Plasmodium falciparum digests up to 75% of erythrocyte (red blood cell [RBC]) hemoglobin and forms hemozoin. Phagocytosed hemozoin and trophozoites inhibit important monocyte functions. Delipidized trophozoites and hemozoin were remarkably less toxic to monocytes. Parasitized RBCs and hemozoin contained large amounts of mostly esterified monohydroxy derivatives (OH-PUFAs), the stable end products of peroxidation of polyenoic fatty acids. The concentrations of OH-PUFA were 1.8 micromoles per liter RBCs in nonparasitized RBCs, 11.1 micromoles per liter RBCs in rings, 35 micromoles per liter RBCs in trophozoites; and approximately 90 micromoles per liter RBC equivalents in hemozoin. In parasitized RBCs and hemozoin a complex mixture of monohydroxy derivatives of arachidonic (HETEs) and linoleic (HODEs) acid was determined. Respectively, 13- and 9-HODE and 9- and 12-HETE were predominant in hemozoin and parasitized RBCs. The estimated concentrations of all HETE isomers were 33 and 39 micromoles per liter RBCs or RBC equivalents in trophozoites and hemozoin, respectively. No evidence of lipoxygenase activity was found, whereas the large number of positional and optical isomers, the racemic structure, and their generation by incubation of arachidonic acid with hemozoin indicated nonenzymatic origin via heme-catalysis. Sub/low micromolar concentrations of 12- and 15-HETE were toxic to monocytes, whereas HODE isomers were ineffective. Low micromolar concentrations of HETE isomers were estimated to be similarly present in monocytes after phagocytosis of trophozoites or hemozoin. Thus, specific products of heme-catalyzed lipid peroxidation appear to contribute to hemozoin toxicity to phagocytes and may thus play a role in increased cytoadherence, vascular permeability, and chemotaxis, as well as in immunodepression in malaria.
Introduction
During the asexual development of Plasmodium falciparum in human erythrocytes (red blood cells [RBCs]), the parasite digests about 75% of the host cell hemoglobin (Hb) but is unable to catabolize the heme liberated.1 To protect the parasite membranes against the toxic effects of free heme, heme is polymerized to hemozoin2 or broken down nonenzymatically.3-5 Hemozoin accumulates in the digestive vacuole, an acidic organelle of the parasite.6Nonenzymatic cleavage of heme liberates iron and generates hydrogen peroxide.7 Likewise, formation of hemozoin is accompanied by (per)oxidation of lipids and proteins present in the digestive vacuole. Parasitized RBCs are avidly phagocytosed by circulating and resident phagocytes,8,9 and ingested hemozoin inhibits important cellular functions,10,11 upsets the productions of several cytokines,12-14 and seems to play a role in malaria immunodepression.11 15
Here we report that delipidized trophozoites and hemozoin exhibited strongly reduced toxicity when phagocytosed by human monocytes. Because hemozoin constitutes a potential catalyst for lipid peroxidation, the oxidative modifications of lipids in parasitized RBCs and hemozoin were investigated. Parasitized RBCs and hemozoin were found to contain large amounts of monohydroxy derivatives of polyenoic fatty acids (OH-PUFAs) in their ester lipids. A detailed structural analysis suggested nonenzymatic, heme-catalyzed lipid peroxidation as origin of these products. Among the most abundant OH-PUFAs detected, only 12- and 15-hydroxy-arachidonic acid (12-HETE, 15-HETE) turned out to be toxic to human monocytes when supplemented at sub/low micromolar concentrations. In contrast, hydroxy-linoleic acid isomers were virtually inactive. These data indicate that nonenzymatic, heme-catalyzed lipid peroxidation is an integral part of the infectious process and that certain peroxidation products appear to play a role in hemozoin toxicity.
Materials and methods
Cultivation of P falciparum and isolation of stage-parasitized RBCs and hemozoin
P falciparum parasites (Palo Alto strain,Mycoplasma free) were kept in culture as described.10 If not stated otherwise, hemozoin and RBCs parasitized with different maturation stages were isolated by the Percoll-mannitol (Sigma Chemical, St Louis, MO) method10from synchronized cultures during the first 2 days of reinfection of fresh RBCs by schizonts. After centrifugation at 5000g on a discontinuous Percoll-mannitol density gradient, hemozoin was collected from the 0% to 40% interface, trophozoites/schizonts from the 40% to 80% interface, and rings from the bottom of the gradient. Parasites were washed twice and reincubated in RPMI 1640 (Sigma) for 1 hour at 37°C before lipid extraction or opsonization. For maturation studies, isolated schizonts were incubated with freshly drawn, washed RBCs at a parasitemia of 5% for reinfection for 12 hours. Thereafter, RBCs were centrifuged through a cushion of 80% Percoll-mannitol to remove extracellular hemozoin and residual trophozoites. The ring-containing bottom fraction (parasitemia, 20%-25% rings) was washed, and lipids were extracted from half of the cells after 1 hour of reincubation. The remaining cells were incubated for another 24 hours to allow maturation of parasites. Lipids were extracted from separated trophozoites at this time. The purity of trophozoites was 95% to 97% as assessed by microscopic inspection. Nonparasitized RBCs were kept and treated as parasitized RBCs. Before lipid extraction, hemozoin collected from the top of the gradient was washed 5 times with ice-cold 10 mM phosphate buffer containing 10 mM mannitol, pH 7.4.
In vitro peroxidation of PUFA by hemozoin
Hemozoin and nonparasitized RBCs were suspended in ice-cold phosphate-buffered saline (PBS), adjusted to pH 5.5 or 7.4 at 2 mM final heme concentration, corresponding to 100 μL packed RBCs per milliliter. Arachidonic or linoleic acid (100 μM, final) (Serva, Heidelberg, Germany) was added as exogenous substrates immediately before cell lysis by sonication. Hemozoin and cell lysates were incubated for 20 hours at room temperature in presence of oxygen. Hydrogen peroxide (100 μM, final) was added once to the hemozoin samples at the start of incubation. In control samples where 100 μM hydrogen peroxide was added to PUFA, no PUFA peroxidation was observed (not shown). In all cases, the reaction was stopped by adding one volume of ice-cold methanol containing acetic acid (1% vol/vol, final). After centrifugation the supernatant was collected and analyzed for OH-PUFA and PUFA by high-performance liquid chromatography (HPLC).
