine P-selectin/IgM during the first 60 seconds was quantified and normalized by dividing by the number of cells delivered across the field of view in the focal plane of the substrate. The percentages of tethers that were transient or that were converted to rolling adhesion are also indicated. The P-selectin/IgM density was 31 sites/μm2. The wall shear stress was 0.5 dyn/cm2. The data represent the mean ± SEM of at least 4 independent experiments.
Effect of coexpression of Core2GlcNAcT-I on tethering of cells expressing FTVII and PSGL-1 constructs to murine P-selectin.
The number of cells that tethered to murine P-selectin/IgM during the first 60 seconds was quantified and normalized by dividing by the number of cells delivered across the field of view in the focal plane of the substrate. The percentages of tethers that were transient or that were converted to rolling adhesion are also indicated. The P-selectin/IgM density was 31 sites/μm2. The wall shear stress was 0.5 dyn/cm2. The data represent the mean ± SEM of at least 4 independent experiments.
Discussion
Studies using blocking mAbs, recombinant proteins, glycosulfopeptides, and crystallography have demonstrated that a small N-terminal region of human PSGL-1 is both necessary and sufficient for binding to the C-type lectin domain of human P-selectin.2Optimal binding requires a stereochemically precise array of 3 tyrosine sulfates, other peptide components, and a core-2 O-glycan capped with sLex.19-21 Loss of either the tyrosine sulfates or the core-2 O-glycan is sufficient to lower binding affinity below the detection limit in biochemical assays and to eliminate or markedly decrease the ability of cells expressing PSGL-1 to tether to or roll on P-selectin under flow.13-15,17-20 We have used complementary biochemical measurements and assays of cell adhesion under flow to analyze structural requirements for binding of recombinant murine PSGL-1 to murine P-selectin. Previous studies with blocking mAbs implicated the N-terminal region of murine PSGL-1 in binding to murine P-selectin.24-26 Our studies of this region suggest that posttranslational modifications of critical residues are required for binding. However, our data imply that murine PSGL-1 differs significantly from human PSGL-1 in the relative contributions of tyrosine sulfate, peptide components, and glycosylation that it uses to bind to P-selectin.
The N-terminal region of murine PSGL-1 has 2 tyrosines, Tyr13 and Tyr15, which could be sulfated, and 2 threonines, Thr14 and Thr17, which could be O-glycosylated. Assays of mutated forms of murine PSGL-1 strongly suggest that only Tyr13 and Thr17 contribute to binding to murine P-selectin. Mutation of either Tyr13 or Thr17 markedly decreased interactions with P-selectin. In contrast, mutation of either Tyr15 or Thr14 did not detectably affect binding, and mutation of both tyrosines or both threonines did not reduce binding more than mutation of Tyr13 or Thr17 alone. The simplest interpretation of the data are that Tyr13 must be sulfated and Thr17 must be modified with a core-2 O-glycan capped with sLex to bind optimally to P-selectin. Validation of this interpretation will require direct studies with semisynthetic glycosulfopeptides, where sulfation and glycosylation can be modified without mutation of amino acids.19,20 Inhibition of sulfation with chlorate markedly diminished binding of murine PSGL-1 to P-selectin, providing independent evidence for the importance of sulfation in binding. The failure of chlorate to diminish the residual binding of Tyr13/15 PSGL-1 to P-selectin suggests that optimal binding requires sulfation of tyrosine. Algorithms for consensus sequences suggest thatO-glycosylation is more likely to occur on Thr17 than on Thr14.44 Tyrosine sulfation occurs primarily in the trans-Golgi network and is therefore likely to occur after the initial steps of O-glycosylation.45O-glycosylation of Thr17 might sterically inhibit sulfation of Tyr15 but not of the potentially more distant Tyr13.
