Here we report that histone deacetylase inhibitors (HDAC-i) comprise a new class of immunosuppressive agents. HDAC-i inhibited CD4 T-cell proliferation in a dose-dependent manner, which was not caused by apoptosis or decreased viability. Although early intracellular signals such as tyrosine kinase activity and elevation of intracellular calcium concentration were not affected, the characteristic aggregation of T cells following activation was completely abrogated. This correlated with diminished activation-induced expression of the adhesion molecules. HDAC-i furthermore inhibited activation-induced CD25 and CD154 expression on CD4 cells, without affecting induction of CD69. HDAC-i inhibited CD154 expression by a mechanism distinctly different from cyclosporine-mediated inhibition. HDAC-i thus inhibited interleukin 2 (IL-2)–induced CD154 expression on effector T cells and constitutively expressed CD154 on various tumor cells, events that were not affected by cyclosporine. Additional studies showed that HDAC-i treatment inhibited c-Myc expression, which was further shown to be important for CD154 gene activation. These results demonstrate pronounced T-cell inhibitory activity of HDAC-i, which may form the basis of novel therapeutic interventions against autoimmune diseases and allograft rejection.
Introduction
Histone deacetylase inhibitors (HDAC-i) are potent inducers of apoptosis and growth inhibition in a variety of transformed cells in vitro and in vivo, including malignancies originating from lymphoid cells.1-3 On the other hand, HDAC-i are relatively nontoxic to normal cells, when measured by viability,2 making pharmacological reagents with histone deacetylase inhibitory activity good candidates for novel antitumor therapy.
Several different agents have been identified as having HDAC-i activity, including trichostatin A, trapoxin, oxamflatin, apicidin, phenylbutyrate, suberoylanilide hydroxamic acid (SAHA), pyroxamide, and FR901228; at least the last 4 are currently in clinical trials for evaluation of their anticancer efficacy.4
Histones are core structural components of nucleosomes and play a key role in regulating DNA accessibility to transcriptional regulation. DNA is normally tightly wrapped around nucleosomes. Acetylation of histones prevents their binding to the negatively charged DNA, leading to a more open DNA structure. The degree of acetylation depends on a dynamic equilibrium between histone acetylase (HAT) and histone deacetylase (HDAC) activity. Inhibition of deacetylase activity thus favors the action of histone acetylases (HATs) leading to hyperacetylation and unfolding of DNA, whereby previously nonaccessible promoter regions become targets for latent or newly transcribed transcription factors, possibly resulting in both positive and negative regulation of transcription.
A number of proteins other than histones are affected by acetylation; these include transcription factors such as p53 and GATA-1.5,6 Moreover, transcription factors such as p300, Rb, GCN5, P/CAF, and Blimp-1 possess/recruit HDAC or HAT activity.7-9 Given the broad spectrum of activities mediated by HDAC-i, it is interesting that only a small number of genes are affected.10 It is currently believed that the initial regulation of gene expression is predominantly regulated by modulation of the promotor acetylation grade through recruitment of transcription factors possessing HDAC/HAT activity.
Several groups have studied HDAC-i–mediated apoptosis and growth inhibition of tumor cells. HDAC-i induce transcriptional activation and protein expression of the cyclin kinase inhibitor p21 cip/waf,11,12,14 and p21 cip/waf expression has been shown to be essential for butyrate-induced growth inhibition of human colon cancer cells.12 In agreement with this, Sandor et al showed that p21 cip/waf–negative NIH3T3 cells lack the ability to be arrested in G1 by FR901228.13
A number of groups have shown that HDAC-i inhibit the constitutive expression of the c-Myc proto-oncogene in various tumor and established cell lines.3,15 c-Myc is an immediate early gene that is involved in both proliferation and apoptosis of normal cells.16,17 Inhibition of c-Myc was previously shown not to be involved in HDAC-i–induced apoptosis of human leukemic lymphoblasts, as transgeneic expression of c-Myc could not inhibit the induction of apoptosis.18
Stra13 is a transcriptional repressor induced by trichostatin A, and ectopic expression of Stra13 inhibits growth of NIH 3T3 cells.19 It is therefore possible that Stra13 expression is causally involved in HDAC-i–mediated growth inhibition; however, experiments with cells containing nonfunctional or deleted Stra13 are required to confirm this hypothesis. Interestingly, Stra13 expression strongly suppresses c-Myc promotor activity in vitro,19making Stra13 expression a possible link to the molecular mechanism behind HDAC-i inhibition of c-Myc expression.
FR901228 has been shown to inhibit the constitutive Fas ligand expression in A1.1 T cells, which correlated with inhibition of c-Myc expression.15 c-Myc was later shown to be involved in FasL expression through a previously unrecognized c-Myc DNA binding domain in the proximal FasL promoter.20 It is therefore tempting to speculate that FR901228 inhibits FasL expression through inhibition of c-Myc expression, although an unrecognized effect of FR901228 cannot be ruled out with the current knowledge.
Trichostatin A has been shown to partially inhibit interleukin 2 (IL-2) signaling in IL-2–dependent hematopoietic cell lines. IL-2 induction of c-Myc, bag-1, and LC-PTP was abolished by trichostatin A; in contrast, IL-2–mediated phosphorylation of SHP-2, Jak1, and Stat5 was not affected, demonstrating specificity of the inhibition.21 Trichostatin A has furthermore been shown to reverse the abnormal expression of CD154, IL-10, and interferon γ (IFN-γ) in SLE T cells.22
Identification and characterization of novel T cell–inhibitory compounds is important for creating new strategies to prevent autoimmune diseases and graft rejection. Although cyclosporine and FK-506 effectively suppress activation of naive T cells, they are poor inhibitors of ongoing effector immune responses, which is reflected in their poor therapeutical effect toward autoimmune diseases.
In this study we describe the immunosuppressive activity of HDAC-i, which, in contrast to previously known immunosuppressors, are able to effectively inhibit CD154 expression on effector T cells, suggesting that this may pave the road for development of effective inhibitors of ongoing immune responses.
Materials and methods
Cells, antibodies, and chemicals
All cells isolated from human blood were grown in media supplemented with 20 U/mL IL-2 (Boehringer Mannheim, Copenhagen, Denmark), 5% human serum or 5% fetal bovine serum (FBS), 2 mM glutamine, and 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid). Jurkat cells were grown in RPMI 1640 supplemented with 10% FBS, 2 mM glutamine, and 2 mM of both penicillin and streptomycin.
Buffy coats from healthy human volunteer donors were obtained from the State Hospital, Copenhagen, Denmark. Peripheral blood mononuclear cells (PBMCs) were obtained using Lymphocyte Separation Media (ICN, Rødovre, Denmark) according to the manufacturer's description. Peripheral blood lymphocytes (PBLs) were obtained from the PBMC fraction by incubation in culture flask with uncoated dynabeads (Dynal, Copenhagen, Denmark), 2 beads per cell, for 2 hours at 37°C. Monocytes and macrophages stick to the culture flask or phagocytose the paramagnetic beads that were depleted by magnetic cell separation (Dynal). CD4 cells were purified from the PBL fraction by incubation with 10 μg/mL of monoclonal antibodies against CD8 (G10-1, ATCC), CD20 (IF5, ATCC), CD14 (F13, ATCC), and CD16 (Beckman Coulter, Birkerød, Denmark) for 20 minutes at 4°C. After washing, cells were depleted twice with swine antimouse immunoglobulin-coupled dynabeads (6 beads per cell, 20 minutes at 4°C) followed by magnetic cell separation. The purity of CD4 cells was routinely 91% to 95% as measured by flow cytometry.