In vitro lipoxygenase activity assay
Trophozoites separated from nonsynchronized cultures, hemozoin, and nonparasitized RBCs were suspended in ice-cold PBS, adjusted to pH 7.4 at 2 mM final heme concentration, corresponding to 100 μL packed RBCs per mL. Arachidonic or linoleic acid (100 μM, final) (Serva) was added as exogenous substrates immediately before cell lysis by sonication. Cell lysates and hemozoin were incubated at room temperature in presence of oxygen. The reaction was stopped after 15 minutes by adding one volume of ice-cold methanol containing acetic acid (1% vol/vol, final). For further treatment, see “In vitro peroxidation of PUFA by hemozoin.”
Lipid extraction and alkaline hydrolysis
Stage-separated parasitized RBCs, hemozoin, and nonparasitized RBCs were sedimented by centrifugation and kept on ice. Lipids were extracted from 0.5 to 1.0 mL RBCs (hematocrit, 70%) or hemozoin suspension according to Bligh and Dyer.16 The chloroform phase was collected, and the solvent was evaporated in a stream of nitrogen. The lipid extracts were reconstituted in methanol and split in 2 aliquots. The first aliquot was directly analyzed for free OH-PUFA, and the second one was subjected to alkaline hydrolysis. The lipid extracts were diluted with anaerobic methanol to 0.85 mL final volume, and the ester lipids were hydrolyzed for 30 minutes at 60°C after addition of 0.15 mL KOH (4% wt/vol). Hydrolysis was stopped by the addition of 0.15 mL glacial acetic acid. After centrifugation, aliquots of the clear supernatant were directly injected to reverse phase (RP)–HPLC.
HPLC analysis of PUFA and OH-PUFA
HPLC analysis was performed on a Shimadzu SPD-M10AVP instrument (Duisburg, Germany) coupled with a diode array detector. RP-HPLC was accomplished on a Nucleosil C-18 column (KS-system, 250 × 4 mm, 5 μm particle size; Macherey/Nagel, Düren, Germany). The free fatty acid derivatives were eluted with the solvent system methanol/water/acetic acid (85:15:0.1, vol/vol) at a flow rate of 1 mL/minute. The absorbances at 235 nm (detection of OH-PUFA) and at 210 nm (detection of PUFA) were recorded simultaneously. The chromatographic scale was calibrated for conjugated dienes (5-point calibration curve) by injecting known amounts of 13(S)-hydroxyoctadeca-9Z,11E-dienoic acid (13-HODE) (Cayman, Ann Arbor, MI). Under these experimental conditions, all major hydroxy polyenoic fatty acid isomers originating from linoleic acid (HODE isomers) and arachidonic acid (HETE isomers) coeluted in the time interval of 5 to 7 minutes. The HPLC fractions containing the hydroxy fatty acids were pooled, the solvent was evaporated under vacuum, and the remaining lipids were reconstituted in 0.2 mL of N-hexane containing 1% (vol/vol) acetic acid. Analysis of the positional HODE and HETE isomers was performed by solid phase (SP)–HPLC on a Zorbax SIL column (250 × 4.6 mm, 5 μm particle size; DuPont, Wilmington, DE). OH-PUFA isomers were eluted with the solvent systemN-hexane/2-propanol/acetic acid (100:2:0.1, vol/vol/vol) at a flow rate of 1 mL/min, and the absorbances at 235 nm were recorded. The enantiomer composition of selected OH-PUFA isomers isolated from rings and trophozoites was analyzed on a Chiralcel OD column (250 × 4.6 mm, 5 μm particle size; Diacel Chemical Industries, Tokyo, Japan) with the solvent systemN-hexane/2-propanol/acetic acid (100:5:0.1, vol/vol/vol). Here again, the absorbance at 235 nm was recorded. The chemical structure of the major OH-PUFAs (12-HETE, 13-HODE, 9-HODE) was confirmed from cochromatography with authentic standards in reverse phase–, normal phase– and chiral phase–HPLC, from UV-spectroscopy and gas chromatography/mass spectroscopy.
Opsonization of hemozoin, trophozoites, and nonparasitized RBCs
Isolated hemozoin (see “HPLC analysis of PUFA and OH-PUFA”) was washed once and finely dispersed with PBS. One volume of freshly prepared human AB+ serum was added immediately to the hemozoin suspension (approximately 50% wt/vol), and the mixture was incubated for 30 minutes at 37°C. Trophozoites (hematocrit, 50%) were incubated with human AB+ serum for 30 minutes at 37°C. Freshly drawn nonparasitized RBCs (hematocrit, 50%) were incubated with human anti-D immunoglobulin G (IgG; Rhesuman Berna, Institut für Serotherapeutica und Vaccine, Bern, Switzerland) as described.10
Delipidization of trophozoites and hemozoin
Trophozoites and hemozoin were delipidized according to Bligh and Dyer.16 To remove lipids completely, the procedure was repeated twice. After the last extraction, delipidized trophozoites and hemozoin were collected from the interphase, the organic solvents were evaporated under nitrogen, and trophozoites and hemozoin were washed 5 times with excess PBS. The delipidized trophozoites and hemozoin were passed through a needle to obtain a fine dispersion before one volume of human AB+ serum was added. After 30 minutes of incubation at 37°C, the delipidezed trophozoites and hemozoin were recovered and washed free of plasma.