Our data suggest that murine PSGL-1 requires only one tyrosine, which may be sulfated, plus a core-2 O-glycan to bind to P-selectin. This differs significantly from human PSGL-1, where each of 3 tyrosine sulfates contributes to the affinity of binding to P-selectin. Indeed, crystallographic studies reveal that at least 2 of the 3 tyrosine sulfates of human PSGL-1 bind directly to amino acids in the lectin domain of human P-selectin.21 The relative affinities of murine and human PSGL-1 for P-selectin are not known. Perhaps the decrease in sulfation of murine PSGL-1 lowers its affinity for murine (or human) P-selectin. Alternatively, N-terminal peptide components unique to murine PSGL-1 may provide compensatory binding sites for P-selectin so there is no net loss of binding affinity. Both biochemical and structural evidence indicates that peptide components other than tyrosine sulfate enhance binding of human PSGL-1 to P-selectin.20,21 These components could be even more important in murine PSGL-1. This might explain why mutation of the tyrosines or prevention of sulfation of murine PSGL-1 reduces, but does not eliminate, binding to P-selectin, whereas mutation of tyrosines or removal of sulfate on human PSGL-1 more profoundly inhibits binding. Thus, peptide and tyrosine sulfate components in the N-terminal region of murine PSGL-1 might be sufficient to mediate the residual binding to P-selectin observed after mutation of both Thr14 and Thr17. Loss ofO-glycosylation might even permit sulfation of Tyr15, which normally may not be modified. However, the Thr14/17Ala PSGL-1 construct bound to P-selectin only when it was coexpressed with both Core2GlcNAcT-I and FTVII. This suggests that a core-2O-glycan capped with sLex, located elsewhere from Thr14 or Thr17 on PSGL-1, augments the binding of the peptide and sulfate components of PSGL-1 to P-selectin. Alternatively, one or more core-2 O-glycans on other glycoprotein(s) may augment cell adhesion to P-selectin. It is possible that Thr17 is notO-glycosylated; instead, mutation of this residue might impair binding indirectly by inhibiting sulfation of Tyr13. This seems less likely because mutation of both tyrosines and both threonines diminished binding much more than mutation of only the threonines or only the tyrosines. Direct binding studies with purified murine PSGL-1 constructs will be required to distinguish these possibilities. Whatever the mechanism, weak binding to P-selectin persists after mutation of Thr14 and Thr17 in murine PSGL-1, whereas binding to P-selectin is virtually eliminated after mutation of Thr16 in human PSGL-1.15 17 Taken together, these data argue for important differences in the relative contributions of tyrosine sulfate, peptide components, and O-glycosylation to binding of murine and human PSGL-1 to P-selectin.
The site-directed mutagenesis results presented here should be interpreted cautiously because mutations may impair function by indirect structural alterations. Furthermore, we coexpressed murine PSGL-1 with specific glycosyltransferases in transfected CHO cells, which may modify PSGL-1 differently than do murine leukocytes. Nevertheless our results suggest both similarities and interesting differences in how murine and human PSGL-1 bind to P-selectin. Direct comparisons of binding affinities of murine and human PSGL-1 with murine and human P-selectin will provide additional insights. Complementary information will be obtained from studies with glycosulfopeptides modeled after PSGL-1, where precise changes in peptide sequence, sulfation, and glycosylation can be made. Finally, it will be important to determine the structures of oligosaccharides on murine leukocytes, particularly those on PSGL-1. Such information may be useful for interpreting the degree to which in vivo studies of selectin/PSGL-1 interactions in mice can be extended to human physiology.
We thank Dr Hendra Setiadi for assistance with site density measurements, Jim Henthorn for assistance with cell sorting, and Drs Dietmar Vestweber and John Lowe for providing reagents.
Prepublished online as Blood First Edition Paper, August 29, 2002; DOI 10.1182/blood-2001-11-0036.
Supported by National Institutes of Health grants HL 65631, AI 44902, and AI 48075. L.X. is the recipient of a Scientist Development Award (0130039N) from the American Heart Association. V.R. was the recipient of a postdoctoral fellowship from the Heartland Affiliate of the American Heart Association.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Rodger P. McEver, Cardiovascular Biology Research Program, Oklahoma Medical Research Foundation, 825 NE 13th St, Oklahoma City, OK 73104; e-mail:rodger-mcever@ouhsc.edu.