Dendritic cells were generated from PBMCs adhering to the culture flask after 2 hours' culture at 37°C (without dynabeads). After extensive washing, adhering cells were cultured for 7 days in media containing 500 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Boehringer Mannheim) and 12.5 U/mL IL-4 (Boehringer Mannheim). The resulting cell population was weakly adherent and expressed surface markers characteristic of dendritic cells (CD1a, CD11c, CD86, HLA-DR high, and CD4 low; not shown).
FR901228 was obtained from the National Cancer Institute (Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis; Bethesda, MD). Trichostatin A and cyclosporine were obtained from Calbiochem (Rødovre, Denmark) and CFSE (5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester) was obtained from Molecular Probes (Rødovre, Denmark).
Cell stimulation
Cells were stimulated by 3 different methods: (1) dynal beads coupled to anti-CD3 and anti-CD28 antibodies, 3 beads per cell; (2) ionomycin (100 ng/mL) and PMA (phorbol 12-myristate 13-acetate; 10 ng/mL); (3) allogenic dendritic cells (25 000 dendritic cells/200 000 CD4 cells). All cells were stimulated at a concentration of 106 cells/mL. Cells were incubated with FR901228 for 1 to 2 hours prior to stimulation.
Measurement of proliferation
Proliferation assay was performed in quadruplicates in 96-well flat-bottomed plates. Cells were stimulated at 106 cell/mL in a final volume of 200 μL. Proliferation was measured by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay (10 μg/mL MTT, 4 hours at 37°C) or by flow cytometric analysis of CFDA-SE–stained cells (cells were stained with 1 μM CFDA-SE for 10 minutes at 25°C and washed extensively prior to stimulation).
Flow cytometry
Cells were labeled with antibodies (Abs) coupled with fluorescein isothiocyanate (FITC) or phycoerythrin (PE): antihuman CD4 Ab (Immunotech), antihuman CD8 Ab (BD, Rødovre, Denmark), antihuman CD25 Ab (BD), anti-CD11a (BD), antihuman CD11c (Beckman Coulter), antihuman CD54 Ab (BD), antihuman CD69 Ab (BD), antihuman CD154 Ab (BD), antihuman CD86 (BD), anti-HLA-DR Ab (BD), and isotype control Ab. Generally, 2 to 4 × 105 cells were stained with 0.1 to 1 μg/mL antibody for 20 minutes at 4°C. Cells were resuspended and analyzed in phosphate-buffered saline (PBS) or 1% paraformaldehyde. Intracellular staining for IL-2 was done according to the manufacturer's protocol. Briefly, cells were stained for surface proteins as described above, fixed and permeabilized using the BD intracellular staining kit, and labeled with PE-coupled antihuman IL-2 Ab or the corresponding control Ab. Intracellular staining of DNA was performed as follows: cells were washed once in 0.03% saponin/PBS and resuspended in 0.03% saponin/PBS containing 1 μg/mL propidium iodide (PI) or 7-amino-actinomycin D (7-AAD) for 25 minutes at 25°C.
Data acquisition and flow cytometric analysis were performed on a BD FACSCalibur using Cellquest software.
Measurement of intracellular calcium concentration
Measurement of intracellular calcium concentration after anti-CD3 stimulation was done on Fura-2 loaded PBLs as described in Skov et al.23
ELISA
Cytokine production was analyzed by sandwich enzyme-linked immunosorbent assay (ELISA), using the manufacturer's protocol (BD).
Construction of CD154 promotor-EGFP construct
Approximately 2 kb upstream from the CD154 transcription start was polymerase chain reaction (PCR) cloned from a Jurkat E6-1 DNA preparation, using primer set 5′ggggtaccccgaattcactggggagagcattcagg; 5′cgggatcccggggagaagtttggttgtatgtttcg. The fragment was purified and subcloned (BamHI/KpnI) into pEGFP-1 vector (BD) in front and in frame of the EGFP coding sequence.
Transient transfections
Jurkat E6-1 or Jurkat Tag cells were transiently transfected with DMRIE-C reagent, used according to the manufacturer's protocol (Invitrogen, Tåstrup, Denmark). Cells were transfected with 1 to 4 μg DNA/2 × 106 cells, using 4 uL DMRIE-C reagent. The transfection was made in pure RPMI 1640 (without supplements) and cells were incubated for 4 to 5 hours at 37°C (final volume 1.2 mL) prior to addition of 2 mL conditioned RPMI 1640 media (containing 10% FBS) and further culturing overnight.
In vivo experiments with NOD mice
Female NOD mice were screened for development of diabetes by twice-weekly measurement of urinary glucose. When urinary glucose was positive the blood glucose was measured twice weekly. The mice were injected twice a week intraperitoneally with 8 μg FR901228 or vehicle control for a maximum of 8 weeks following initiation of diabetes. Treatment was initiated at the beginning of diabetes development when the blood glucose level rose above 10 mM. Mice with a blood glucose level exceeding 15 mM at the time of treatment initiation were not included in the study. Mice were monitored for at least 3 weeks following the initial treatment; during this period mice with blood glucose levels exceeding 20 mM were injected subcutaneously with 1.0 to 1.5 U insulin twice daily to prevent possible clinical effects of diabetes. From 3 to 16 weeks after treatment initiation, mice were killed if the blood glucose level exceeded 20 mM in 2 consecutive measurements.
Results
HDAC-i inhibit activation-induced proliferation of normal T cells
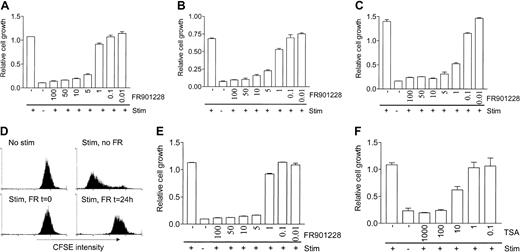
We investigated the effect of HDAC-i on activation-induced T cell proliferation. Peripheral blood lymphocytes (PBLs) were treated with various concentrations of the HDAC-i FR901228 1 hour prior to stimulation with antibodies against CD3 and CD28 coupled with paramagnetic beads (3 × 28 beads), ionomycin/PMA, or in vitro–derived allogeneic dendritic cells. Concentrations of FR901228 down to 5 to 10 ng/mL inhibited proliferation of PBL cultures (Figure 1A-C). Proliferation induced by allogeneic dendritic cells was significantly inhibited by FR901228 at concentrations down to 1 ng/mL (Figure 1C). Presence of 1 ng/mL FR901228 or less had no significant effect on 3 × 28 beads or ionomycin/PMA–induced proliferation (Figure 1A-B). To verify these results we used CFSE-labeled PBL cultures stimulated with 3 × 28 beads for 6 days (Figure 1D); in this assay proliferation is measured by flow cytometry as decrease in CFSE fluorescence intensity due to segregation of CFSE between daughter cells. Addition of 20 ng/mL FR901228 at the time of stimulation (Figure 1D) or up to 6 hours after stimulation (not shown) completely inhibited proliferation. Furthermore, FR901228 added to the culture 24 hours after stimulation with 3 × 28 beads stopped proliferation at this time point, demonstrating that FR901228 can inhibit ongoing proliferation (Figure1D). Simultaneous staining for CD4 and CD8 showed that FR901228 inhibited CD4 and CD8 T cells to a similar extent (not shown).
HDAC-i inhibits proliferation of T cells.