Assay of oxidative burst in variously treated monocytes
Human peripheral monocytes were isolated from freshly prepared buffy coats by Ficoll (Sigma) density centrifugation.10For experiments with suspended monocytes, monocytes were kept at 107 cells/mL in AIM V serum-free cell culture medium (GIBCO-BRL, Karlsruhe, Germany) in a humidified CO2/air incubator at 37°C. The monocytes were distributed in 5 aliquots, and trophozoites, delipidized trophozoites, hemozoin, and opsonized nonparasitized RBCs were added at 50 RBCs (or RBC equivalents in case of hemozoin) per monocyte. To standardize phagocytosis conditions, the heme meal uniformly consisted of 100 fmol heme/monocyte. The last monocyte aliquot served as a negative control without phagocytosis. Immediately afterward, the monocytes were centrifuged at 500g for 5 seconds to allow rapid contact with RBCs or hemozoin. The monocytes were incubated in the humidified CO2/air incubator at 37°C until measurement of oxidative burst. At indicated times, aliquots were transferred to a test tube for measuring luminescence in a luminol-dependent assay after stimulation of monocytes by addition of 100 nM phorbol 12-myristate 13-acetate (PMA) (Sigma) as described.10 The effect of 12-HETE, 15-HETE, 13-HODE, 13(S)-hydroperoxy-(9Z,11E)-octadecadienoic acid (HpODE) (Cayman), and arachidonic acid (Serva) on the PMA-elicited burst was measured in adherent monocytes to allow the removal of additions before luminescence measurement. Suspended monocytes (2 mL) were plated at 107 cells/mL AIM V serum-free cell culture medium in plastic dishes (3-cm diameter). After 30 minutes of adhesion period in a humidified CO2/air incubator at 37°C, nonadherent cells were removed by repeated washings. During the preincubation with additions, monocytes were kept in RPMI 1640 to avoid binding of added substances to extracellular proteins. Immediately before the luminescence assay, the medium was removed and PBS containing 10 mM glucose and 100 nM PMA was added. At burst peak, the supernatant was transferred to a lumimeter tube, and generation of oxygen radicals was quantified by measuring luminescence in a luminol-dependent assay.10
Results
Inhibition of PMA-elicited oxidative burst of monocytes fed with trophozoites, delipidized trophozoites, or hemozoin
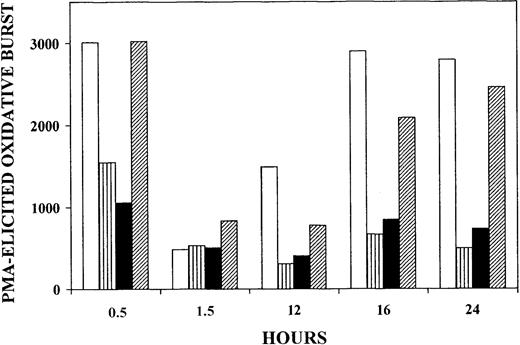
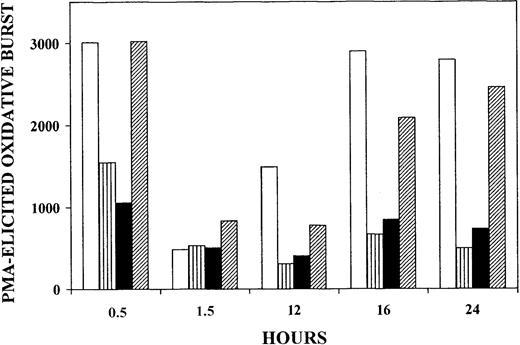
Thirty minutes after onset of phagocytosis of nonparasitized RBCs, PMA-elicited oxidative burst was virtually unaltered when compared with control monocytes without phagocytosis. However, after 90 minutes the oxidative burst was down to about 15% of the initial level but recovered completely after 16 hours of incubation. In contrast, when trophozoites or hemozoin were phagocytosed (Figure1 striped and solid bars, respectively), the oxidative burst was irreversibly inhibited by more than 50% after 30 minutes, decreased to about 15% after 90 minutes, and did not recover significantly at later time points. When delipidized trophozoites were phagocytosed (Figure 1 hatched bars), oxidative burst was not impaired after 30 minutes, despite comparable intensity of phagocytosis indicated by similar numbers of ingested particles, as assessed by microscopic inspection (not shown). After 90 minutes, the oxidative burst was down to about 30% of the initial level but recovered time dependently, reaching 90% of the initial level after 24 hours. In a separate series of experiments, phagocytosis of delipidized hemozoin poorly affected PMA-elicited burst (−17% and −21% burst inhibition at 20 minutes and 17 hours, respectively). These data suggest that irreversible inactivation of monocyte oxidative burst was linked to alteration in the lipid compartment of parasitized RBCs and hemozoin, and lipid peroxidation was likely to be involved. It should be noted that, as outlined in “Discussion,” monocytes phagocytosed similar quantities of trophozoites or hemozoin (approximately 10 trophozoites or trophozoite equivalents per monocyte)10 and ingested approximately the same amount of HETEs (approximately 33 and 39 micromoles per liter monocytes, respectively).
Inhibition of PMA-elicited oxidative burst of human monocytes fed with trophozoites, delipidized trophozoites, or hemozoin.
Suspended monocytes were incubated after addition of nonparasitized RBCs (■), trophozoites (▥), isolated hemozoin (▪), or delipidized trophozoites (▨). Cells and hemozoin were opsonized as indicated and added at time zero to the monocyte suspension at 50 RBC/RBC equivalents (hemozoin) per monocyte. In all cases the same amount of heme was fed to the monocytes. The monocytes were kept in the humidified CO2/air incubator at 37°C, and PMA-elicited oxidative burst was quantified by measuring luminescence in a luminol-dependent assay at the indicated times. Luminescence values are expressed as counts per second per 411 monocytes. For details, see “Materials and methods.” One experiment representative of 4 with similar results is shown.
Inhibition of PMA-elicited oxidative burst of human monocytes fed with trophozoites, delipidized trophozoites, or hemozoin.
Suspended monocytes were incubated after addition of nonparasitized RBCs (■), trophozoites (▥), isolated hemozoin (▪), or delipidized trophozoites (▨). Cells and hemozoin were opsonized as indicated and added at time zero to the monocyte suspension at 50 RBC/RBC equivalents (hemozoin) per monocyte. In all cases the same amount of heme was fed to the monocytes. The monocytes were kept in the humidified CO2/air incubator at 37°C, and PMA-elicited oxidative burst was quantified by measuring luminescence in a luminol-dependent assay at the indicated times. Luminescence values are expressed as counts per second per 411 monocytes. For details, see “Materials and methods.” One experiment representative of 4 with similar results is shown.
Quantification of PUFAs and OH-PUFAs in parasitized RBCs and hemozoin
Total PUFAs have been quantified in parasitized RBCs and hemozoin. The levels of extracted PUFAs were 713 ± 49 mg/L RBCs in nonparasitized RBCs; 706 ± 56 mg/L RBCs in rings; 848 ± 119 mg/L RBCs in trophozoites; and 2383 ± 405 mg/L RBC equivalents in hemozoin from 4 separate experiments. Peroxidation of PUFA leads to the formation of hydroperoxy-PUFA that is rapidly reduced to the corresponding stable OH-PUFA. The OH-PUFA/PUFA ratio expressed as a percentage of OH-PUFA related to total PUFA appears to be a suitable measure of peroxidation of cell lipids. The OH-PUFA/PUFA ratio was determined in a single HPLC run, recording the column eluent simultaneously at 235 nm (OH-PUFA) and 210 nm (PUFA) (not shown).
As shown in Figure 2, in the lipid extracts of nonparasitized RBCs, a OH-PUFA/PUFA ratio of 0.08% was determined. In contrast, much higher OH-PUFA/PUFA ratios were found in parasitized RBCs. The ratio increased with parasite maturation and attained 0.48% in rings, 1.26% in trophozoites, and 1.15% in isolated hemozoin. The above percentage values of OH-PUFAs were converted to concentrations expressed as micromoles per liter RBCs or micromoles per liter RBC equivalents for hemozoin, under assumption of heme concentration of 20 millimoles per liter RBCs, and substantial constancy of total heme throughout the parasite development and in hemozoin.17 The concentrations of OH-PUFAs were 1.8 micromoles per liter RBCs in nonparasitized RBCs; 11.1 micromoles per liter RBCs in rings; 35 micromoles per liter RBCs in trophozoites; and 90 micromoles per liter RBC equivalents in hemozoin isolated on day 2 after reinfection. The concentration of OH-PUFA in hemozoin increased time dependently with the duration of culture. For example, after 6 complete growth cycles 1400 micromoles OH-PUFA per liter RBC equivalents was measured, corresponding to 4.6% of total PUFAs. Notably, most of the OH-PUFAs was localized in the ester-lipid fraction. The amount of nonesterified OH-PUFAs was negligible in rings and trophozoites but made up approximately 7% of total OH-PUFAs in hemozoin (not shown).
Quantification of OH-PUFA in stage-separated parasitized RBCs and hemozoin.
The OH-PUFA/PUFA ratio is expressed as percentage of OH-PUFA ± SD from 4 separate experiments related to total PUFAs in nonparasitized RBCs, rings, trophozoites, and hemozoin. The ring and trophozoite data were corrected to 100% parasitemia. Rings and trophozoites were harvested 12 and 36 hours after reinfection from synchronized cultures (parasitemia, 23%). Control RBCs kept in culture for the same time periods were treated identically. Lipids were extracted from the intact cells or hemozoin and analyzed for OH-PUFA and PUFA by HPLC. The levels of extracted PUFAs from 4 separate experiments were 713 ± 49 mg/L RBCs in nonparasitized RBCs, 706 ± 56 mg/L RBCs in rings, 848 ± 119 mg/L RBCs in trophozoites, and 2383 ± 405 mg/L RBC equivalents in hemozoin. For details, see “Materials and methods.”
Quantification of OH-PUFA in stage-separated parasitized RBCs and hemozoin.
The OH-PUFA/PUFA ratio is expressed as percentage of OH-PUFA ± SD from 4 separate experiments related to total PUFAs in nonparasitized RBCs, rings, trophozoites, and hemozoin. The ring and trophozoite data were corrected to 100% parasitemia. Rings and trophozoites were harvested 12 and 36 hours after reinfection from synchronized cultures (parasitemia, 23%). Control RBCs kept in culture for the same time periods were treated identically. Lipids were extracted from the intact cells or hemozoin and analyzed for OH-PUFA and PUFA by HPLC. The levels of extracted PUFAs from 4 separate experiments were 713 ± 49 mg/L RBCs in nonparasitized RBCs, 706 ± 56 mg/L RBCs in rings, 848 ± 119 mg/L RBCs in trophozoites, and 2383 ± 405 mg/L RBC equivalents in hemozoin. For details, see “Materials and methods.”
Separation, identification, and quantification of OH-PUFA isomers in parasitized RBCs and hemozoin: evidences against lipoxygenase involvement
To determine their biosynthetic pathway, the structure of OH-PUFAs found in parasitized RBCs was analyzed in more detail. In biologic systems and in presence of heme, lipid peroxidation can proceed enzymatically via lipoxygenase catalysis or nonenzymatically via heme-dependent catalysis. Lipoxygenases form specific patterns of oxygenated PUFAs with a strong preponderance of a single product isomer.
For instance, 15-lipoxygenase and 5-lipoxygenase convert arachidonic acid almost exclusively to 15S-HETE or 5S-HETE, respectively, with other HETE isomers being minor side products.18 19 In contrast, nonenzymatic peroxidations form a complex product pattern, which involves all positional and optical isomers. Thus, the complexity of the product pattern and the enantiomer composition may help to define the mechanism of OH-PUFA formation. In hemozoin and parasitized RBCs a complex mixture of hydroxy-linoleic acid and hydroxy-arachidonic acid isomers was detected.