PBL cultures (A-D,F) or purified CD4 T cells (E) were treated without or with the indicated amount of FR901228 (A-E) or trichostatin A (F) for 2 hours prior to stimulation with 3 × 28 beads (A,E,F), ionomycin/PMA (B), or allogeneic dendritic cells (C). Proliferation was measured by MTT assay after 3 (A-B) or 6 (C) days of stimulation (stim). Panel D shows a flow-cytometric profile of CFSE-labeled PBL cultures 6 days after stimulation with 3 × 28 beads in the presence or absence of 20 ng/mL FR901228, added before or after stimulation as indicated.
HDAC-i inhibits proliferation of T cells.
PBL cultures (A-D,F) or purified CD4 T cells (E) were treated without or with the indicated amount of FR901228 (A-E) or trichostatin A (F) for 2 hours prior to stimulation with 3 × 28 beads (A,E,F), ionomycin/PMA (B), or allogeneic dendritic cells (C). Proliferation was measured by MTT assay after 3 (A-B) or 6 (C) days of stimulation (stim). Panel D shows a flow-cytometric profile of CFSE-labeled PBL cultures 6 days after stimulation with 3 × 28 beads in the presence or absence of 20 ng/mL FR901228, added before or after stimulation as indicated.
FR901228 inhibited proliferation of highly purified CD4 cells at concentrations comparable to those that inhibit PBL cultures (Figure1E), implying that HDAC-i directly target T cells. Trichostatin A, a structurally unrelated HDAC inhibitor, also inhibited stimulation-induced proliferation of PBL (Figure 1F) and purified CD4 cultures (not shown), strongly suggesting that inhibition of HDAC activity, and not a secondary effect, is responsible for the proliferation arrest.
HDAC-i inhibit T cells prior to S-phase entry and do not induce apoptosis in stimulated naive T cells
To investigate the cause of proliferation inhibition, FR901228-treated cells were analyzed for viability, cell cycle progression, and apoptosis. Viability was measured by cell membrane integrity. 7-AAD staining of DNA was used to follow progression from G1 to S to G2 phase of the cell cycle. Annexin-V and sub-G1 DNA staining were used to monitor apoptosis.
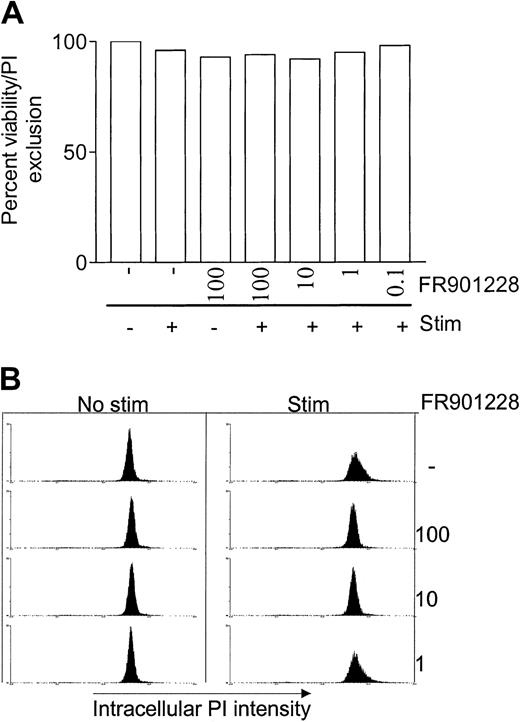
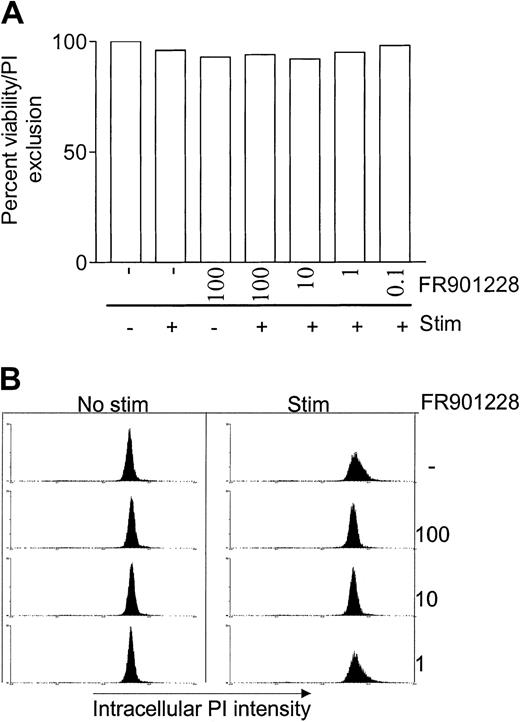
Addition of FR901228 at doses up to 100 ng/mL had very little effect on PBL viability within 24 hours (Figure2A), emphasizing that the inhibitory effect of HDAC-i is not caused by a cytotoxic effect. These results correlate with a previous report in which 24 hours' exposure of PBMC cultures to FR901228 was shown to have an LC50 value of about 965 nM.2 In the previous study PBMC cells were exposed to FR901228 for 24 hours but viability was measured after 4 days; the LC50 value immediately after 24 hours' exposure is thus almost certainly higher.
HDAC-i influence on viability, cell cycle, and apoptosis.
PBL cultures were treated with or without the indicated amount of FR901228 and stimulated for 24 hours with 3 × 28 beads. Viability was measured as exclusion of PI by flow cytometry (A). Cell cycle distribution and apoptosis was measured by intracellular PI staining (B) as described in “Materials and methods.”
HDAC-i influence on viability, cell cycle, and apoptosis.
PBL cultures were treated with or without the indicated amount of FR901228 and stimulated for 24 hours with 3 × 28 beads. Viability was measured as exclusion of PI by flow cytometry (A). Cell cycle distribution and apoptosis was measured by intracellular PI staining (B) as described in “Materials and methods.”
Intracellular DNA staining after 24 hours of stimulation showed that FR901228 arrested 3 × 28 bead–stimulated T cells in the G1 phase, prior to S-phase entry (Figure 2B). Concentrations of FR901228 of 1 ng/mL or below had no effect on cell cycle progression, and cells can be seen to enter the S phase similarly to stimulated cells that were not exposed to FR901228 (Figure 2B). These data correlate with the level of FR901228 that caused inhibition of proliferation. The intracellular DNA staining did not detect signs of apoptosis by highly condensed DNA, which can be seen as a sub-G1 peak (Figure 2B). This observation was supported by negative annexin V staining of naive PBLs exposed to FR901228 for 24 hours (not shown).
In conclusion, FR901228 arrests recently activated naive PBLs in the G1 phase without affecting viability.