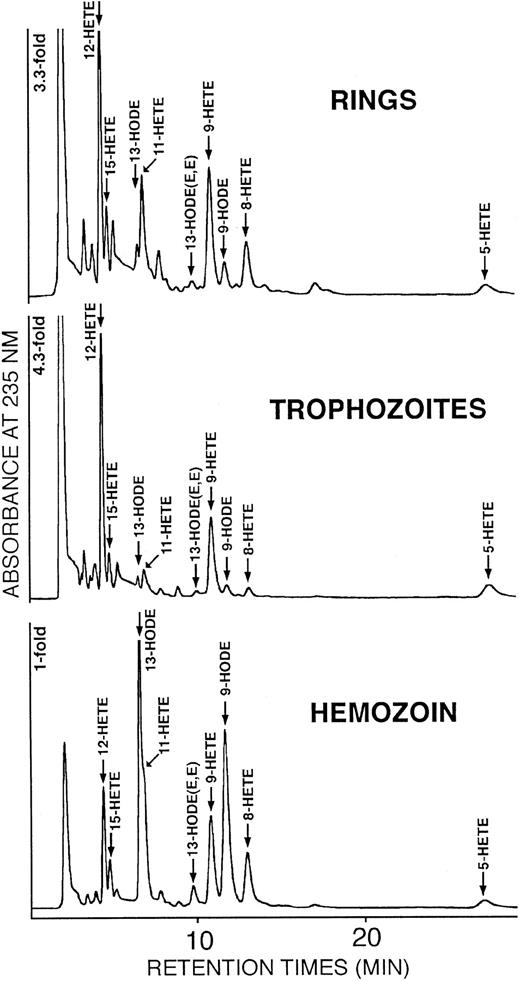
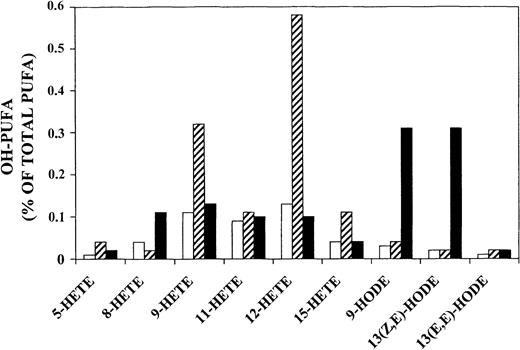
In rings and trophozoites (Figure 3 upper and middle panel and Figure 4) HETEs were predominant (90% and 93% of total OH-PUFAs, respectively), and 12-HETE was the major oxygenation product. In hemozoin (Figure 3 lower panel and Figure 4) hydroxy-linoleic acid isomers (9-HODE and 13-HODE) were slightly predominant and HETEs represented only 44% of total OH-PUFAs. Smaller amounts of 6 hydroxy-arachidonic acid isomers (5-, 8-, 9-, 11-, 12-, 15-HETE) were also detected in hemozoin. Taking into account that hemozoin contained more total PUFAs (2383 versus 848 mg/L RBCs) but less HETEs (44% of OH-PUFAs) compared with trophozoites (93% of OH-PUFA), it finally results that cellular concentrations of HETEs calculated from the data of Figures 2 and 4 were 33 to 39 micromoles per liter RBCs or RBC equivalents in trophozoites and hemozoin, respectively. HODEs were 2.5 and 50 micromoles per liter RBCs or RBC equivalents in trophozoites and hemozoin, respectively.
Separation and identification of OH-PUFA isomers in stage-separated parasitized RBCs and hemozoin.
Rings, trophozoites, and hemozoin were separated from synchronized cultures, and the lipids were extracted. The solvents were removed, the remaining lipids were hydrolyzed under alkaline conditions, and OH-PUFA was analyzed by RP-HPLC (see “Materials and methods” for details). Aliquots of the OH-PUFA fraction were dried under vacuum, the remaining lipids were reconstituted in N-hexane containing 0.1% acetic acid, and aliquots were analyzed by SP-HPLC as described in “Materials and methods.” The chemical structure of the major OH-PUFAs (12-HETE, 13-HODE, 9-HODE) was confirmed from cochromatography with authentic standards in reverse phase–, normal phase–, and chiral phase–HPLC, from UV-spectroscopy and gas chromatography/mass spectroscopy.
Separation and identification of OH-PUFA isomers in stage-separated parasitized RBCs and hemozoin.
Rings, trophozoites, and hemozoin were separated from synchronized cultures, and the lipids were extracted. The solvents were removed, the remaining lipids were hydrolyzed under alkaline conditions, and OH-PUFA was analyzed by RP-HPLC (see “Materials and methods” for details). Aliquots of the OH-PUFA fraction were dried under vacuum, the remaining lipids were reconstituted in N-hexane containing 0.1% acetic acid, and aliquots were analyzed by SP-HPLC as described in “Materials and methods.” The chemical structure of the major OH-PUFAs (12-HETE, 13-HODE, 9-HODE) was confirmed from cochromatography with authentic standards in reverse phase–, normal phase–, and chiral phase–HPLC, from UV-spectroscopy and gas chromatography/mass spectroscopy.
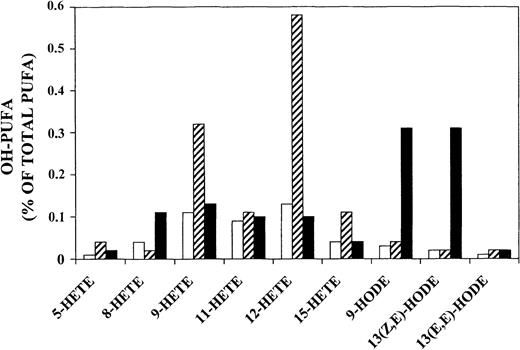
Quantification of OH-PUFA isomers in stage-separated parasitized RBCs and hemozoin.
The OH-PUFA/PUFA ratio is expressed as percentage of specific OH-PUFA isomers related to total PUFAs in rings (■), trophozoites (▨), and hemozoin (▪). The chromatograms shown in Figure 3 were quantified by measuring peak areas.
Quantification of OH-PUFA isomers in stage-separated parasitized RBCs and hemozoin.
The OH-PUFA/PUFA ratio is expressed as percentage of specific OH-PUFA isomers related to total PUFAs in rings (■), trophozoites (▨), and hemozoin (▪). The chromatograms shown in Figure 3 were quantified by measuring peak areas.
Such a complex product pattern strongly suggests nonenzymatic, heme-catalyzed lipid peroxidation as the biosynthetic route. However, the preponderance of 9-HETE and 12-HETE in trophozoites suggests an oxygenation process specifically induced or modified by the parasite. As further evidence against the involvement of a parasite-specific lipoxygenase, the enantiomer composition of 12-HETE isolated from trophozoites was analyzed.
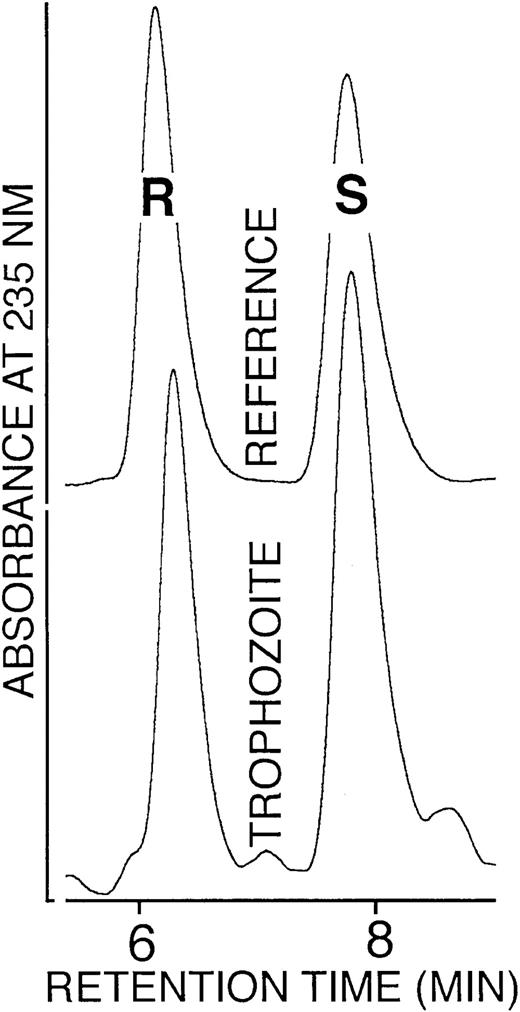
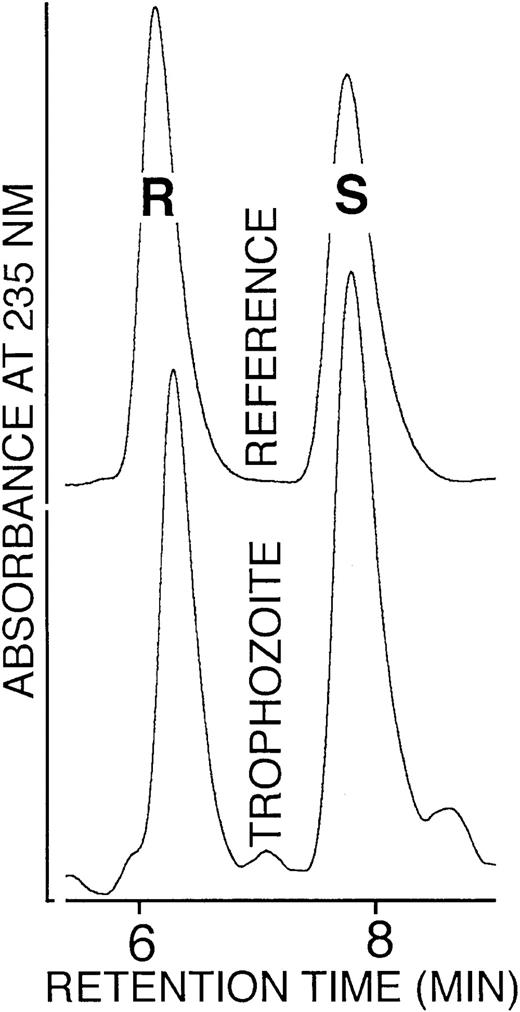
As shown in Figure 5, a close to 1:1 mixture of 12S- and 12R-HETE was found. A similar 1:1 S/R ratio was also detected for 9- and 13-HODE isolated from trophozoites (not shown). Such racemic enantiomer composition is not compatible with a lipoxygenase-mediated origin. Finally, incubation of trophozoite lysates or hemozoin with linoleic or arachidonic acid did not produce OH-PUFA, confirming the absence of functional lipoxygenase (not shown). Moreover, a search of parasite databases did not provide any evidence for a parasite-specific lipoxygenase. Taken together, these data rule out a lipoxygenase-mediated origin of OH-PUFA.
Enantiomer composition of 12-HETE isolated from trophozoites.
The 12-HETE was prepared from trophozoites by lipid extraction, alkaline hydrolysis, and sequential RP-HPLC and SP-HPLC. The SP-HPLC eluate fractions containing 12-HETE were collected, the solvent was removed under vacuum, the remaining lipids were reconstituted in N-hexane containing 0.1% acetic acid, and aliquots were analyzed by chiral phase HPLC as described in “Materials and methods.”
Enantiomer composition of 12-HETE isolated from trophozoites.
The 12-HETE was prepared from trophozoites by lipid extraction, alkaline hydrolysis, and sequential RP-HPLC and SP-HPLC. The SP-HPLC eluate fractions containing 12-HETE were collected, the solvent was removed under vacuum, the remaining lipids were reconstituted in N-hexane containing 0.1% acetic acid, and aliquots were analyzed by chiral phase HPLC as described in “Materials and methods.”
To confirm the ability of hemozoin to generate OH-PUFAs from PUFAs via heme catalysis, native and delipidized hemozoins were incubated in vitro with linoleic or arachidonic acid at pH 7.4, which represents the cytosolic pH of parasitized RBCs, and at pH 5.5 to mimic the conditions in the digestive vacuole. During a 20-hour incubation period about 1% of the supplemented PUFA was transformed to the corresponding OH-PUFA, and there was no significant difference between the 2 pH values (not shown). Moreover, the extent of PUFA peroxidation was essentially the same when the incubation was performed in absence or presence of hydrogen peroxide (not shown).