HDAC-i do not inhibit the proximal signaling after CD3/CD28 stimulation
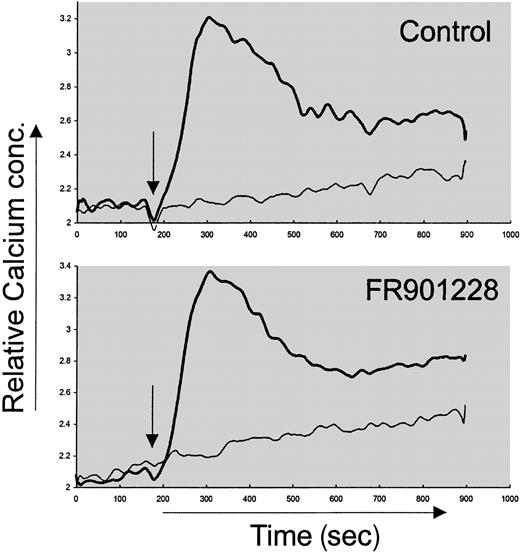
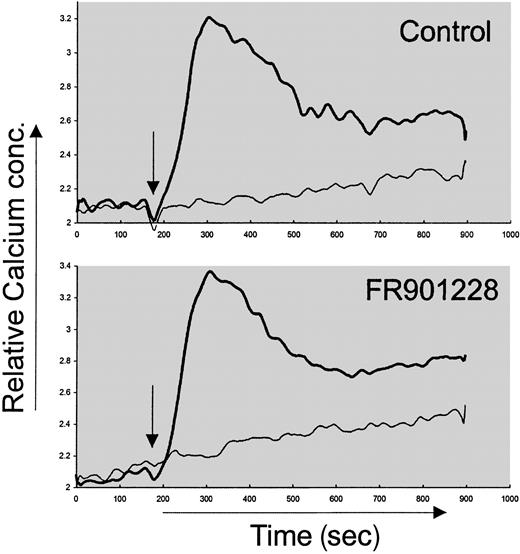
We wished to examine the mechanism behind HDAC-i inhibition of activation-induced proliferation. The proximal intracellular signal pathways in naive CD4 cells were not inhibited by HDAC-i. Thus, the immediate tyrosine phosphorylation after anti-CD3 stimulation of naive CD4 T cells was not affected by FR901228 or trichostatin A (not shown). This is in agreement with earlier studies measuring phosphotyrosine content after epidermal growth factor (EGF) stimulation of the MCF-10A immortalized cell line in the presence of FR901228.13 Furthermore, the rise in intracellular calcium concentration after anti-CD3 stimulation of PBLs was not affected by FR901228 (Figure 3). These data suggest that the very proximal signals after TCR/CD3 engagement were not altered by HDAC-i.
Calcium measurement after FR901228 pretreatment of CD3-activated PBL cultures.
Increase in intracellular calcium content was measured after anti-CD3 stimulation of Fura-2–loaded cells preincubated with or without 20 ng/mL FR901228. Thick line represents anti-CD3 stimulation; thin line, control stimulation with PBS. Stimulation was initiated 2 minutes after start of recording and is indicated by arrow.
Calcium measurement after FR901228 pretreatment of CD3-activated PBL cultures.
Increase in intracellular calcium content was measured after anti-CD3 stimulation of Fura-2–loaded cells preincubated with or without 20 ng/mL FR901228. Thick line represents anti-CD3 stimulation; thin line, control stimulation with PBS. Stimulation was initiated 2 minutes after start of recording and is indicated by arrow.
HDAC-i inhibit activation-induced CD154 and CD25 expression on CD4 cells
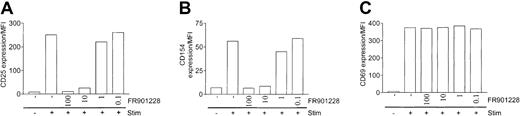
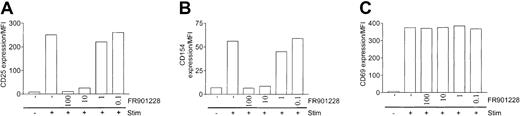
To further characterize HDAC-i inhibition of T cells, we measured changes in surface expression of activation molecules. As expected, there was a robust induction of the IL-2 receptor α-chain (CD25), the CD40 ligand (CD154), and the CD69 molecule on the surface of peripheral CD4 T cells 24 hours after 3 × 28 bead stimulation (Figure 4A-C). Inclusion of FR901228 down to 10 ng/mL completely suppressed the induction of CD25 and CD154 (Figure 4A-B). Strikingly, induction of CD69 was only minimally affected by FR901228, even at concentrations up to 100 ng/mL (Figure 4C). Similar results were obtained using trichostatin A (not shown). These results suggest that HDAC-i impose a selective, but not complete, inhibition of CD4 T-cell activation. The inhibition of CD25 and CD154 could not be bypassed by ionomycin/PMA stimulation (not shown), further implying that HDAC-i inhibition lies downstream of the rise in intracellular calcium concentration.
Expression of activation markers on activated CD4 T cells treated with HDAC-i.
CD25 (A), CD154 (B), and CD69 (C) were measured by flow cytometry 24 hours after stimulation with 3 × 28 beads with or without the indicated amount of FR901228. Results are shown as mean fluorescence intensity (MFI) of gated CD4 T cells.
Expression of activation markers on activated CD4 T cells treated with HDAC-i.
CD25 (A), CD154 (B), and CD69 (C) were measured by flow cytometry 24 hours after stimulation with 3 × 28 beads with or without the indicated amount of FR901228. Results are shown as mean fluorescence intensity (MFI) of gated CD4 T cells.
Activation-induced T-cell aggregation is abrogated by HDAC-i
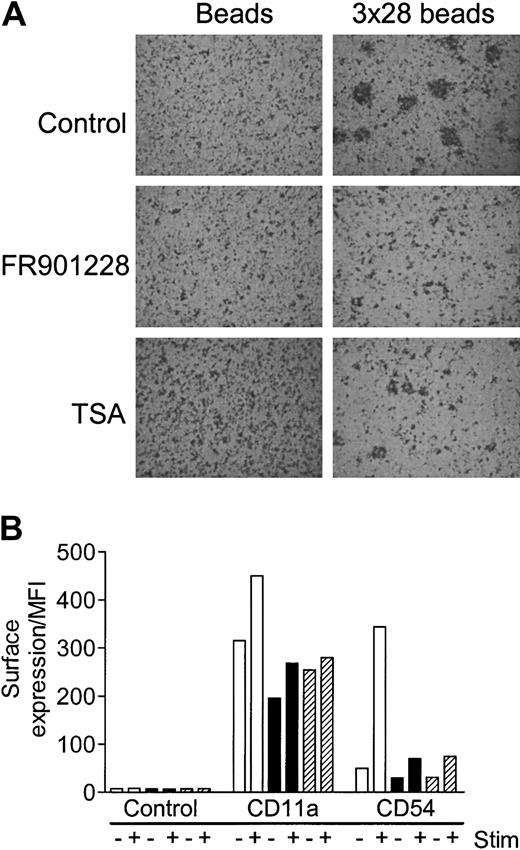
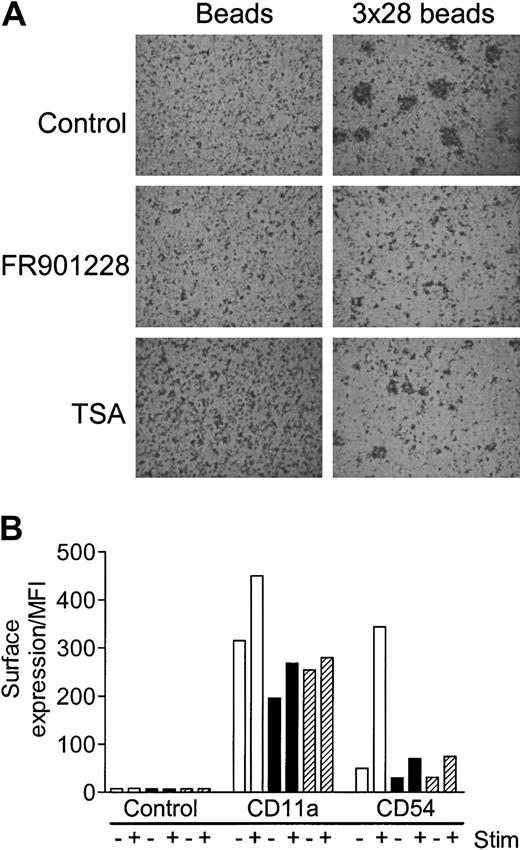
Activated T cells rapidly adhere into characteristic aggregates; these aggregates can be formed as homotypic aggregates between T cells themselves or heterotypic aggregates between T cells and antigen-presenting cells (APCs). The specific adhesion is caused by increased surface expression and/or activation of adhesion molecules, primarily LFA-1, ICAM-1, ICAM-3, and CD2 on the T cell and ICAM-1, ICAM-2, and LFA-3 on the APC. Aggregation of PBLs 24 hours after 3 × 28 bead stimulation was completely abolished by FR901228 or trichostatin A (Figure 5A). Aggregation of highly purified 3 × 28 bead–activated CD4 cells was also inhibited by HDAC-i (not shown). A likely explanation for the deficient aggregation is that expression of adhesion molecules is compromised by HDAC-i. To investigate this in greater detail, expression of CD11a (the LFA-1–specific α-chain) and CD54 (ICAM-1) was monitored. FR901228 and trichostatin A completely inhibited activation-induced expression of CD11a and CD54 expression on CD4 T cells (Figure 5B). There was a significant expression of CD11a and a weak expression of CD54 on nonactivated CD4 T cells; basal expression of both molecules was reduced by HDAC-i treatment, although not to undetectable levels (Figure 5B). In conclusion, HDAC-i abrogates activation-induced aggregation, possibly, at least in part, owing to diminished expression of LFA-1 and ICAM-1 molecules.