12- and 15-HETE mimic the toxic effects of hemozoin phagocytosis
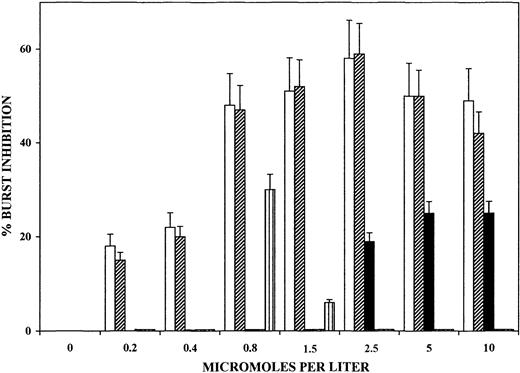
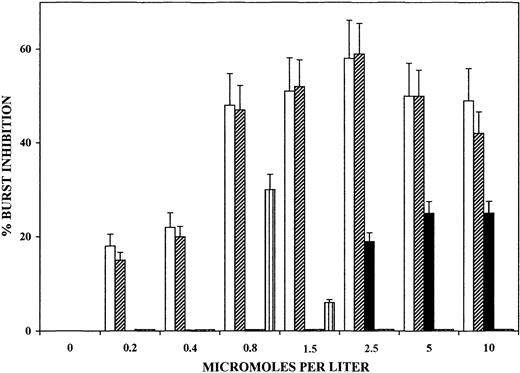
The large amounts of OH-PUFA detected in trophozoites and isolated hemozoin as well as the inhibition of the PMA-elicited oxidative burst by lipophilic compounds of trophozoites prompted us to investigate the direct effects on oxidative burst of selected OH-PUFAs and their nonoxygenated parent compounds. As shown in Figure6, 12- and 15-HETE dose dependently inhibited PMA-elicited oxidative burst in monocytes. Inhibition by HETE was evident already at submicromolar concentration and attained approximately 60% inhibition at 1 μM concentration. A 10-minute preincubation period of monocytes with the additives was sufficient to cause inhibition, and there was no significant increase after longer incubations (not shown). In contrast, 13-HODE and 13-HpODE, the peroxidation products of linoleic acid, were ineffective at up to 10 μM concentration. Arachidonic acid elicited 20% to 25% burst inhibition at 2.5 to 10 μM concentration. Supplementation of delipidized hemozoin with 15-HETE at hemozoin-compatible amounts and stoichiometry increased burst inhibition to the levels elicited by native hemozoin (not shown).
Dose-dependent inhibition of PMA-elicited oxidative burst of human monocytes by selected OH-PUFA.
Adherent monocytes were incubated for 10 minutes with 12-HETE (■), 15-HETE (▨), arachidonic acid (▪), 13-HODE (▥), and 13-HpODE (dotted bars, close to nil) in the humidified CO2/air incubator at 37°C. After changing medium to remove additions, PMA-elicited oxidative burst was quantified at burst peak by measuring luminescence in a luminol-dependent assay. Values represent the percentages of inhibition of PMA-elicited oxidative burst in treated versus untreated control monocytes. For details, see “Materials and methods.” Data are expressed as means ± SD of 3 separate experiments.
Dose-dependent inhibition of PMA-elicited oxidative burst of human monocytes by selected OH-PUFA.
Adherent monocytes were incubated for 10 minutes with 12-HETE (■), 15-HETE (▨), arachidonic acid (▪), 13-HODE (▥), and 13-HpODE (dotted bars, close to nil) in the humidified CO2/air incubator at 37°C. After changing medium to remove additions, PMA-elicited oxidative burst was quantified at burst peak by measuring luminescence in a luminol-dependent assay. Values represent the percentages of inhibition of PMA-elicited oxidative burst in treated versus untreated control monocytes. For details, see “Materials and methods.” Data are expressed as means ± SD of 3 separate experiments.
Discussion
Large amounts of OH-PUFA, the stable derivatives of peroxidized PUFA, were found to accumulate in P falciparum–parasitized RBCs and isolated hemozoin. The OH-PUFA/PUFA ratio, expressed as percentage of OH-PUFA related to total PUFA, increased stage dependently from 0.08% in nonparasitized RBCs to 0.48% in rings, 1.26% in trophozoites, and 1.15% in hemozoin. Similar ratios have been reported in biologic membranes20and lipoproteins21 treated in vitro with high concentrations of lipoxygenase. Thus, our data indicate a severe oxidative stress in the lipids of parasitized RBCs and hemozoin. Permanence of hemozoin in culture during longer incubation periods was accompanied by further increase in esterified OH-PUFA, that attained 4.6% of total PUFAs after 6 complete growth cycles. Six HETE-isomers (5-, 8-, 9-, 11-, 12-, and 15-HETE) and 2 major isomers of HODE (9- and 13-HODE) were found in parasitized RBCs and isolated hemozoin. Although the HETE isomers were predominant in parasitized RBCs (90% and 93% of total OH-PUFAs in rings and trophozoites, respectively), the HODE isomers amounted to 56% of total OH-PUFAs in isolated hemozoin. Importantly, most HETE and HODE isomers were present in the ester lipid fraction. Rings and trophozoites had negligible amounts of free OH-PUFA, and in hemozoin only 7% of total OH-PUFA was free. These data indicate that membrane phospholipids were the major substrates of lipid peroxidation in parasitized RBCs and hemozoin.
OH-PUFAs, including HETE and HODE, can be synthesized in vivo via the lipoxygenase reaction and/or by nonenzymatic lipid peroxidation. Lipoxygenases with different positional specificity are expressed in a number of mammalian cell types and in several circulating blood cells, including reticulocytes but not in mature RBCs.22-24 The expression of lipoxygenase isoforms in P falciparum has not been investigated so far. We did not detect any lipoxygenase activity in various maturation stages of the parasite. Moreover, we were unable to extract lipoxygenase sequences from parasite genomic databases. The most likely alternative biosynthetic pathway for the OH-PUFAs detected in parasitized RBCs is heme-catalyzed lipid peroxidation, in agreement with the complex pattern of oxygenation products and the 1:1 enantiomer composition of the major positional isomers.
The developing P falciparum relies heavily on host Hb as a source of amino acids and iron.1 Hb is transferred to the parasite digestive vacuole, where it is degraded to small peptides and heme that is predominantly polymerized to hemozoin.2 High concentrations of hemozoin and the acidic pH in the digestive vacuole are likely to favor unspecific heme-catalyzed lipid peroxidations, leading to a complex pattern of oxygenation products. It is well known (reviewed in Everse and Hsia25) that free or protein-bound heme can catalyze peroxidation of PUFAs to generate keto-, hydroxy-, and dihydroxy-PUFAs25,26 as well as several terminal hydroxy-aldehydes, such as 4-hydroxynonenal and malonaldehyde.27,28 Moreover, native and artificial hemozoins (beta-hematin) were able to oxygenate exogenous arachidonic acid to 5-, 12-, and 15-HETE in the presence of 500 μM hydrogen peroxide.29 Hb- or hemozoin-heme may come into contact with phospholipid micelles or with small lipid-containing membrane fragments present as remnants of the vesicles that carry portions of RBC cytoplasm into the digestive vacuole. Phospholipids, strongly increased in the parasitized RBCs,30 and selected PUFAs appear to play an important role as seeding molecules to initiate polymerization of heme to hemozoin.31-33
For a long time hemozoin has been considered a metabolically inert side product of Hb digestion. More recently hemozoin was shown to display toxic effects when phagocytosed by monocytes. For instance, hemozoin-laden monocytes were unable to repeat phagocytosis, to produce oxidative burst,10 and to kill ingested bacteria.34 Moreover, hemozoin loading upsets cytokine production in monocytes and polymorphonuclear leukocytes12-14 and appeared to be involved in malaria-associated immunodepression.11 15
The data presented here are the first indication that specific components of the lipid fraction of trophozoites and hemozoin were responsible for the abrogation of oxidative burst. Trophozoites and hemozoin contained similar amounts of HETEs (approximately 33-39 micromoles per liter RBCs or RBC equivalents, respectively), and monocytes phagocytosed similar quantities of trophozoites and hemozoin (approximately 10 trophozoites or trophozoite equivalents per monocyte).10 Thus, in both cases monocytes ingested approximately the same amount of HETEs, namely approximately 33 to 39 micromoles per liter monocytes, respectively, assuming homogeneous distribution and a monocyte spherical volume of 1000 fL.35The supplemented 12- or 15-HETE that elicited burst inhibition at micromolar concentration (Figure 6) was, therefore, in the same concentration range as in trophozoite- or hemozoin-fed monocytes, even assuming incomplete hydrolysis of esterified OH-PUFAs by monocyte membrane phospholipase A36 or by lysosomal phospholipases preferentially active on peroxidized phospholipids.37 It is in fact possible that free OH-PUFAs as well as other toxic lipid derivatives were generated in the phagocyte, in analogy to the formation of large amounts of 4-hydroxynonenal previously observed in hemozoin-fed monocytes.38
Other reported effects of 15-HETE may also point toward its role in malaria pathophysiology. 15-HETE was found to stimulate RBC adherence to capillary endothelia,39 enhance vascular permeability and edema,40 and induce chemotaxis and chemokinesis.41 These effects may mechanistically explain hallmarks of severe malaria such as adherence of parasitized RBCs to endotelia,42 cerebral edema, and recruitment of phagocytes around cerebral capillaries.43
It has been reported that prostaglandins PGD2, PGE2, and PGF2a were formed by P falciparum–parasitized RBCs44 and by other hemozoin-forming parasites such as Schistosoma mansoni45 without apparent evidence of cyclooxygenase activity.46 Prostaglandins have been attributed important roles in parasitic infections and contribute significantly to parasite-induced immunodepression.44,46,47 Interestingly, free and bound heme exhibit selective “quasi-cyclooxygenase” activity that converts arachidonic acid into PGE2 and PGF2a,48 the same prostaglandins generated byP falciparum44 and S mansoni.46 Thus, it is plausible to assume that also specific prostaglandins are generated nonenzymatically by heme-catalysis in Hb-catabolyzing parasites.
In conclusion, hemozoin formation in the parasitized RBCs and its long-term stability in host phagocytes appear to constitute 2 functionally synergic phenomena in P falciparum malaria. The first phenomenon is due to the absence of heme-catabolyzing enzymes in the parasite49 and is the prerequisite for the formation of a series of powerful lipid derivatives via heme catalysis. The second is due to the inability of host phagocytes to degrade hemozoin and to the extremely long persistence of hemozoin in resident phagocytes.50 As final consequences, phagocytosed hemozoin may ferry large amounts of diverse, partially very complex bioactive lipid derivatives into the host phagocytes. However, persistent hemozoin ensures the continuing production by phagocytes of lipid derivatives during and after acute malaria, inflicting damages to the host even after the elimination of the living parasite. In fact, it has been shown that monocytes fed with hemozoin produce and accumulate large amounts of lipoperoxides and 4-hydroxynonenal over long periods of time.38 It is unlikely that both phenomena are the result of evolutionary accidents. We believe instead that they represent a powerful and original strategy of subversion of host defense developed to perfection by P falciparum, one of the most successful parasites worldwide.
Prepublished online as Blood First Edition Paper, August 29, 2002; DOI 10.1182/blood-2002-03-0979.
Supported by grants from Humboldt-University Medical School (Charité), Berlin, Germany (E.S. and H.K.); from Deutsche Forschungsgemeinschaft (Grant Ku961/7-1) (H.K.); from Compagnia di San Paolo, Torino, Italy, the Italian Ministry of University (COFIN-MURST 1999), and the University of Torino Medical School Intramural Funds (P.A.); and from the Italy-Germany Science Cooperation Program (Villa Vigoni Project) (E.S. and P.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Paolo Arese, Dipartimento di Genetica, Biologia e Biochimica, Università di Torino, Via Santena 5 bis, 10126 Torino, Italy; e-mail: paolo.arese@unito.it.