HDAC-i influence on aggregation and expression of adhesion molecules after T-cell activation.
PBL cultures were preincubated with or without 20 ng/mL FR901228 or 100 ng/mL trichostatin A and stimulated for 24 hours with 3 × 28 beads or uncoupled beads. (A) A characteristic example of the HDAC-I–mediated inhibition of aggregation; similar results were always observed upon visual inspection of stimulated cells treated with HDAC-i. Original magnification, × 20. (B) CD11a and CD54 were measured by flow cytometry 24 hours after stimulation with control (−) or 3 × 28 beads (+) without (white bars) or with (black bars) 20 ng/mL FR901228 or 100 ng/mL trichostatin A (hatched bars). Results are shown as MFI of gated CD4 T cells.
HDAC-i influence on aggregation and expression of adhesion molecules after T-cell activation.
PBL cultures were preincubated with or without 20 ng/mL FR901228 or 100 ng/mL trichostatin A and stimulated for 24 hours with 3 × 28 beads or uncoupled beads. (A) A characteristic example of the HDAC-I–mediated inhibition of aggregation; similar results were always observed upon visual inspection of stimulated cells treated with HDAC-i. Original magnification, × 20. (B) CD11a and CD54 were measured by flow cytometry 24 hours after stimulation with control (−) or 3 × 28 beads (+) without (white bars) or with (black bars) 20 ng/mL FR901228 or 100 ng/mL trichostatin A (hatched bars). Results are shown as MFI of gated CD4 T cells.
We have previously shown that the molecular pathway leading to IL-2–induced T-cell aggregation is not directly linked to proliferation.24 Thus, although lack of aggregation following T-cell activation is a significant sign of inhibition, it is not necessarily involved in growth inhibition.
HDAC-i inhibit IL-2 production by activated peripheral T cells
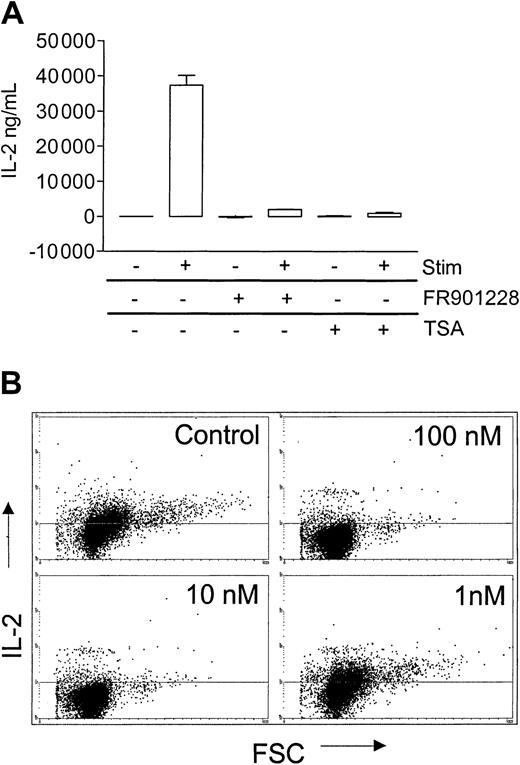
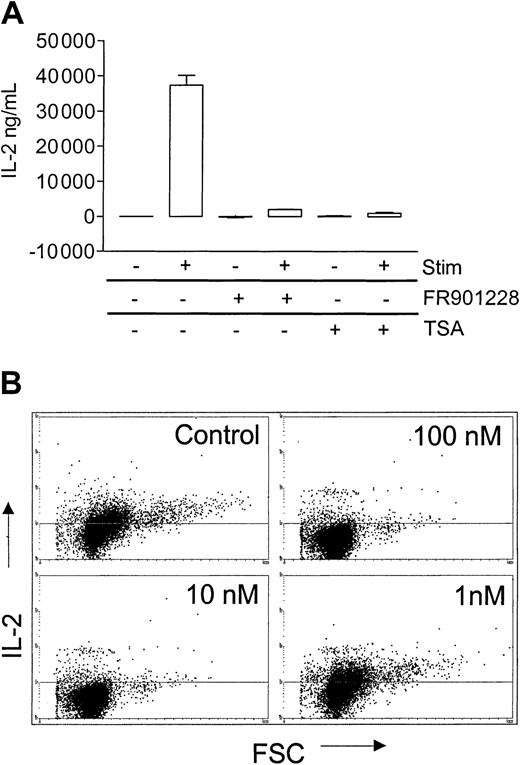
A hallmark of CD4 T-cell activation is production of IL-2. In order to make efficient amounts of IL-2, T cells need costimulation, normally from the CD28 molecule. FR901228 or trichostatin A markedly inhibited IL-2 production, as measured by ELISA 24 hours after 3 × 28 bead stimulation of PBL cultures (Figure6A), a result that was confirmed by intracellular IL-2 staining of CD4 T cells (Figure 6B). In agreement with the earlier observed inhibitory capacity of FR901228, 100 nM and 10 nM completely inhibited IL-2 production, whereas 1 nM had no effect (Figure 6B). (Brefeldin A/monensin, which causes a smaller increase in intracellular IL-2 staining, was omitted in these experiments; in our hands, this, however, more appropriately reflects the actual level of IL-2).
HDAC-i influence on IL-2 production from activated CD4 T cells.
PBL cultures were preincubated with or without 20 ng/mL FR901228 or 100 ng/mL trichostatin A (TSA) and stimulated for 24 hours with 3 × 28 beads. (A) IL-2 was measured in the supernatant by ELISA. (B) Intracellular IL-2 was measured by flow cytometry on gated CD4 cells.
HDAC-i influence on IL-2 production from activated CD4 T cells.
PBL cultures were preincubated with or without 20 ng/mL FR901228 or 100 ng/mL trichostatin A (TSA) and stimulated for 24 hours with 3 × 28 beads. (A) IL-2 was measured in the supernatant by ELISA. (B) Intracellular IL-2 was measured by flow cytometry on gated CD4 cells.
The diminished IL-2 production was not by itself responsible for the lack of proliferation, as addition of 1000 IU/mL IL-2 could not restore proliferation (not shown). This finding is not surprising, given the fact that HDAC-i treatment also inhibits CD25 expression.
Molecular mechanism behind HDAC-i inhibition of CD154 expression
To understand the molecular mechanism behind HDAC-i inhibition of T-cell activation, regulation of CD154 was investigated more thoroughly.
CD154 expression by CD4 T cells is vital for activation of the cellular and humoral immune response.25 Moreover, absent or reduced interaction between CD154 and CD40 expressed on APCs has been linked to induction of immunological tolerance,26-28 making the CD154 molecule a key target for modulation of the immune system.
CD154 is expressed on recently TCR/CD3-activated CD4 T cells through a cyclosporine/FK-506–sensitive nuclear factor of activated T cells (NF-AT)–dependent pathway.29-31 CD154 is furthermore induced on activated effector CD4 T cells by IL-2 and is constitutively expressed on some cancer T cells.32,33Cytokine-mediated expression of CD154 on effector T cells cannot be inhibited by cyclosporine or FK-506 and is thus apparently not dependent upon NF-AT activity.32
Figure 7A shows that FR901228 inhibited CD154 expression on previously activated effector CD4 T cells induced through either TCR/CD3 or IL-2 stimulation, in contrast to cyclosporine, which inhibited only TCR/CD3-induced CD154 expression. The near-constitutive CD154 expression on long-term expanded CD4 T cells, here generated by expansion of CD4 T cells for 21 days with 3 × 28 beads and IL-2, was also completely inhibited by FR901228 or trichostatin A (not shown).
Mechanism behind HDAC-i inhibition of CD154 expression.
(A) Previously activated PBL cultures were stimulated for 3 days with 3 × 28 beads, then cultured for 24 hours with 3 × 28 beads and 100 U/mL IL-2; 3 × 28 beads and 10 μg/mL blocking anti–IL-2 Ab; or 100 U/mL IL-2, 10 μg/mL blocking anti–IL-2 Ab, as indicated. Prior to stimulation, for the last 24 hours, cells were incubated without (black bars) or with 500 ng/mL cyclosporine (white bars) or with 20 ng/mL FR901228 (hatched bars). (B) Flow cytometric analysis of CD154 surface expression on mycosis fungoides cells and intracellular CD154 expression on Jurkat cells 24 hours after addition of vehicle control, 20 ng/mL FR901228, or 500 ng/mL cyclosporine, as indicated. Control stain indicates staining with IgG1-PE Ab. (C) Jurkat cells were transiently transfected with empty vector or CD154 promotor–GFP construct as described in “Materials and methods” and stimulated with or without anti-CD3 mAb for 24 hours. Figure shows the flow cytometric profile of GFP expression in the cells. (D) Jurkat cells were transiently transfected with CD154 promotor–GFP construct and stimulated with or without anti-CD3 Ab for 24 hours in the presence or absence of 20 ng/mL FR901228, 100 ng/mL trichostatin A, or 500 ng/mL cyclosporine. Figure shows CD154 promotor activity as MFI of gated GFP-expressing cells multiplied by the percentage of GFP-positive cells in order to take into account both the level and percentage of GFP expression. Error bars indicate SEM.
Mechanism behind HDAC-i inhibition of CD154 expression.
(A) Previously activated PBL cultures were stimulated for 3 days with 3 × 28 beads, then cultured for 24 hours with 3 × 28 beads and 100 U/mL IL-2; 3 × 28 beads and 10 μg/mL blocking anti–IL-2 Ab; or 100 U/mL IL-2, 10 μg/mL blocking anti–IL-2 Ab, as indicated. Prior to stimulation, for the last 24 hours, cells were incubated without (black bars) or with 500 ng/mL cyclosporine (white bars) or with 20 ng/mL FR901228 (hatched bars). (B) Flow cytometric analysis of CD154 surface expression on mycosis fungoides cells and intracellular CD154 expression on Jurkat cells 24 hours after addition of vehicle control, 20 ng/mL FR901228, or 500 ng/mL cyclosporine, as indicated. Control stain indicates staining with IgG1-PE Ab. (C) Jurkat cells were transiently transfected with empty vector or CD154 promotor–GFP construct as described in “Materials and methods” and stimulated with or without anti-CD3 mAb for 24 hours. Figure shows the flow cytometric profile of GFP expression in the cells. (D) Jurkat cells were transiently transfected with CD154 promotor–GFP construct and stimulated with or without anti-CD3 Ab for 24 hours in the presence or absence of 20 ng/mL FR901228, 100 ng/mL trichostatin A, or 500 ng/mL cyclosporine. Figure shows CD154 promotor activity as MFI of gated GFP-expressing cells multiplied by the percentage of GFP-positive cells in order to take into account both the level and percentage of GFP expression. Error bars indicate SEM.
Recently, Storz et al showed that T cells from patients with the rare skin cancer mycosis fungoides have constitutive surface expression of CD154.33 We could confirm their results in various MF cell lines generated in our laboratory; again, CD154 expression was not affected by cyclosporine but was completely inhibited by FR901228 (Figure 7B). A fraction of Jurkat T cells have constitutive expression of CD154 when measured by intracellular staining (CD154 is weakly expressed on the surface of Jurkat cells). CD154 expression was again completely inhibited by FR901228 and not affected by cyclosporine (Figure 7B). These data suggest that HDAC-i inhibit CD154 expression on activated T cells and cancer T-cell lines by a mechanism distinct from calcineurin/NF-AT inhibition.
To investigate whether HDAC-i directly inhibit transcriptional activation of the CD154 gene, Jurkat cells were transiently transfected with a vector containing 2 kb of the proximal CD154 promoter in front of a green fluorescent protein (GFP) coding sequence. As expected, there was constitutive, although relatively weak, CD154 promotor activity in Jurkat cells, which was enhanced by stimulation with anti-CD3 stimulation (Figure 7C). FR901228 or trichostatin A completely inhibited basal and TCR-induced CD154 promoter activity (Figure 7D). As expected, cyclosporine inhibited the TCR-induced, but not the basal CD154, promotor activity; interestingly, cyclosporine had a slight enhancing effect on the basal CD154 promotor activity. The results confirm our earlier observations and demonstrate that HDAC-i directly inhibit CD154 promotor activity. We did not succeed in making transient transfections of the mycosis fungoides cell lines; however, reverse transcriptase polymerase chain reaction (RT-PCR) experiments showed that HDAC-i inhibited CD154 mRNA expression in these cells (not shown).
Involvement of c-Myc in CD154 expression
HDAC-i treatment down-regulates the c-Myc transcription factor in various cancer cells.15,18 Because TCR/CD3 and IL-2 stimulation both lead to c-Myc expression in activated T cells, we speculated that inhibition of c-Myc could be causally involved in down-regulation of CD154 promotor activity after HDAC-i treatment. If this is the case, c-Myc must be critically involved in CD154 expression—a hypothesis that has not been previously investigated. Interestingly, we found that the proximal CD154 promotor contains a nonhomologous c-Myc binding motif completely identical to the recently discovered motif in the Fas-ligand promotor.20 34
As shown in Figure 8A, FR901228 could indeed inhibit activation-induced c-Myc expression in activated T cells from peripheral blood in a dose-dependent manner. Jurkat cells also have constitutive expression of c-Myc that could be completely inhibited by FR901228 (Figure 8A) or trichostatin A (not shown). IL-2–stimulated c-Myc expression in previously activated T cells was also inhibited by FR901228 (not shown), which is in agreement with a previous report from Koyama et al, who showed that c-Myc, bag-1, and LC-PTP gene expression was inhibited by trichostatin A in IL-2–dependent cell lines.21 In conclusion, HDAC-i inhibit activation-induced and constitutive c-Myc expression in activated T cells from peripheral blood and Jurkat cells.
Involvement of c-Myc in CD154 expression.
(A) Western blot of c-Myc expression in PBL or Jurkat T cells 18 hours after addition of the indicated amount (ng/mL) of FR901228. PBLs were stimulated (stim) with or without 3 × 28 beads as indicated. (B) PBLs were incubated with the indicated phosphothioate oligo probes, 10 μM, for 6 hours prior to stimulation with or without 3 × 28 beads as indicated. CD154 expression was measured after 18 hours by flow cytometry on gated CD4 T cells. (C) Jurkat cells were transiently transfected, in a 1:3 ratio, with CD154 promotor–GFP construct in combination with empty vector, c-Myc vector, or Madmyc vector as indicated. Cells were then stimulated with (black bars) or without (white bars) anti-CD3 Ab for 24 hours. Figure shows CD154 promotor activity as MFI of gated GFP-expressing cells multiplied by the percentage of GFP-positive cells in order to take into account both the level and percentage of GFP expression.
Involvement of c-Myc in CD154 expression.
(A) Western blot of c-Myc expression in PBL or Jurkat T cells 18 hours after addition of the indicated amount (ng/mL) of FR901228. PBLs were stimulated (stim) with or without 3 × 28 beads as indicated. (B) PBLs were incubated with the indicated phosphothioate oligo probes, 10 μM, for 6 hours prior to stimulation with or without 3 × 28 beads as indicated. CD154 expression was measured after 18 hours by flow cytometry on gated CD4 T cells. (C) Jurkat cells were transiently transfected, in a 1:3 ratio, with CD154 promotor–GFP construct in combination with empty vector, c-Myc vector, or Madmyc vector as indicated. Cells were then stimulated with (black bars) or without (white bars) anti-CD3 Ab for 24 hours. Figure shows CD154 promotor activity as MFI of gated GFP-expressing cells multiplied by the percentage of GFP-positive cells in order to take into account both the level and percentage of GFP expression.
To examine whether activation-induced c-Myc is involved in CD154 expression, normal peripheral blood T cells were incubated with or without an antisense c-Myc construct. As shown in Figure 8B, the antisense construct reduced 3 × 28 bead–induced CD154 expression on gated CD4 T cells by approximately 50%; no significant effects were observed in cells preincubated with control, sense or nonsense constructs.
To more directly investigate the link between c-Myc and CD154, we used Jurkat cells as a model. Jurkat cells were transiently transfected with the CD154 promotor–GFP construct in combination with a c-Myc or a dominantly negatively acting Madmyc construct. Figure 8C shows that overexpression of c-Myc enhances the basal level of CD154 promotor activity; in contrast, overexpression of the dominant-negative acting Madmyc construct inhibited CD154 promotor activity. The same effect was observed on CD3-induced CD154 promotor activity: c-Myc potentiated and Madmyc coexpression inhibited CD154 promotor activity (Figure8C). These results strongly suggest that c-Myc expresssion is essential for CD154 expression in Jurkat cells, both the NF-AT dependent and independent pathways. It should be noted that the modulation of CD154 promotor activity varied with the ratio and end concentration of the transfected plasmids.
To investigate whether c-Myc down-regulation by HDAC-i is solely responsible for CD154 inhibition, we tried to rescue CD154 expression in HDAC-I–treated Jurkat cells by overexpression of c-Myc. Strikingly, we were not able to circumvent CD154 inhibition by ectopic expression of c-Myc; careful titration of the level of c-Myc transfected did not change the result (not shown). A trivial explanation could be that HDAC-i treatment inhibits the cytomegalo-virus (CMV) promotor that controls the ectopic c-Myc expression. This, however, was not the case, as expression of a CMV-GFP construct was merely enhanced by FR901228 treatment (not shown). We thus conclude that c-Myc is critically involved in CD154 promotor activity. However, ectopic expression of c-Myc is not able to rescue HDAC-I–mediated CD154 inhibition, even though HDAC-i treatment directly inhibits c-Myc expression, suggesting that a heretofore unidentified factor modulated by HDAC-i is furthermore essential for CD154 expression.
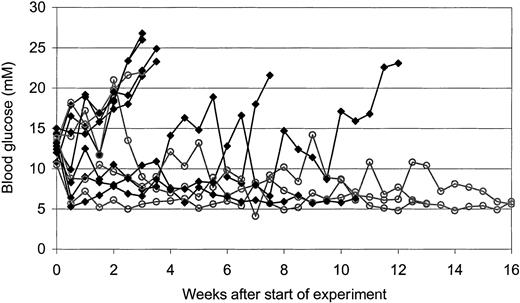
HDAC-i inhibit diabetes development in NOD mice
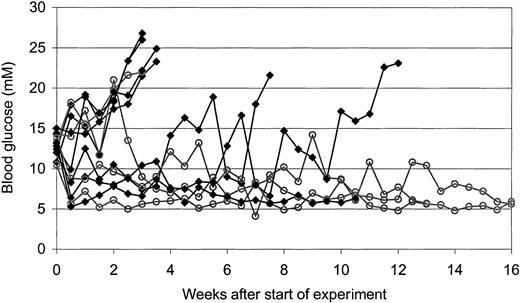
To investigate the T cell–inhibitory capacity of HDAC-i in vivo, we made use of NOD mice that spontaneously develop diabetes due to T cell–mediated destruction of the insulin producing β cells in the pancreas. Mice were treated with vehicle or about 0.5 mg/kg FR901228 intraperitoneally 2 times a week for a period of 8 weeks. To evaluate whether FR901228 could inhibit an ongoing autoaggressive immune attack, treatment was first initiated when the mice developed signs of diabetes (blood glucose levels above 10 mM and glucosuria).
In the control group receiving vehicle, 6 of 7 mice developed overt diabetes (defined as blood glucose exceeding 20 mM in 2 consecutive measurements); in contrast, in the group treated with FR901228, 1 of 4 mice developed diabetes (Figure 9). These results suggest that FR901228 can inhibit ongoing immune responses in vivo.
In vivo effect of FR901228 on glucose level in diabetic NOD mice.
Development in blood glucose level of NOD mice treated with vehicle control (▪) or FR901228 (○) twice a week after their blood glucose level exceeded 10 mM.
In vivo effect of FR901228 on glucose level in diabetic NOD mice.
Development in blood glucose level of NOD mice treated with vehicle control (▪) or FR901228 (○) twice a week after their blood glucose level exceeded 10 mM.
Discussion
Inhibition of histone deacetylases results in a profound inhibition of both CD4 and CD8 T-cell activation. We observed similar inhibition using 2 structurally unrelated HDAC-i, FR901228 and trichostatin A, suggesting that inhibition of deacetylase activity, and not a secondary effect, is the mechanism of action. In favor of this argument, we found that FR901228 and trichostatin A completely resemble each other when measuring changes in expression of 16 different surface molecules on activated CD4 T cells; exceptions were not observed (S.S., unpublished observations). Furthermore, the level of FR901228-induced histone H3-acetylation of PBLs correlated with the observed inhibition (not shown).
Previous studies have shown that HDAC-i inhibit tumor cell growth in both the G1 and G2 cell cycle phases.11,14,18 We did not observe a G2 arrest, most likely because the naive T cells stimulated in the presence of HDAC-i were arrested in the G1 phase before they had a chance to enter the G2 phase. It has been noted that the G2 arrest is associated mainly with apoptosis/cytotoxicity,13which could very well account for the specific induction of apoptosis in tumor cells compared with normal, noncycling cells. In line with this, Byrd et al showed very limited cytotoxicity on PBMC cultures using FR901228.2 A recent phase 1 clinical trial of FR901228 showed a maximal tolerated dose of 24.9 mg/m,2which gave a mean maximum plasma concentration of 472.6 ng/mL.35
It thus seems reasonable to conclude that the inhibition of T-cell proliferation we observed (full inhibition at 10-20 ng/mL FR901228) is not caused by aberrant cytotoxic effects.
We found that HDAC-i inhibited IL-2 production from activated CD4 T cells; this does not correlate with a previous study by Wang et al, who showed that FR901228 could not inhibit CD3-induced IL-2 production from the A1.1 T cell hybridoma line.15 The reason for the apparent difference is not obvious, but different signal pathways could of course be responsible for IL-2 production in each circumstance. Our data concerning inhibition of IL-2 production from PBL cultures by FR901228 or trichostatin A were reproduced with cells from 4 different donors; no exceptions were observed. We thus conclude that HDAC-i inhibits IL-2 production by recently activated CD4 T cells from peripheral blood.
Although inhibition of T-cell proliferation is vital for immunosuppression, it is equally important to inhibit the stimulatory “effector” molecules expressed on activated T cells.
We have focused our attention on the CD154 molecule because of its wide-ranging importance in activation of the main parts of the acquired immune system, including activation of CD8 T cells, B cells, dendritic cells, and macrophages.25
The importance of CD154 expression is convincingly illustrated in knockout mice missing either CD154 or CD40 and in humans with a genetic defect in the CD154 gene, all of which have a severely impaired immune system.25 CD154 is expressed mainly on recently activated CD4 T cells by an NF-AT–dependent mechanism involving the cyclosporine/FK-506–sensitive phosphatase calcineurin.29-31 We have previously demonstrated that IL-2 and IL-15 can induce CD154 expression on activated effector CD4 T cells by a signal pathway that is independent of calcineurin activation.32 Constitutive or elevated CD154 expression is often seen in situ during autoimmune attacks and blockade of CD154/CD40 interaction has proven to be highly beneficial for treatment of autoimmune disorders,36-41 making pharmacological inhibitors of CD154 highly relevant for treatment of autoimmune disorders.
Although therapy with cyclosporine and FK-506 effectively prevents graft rejection and is currently standard therapy, their effectiveness toward prevention of an ongoing autoimmune attack is minimal. It is tempting to speculate that the relatively weak therapeutic effect of cyclosporine toward autoimmune diseases, to a significant part, stems from the lack of effective down-modulation of CD154 expression on activated T cells.
In this report we show that FR901228 and trichostatin A inhibit CD154 by a mechanism that is distinctly different from that of cyclosporine or FK-506. HDAC-i could thus broadly inhibit constitutive CD154 expression in cancer cells and CD154 induced by TCR or IL-2, whereas cyclosporine/FK-506, as previously shown, inhibited only TCR-induced CD154 expression. The most straightforward interpretation would be that HDAC-i modulates a common downstream signal that is essential for CD154 promotor activation, with calcineurin being upstream from this HDAC-i sensitive step during TCR-induced CD154 activation. During cytokine-induced or constitutive CD154 expression, heretofore unidentified signals (not including calcineurin, however) converge to the HDAC-I–sensitive signal pathway.
We hypothesized that this converging downstream signal molecule, involved in CD154 gene activity, could be the transcription factor c-Myc. Interestingly, we found that the proximal promotor of CD154 contains a potential c-Myc binding domain that is completely identical to the noncanonical c-Myc binding domain, which is important for Fas ligand gene activation.20 34 Previous studies have shown that HDAC-i potently inhibits expression of the c-Myc transcription factor in various cancer cells. We observed a similar effect in activated T cells from peripheral blood: both CD3xCD28– and IL-2–induced c-Myc expression was completely abrogated by FR901228 or trichostatin A. Using a dominantly acting c-Myc construct (Madmyc), we could show that c-Myc expression was important for both the constitutive and TCR-induced CD154 expression in Jurkat cells; in line with these results, overexpression of normal c-Myc could enhance CD154 promotor activity. This suggests a critical involvement of c-Myc in CD154 expression. We wanted to verify the c-Myc requirement for CD154 expression in more normal T cells from peripheral blood; however, due to inefficient transfection of the peripheral blood T cells, we turned to antisense down-modulation of c-Myc expression, which indeed significantly reduced CD154 expression. From these results we conclude that c-Myc expression is crucial for CD154 expression, both the NF-AT–dependent and -independent signal pathway.
Because FR901228 could inhibit both CD154 and c-Myc and our results furthermore showed a critical importance of c-Myc in CD154 expression, we were somewhat surprised that overexpression of c-Myc did not rescue CD154 expression in FR901228-treated cells, although the c-Myc construct was effectively expressed. The most likely explanation of these results is that HDAC-i, besides inhibiting c-Myc, also modulate a heretofore unknown factor that is also essential for CD154 expression. Thus, HDAC-i inhibits CD154 expression through modulating at least 2 signal pathways that are each essential, one being inhibition of c-Myc expression and the other currently unknown.
Lack of c-Myc expression is not involved in all aspects of HDAC-i biology. It has previously been shown that HDAC-I–mediated apoptosis in cancer cells is not dependent upon c-Myc inhibition, as reconstitution of c-Myc expression actually elevated HDAC-I–mediated apoptosis.18
Our preliminary in vivo studies suggest that FR901228 treatment can inhibit hyperglycemia and reverse diabetes development in NOD mice, which supports the immunosuppressive effects of FR901228 we observed in vitro. It should be stressed that treatment was initiated only after development of hyperglycemia and glucosuria; thus successful intervention at this late stage requires a very efficient blockade of ongoing immune responses in mice, which is, however, more relevant to a therapeutical situation where an ongoing autoimmune attack has to be neutralized.
Blocking CD154/CD40 interaction significantly inhibits development and progression of various autoimmune diseases and allograft rejection, most likely through inhibition of the critical CD40 stimulation of antigen-presenting cells. A recent study by Homann et al has furthermore demonstrated that even a transient blockade of CD154/CD40 interaction during the primary T-cell activation can lead to specific tolerance toward the stimulatory antigen. This specific tolerance could be adoptively transferred by an antigen-presenting cell with similarities to both dendritic cells and natural killer (NK) cells.42
In conclusion, our studies suggest that HDAC-i may be potential candidates for clinical treatment of autoimmune disorders and allograft rejection. However, when used as antitumor therapy, the primarily unwanted immunosuppressive activity of HDAC-i should be taken into account—although the transient and noncytotoxic nature of the suppression may be tolerable in this circumstance.
Prepublished online as Blood First Edition Paper, October 17, 2002; DOI 10.1182/blood- 2002-07-2073.
Supported by grants from The Johann Weimann Foundation and The Carlsberg Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Søren Skov, Cell Cybernetics Laboratory, Department of Medical Microbiology and Immunology, The Panum Institute, University of Copenhagen, Blegdamsvej 3, Building 22-5, Copenhagen, Denmark; e-mail: s.skov@immi.ku.dk.