The longevity of dendritic cells (DCs) is a critical regulatory factor influencing the outcome of immune responses. Recently, we demonstrated that the immunosuppressive drug rapamycin (Rapa) specifically induces apoptosis in DCs but not in other myeloid cell types. The present study unraveled the mechanism used by Rapa to induce apoptosis in human monocyte-derived DCs. Our data demonstrate that granulocyte-macrophage colony-stimulating factor (GM-CSF) preserves DC survival specifically via the phosphatidylinositol-3 lipid kinase/mammalian target of rapamycin (PI3K/mTOR) signaling pathway, which is abrogated by Rapa at the level of mTOR. Disruption of this GM-CSF signaling pathway induced loss of mitochondrial membrane potential, phosphatidyl-serine exposure, and nuclear changes. Apoptosis of these nonproliferating DCs was preceded by an up-regulation of the cell cycle inhibitor p27KIP1. Overexpression of p27KIP1 in DCs using adenoviral gene transduction revealed that apoptosis is directly regulated by p27KIP1. Furthermore, both overexpression of p27KIP1 and disruption of the GM-CSF/PI3K/mTOR signaling pathway decreased the expression of the antiapoptotic protein mcl-1. This mTOR/p27KIP1/mcl-1 survival seems unique for DCs and may provide novel opportunities to influence immune responses by specific interference with the life span of these cells.
Introduction
Apoptosis, or programmed cell death, is a physiologic process that is required for the normal development and maintenance of tissue homeostasis.1 It is an active process that is regulated by gene products, which either block or accelerate programmed cell death. In most cells, the apoptotic program is always ready to be executed unless continuously inhibited by extracellular survival factors.2 Apoptosis regulates many aspects of immunologic homeostasis, including initiation, magnitude, and termination of immune responses. Dendritic cells (DCs) play a critical role in the diverse facets of immune regulation, ranging from tolerance induction and the prevention of autoimmunity to the induction of antitumor immunity and the protection against infectious agents.3 4
DCs are the most potent antigen-presenting cells. They play a major role in the uptake, transport, and presentation of antigens and have the unique capacity to stimulate naive T lymphocytes.5 In addition to their polarizing capacity on contact with naive T cells,4 they can interact with B cells6 and natural killer (NK) cells7 and thus direct the character of the immune response. Although of possible biologic importance to down-regulate immune responses, apoptosis in DCs has been scarcely investigated. Several different death receptors have been identified on DCs, including Fas (CD95), tumor necrosis factor (TNF) receptor, and TNF receptor–related apoptosis-inducing ligand receptor (TRAIL-R), suggesting a role for death ligand–induced DC apoptosis.8-10 DC apoptosis can also be triggered by UVB radiation,11 glucocorticoids, reactive haptens, infectious pathogens, tumor cells, and NK cells.12 Although recently, specific nuclear factor κB (NF-κB) subunits were described to be important for DC survival in mice,13 the molecular mechanisms underlying DC apoptosis have not yet been unraveled. DCs can also actively protect themselves against cell death. Moreover, DCs protect themselves from cytotoxic T lymphocyte (CTL) attack,14 suggesting that the survival of DCs is an important regulatory mechanism in immune responses.
Previously, we demonstrated that rapamycin (Rapa) specifically induces apoptosis in both monocyte-derived DCs and DCs generated from CD34+ precursors but not in monocytes or macrophages.15 Rapa, which is an immunosuppressive drug introduced to prevent allograft rejection,16 is extensively studied for its effect on T lymphocytes and is known mainly for its antiproliferative effect.17 The drug is structurally related to FK506 that also inhibits T-cell proliferation. Although FK506 and Rapa bind to the same intracellular protein, FKBP-12, the resulting complexes interfere with distinct signaling pathways.18 19 FK506 inhibits production of interleukin 2 (IL-2), via inhibition of calcineurin, whereas Rapa inhibits growth factor signaling rather than growth factor synthesis.
The Rapa/FKBP12 complex acts to inhibit the activity of mammalian target of rapamycin (mTOR).17,20 mTOR is a member of the lipid kinase family with homology to phosphatidylinositol-3 lipid kinase (PI3K). In T cells, Rapa abrogates the IL-2–induced activity of mTOR, thereby blocking G1-S transition and proliferation. A potential mechanism by which PI3K and mTOR exert their proliferative effects is by down-regulation of the cyclin-dependent kinase (CDK) inhibitor p27KIP1.21 However, up-regulation of p27KIP1 is not only linked to cell cycle arrest in G0/1,22 but it seems also associated with apoptosis induced by growth factor withdrawal or PI3K inhibition.23 24
In the present study we examined the mechanism used by Rapa to induce apoptosis in primary human DCs. Our data demonstrate that the survival of monocyte-derived DCs, which are nonproliferating cells, requires granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling via the PI3K/mTOR signaling pathway. Inhibition of mTOR via Rapa leads to increased expression of the cell cycle inhibitor p27KIP1 in nonproliferating DCs. Overexpression of p27KIP1 by using adenoviral gene transduction was shown to be directly responsible for the down-regulation of the antiapoptotic bcl-2 family protein mcl-1 and the induction of apoptosis.
Materials and methods
Reagents
LY294002 (Alexis, San Diego, CA) and PD98059, SB203580, and Rapamycin (all from Calbiochem, Cambridge, MA) were dissolved in dimethyl sulfoxide (DMSO) and used at the concentrations indicated. FK506 (Prograft; Fujisawa Benelux, Houten, The Netherlands) was added to the cultures at 5 × 10−6 M.
Cell culture
Monocyte-derived DCs were generated as described previously.25 In brief, human monocytes were isolated from a buffy coat obtained from healthy donors using Ficoll-Hypaque (Sigma, St Louis, MO) and Percoll (Pharmacia, Uppsala, Sweden) density gradient centrifugation, followed by plastic adherence (2 hours) in T75 culture flasks (15 × 106 cells/flask; Nunc/Life Technologies, Breda, The Netherlands). Adherent monocytes were cultured in RPMI 1640 containing 10% heat-inactivated fetal calf serum (FCS) and penicillin/streptomycin (P/S) supplemented with 5 ng/mL GM-CSF (Sandoz, Uden, The Netherlands) and 10 ng/mL IL-4 (Sanvertech, Heerhugowaard, The Netherlands) for 6 days. Differentiation into CD1a+CD14−CD83− immature DCs was confirmed by fluorescence-activated cell sorter (FACS) analysis.
Induction and detection of apoptosis
Apoptosis induction experiments were performed in 12- or 24-well culture plates (Costar, Cambridge, MA) containing 5 × 105 DC/mL.
Determination of nuclear condensation and fragmentation.
Cells were harvested at the indicated time points and fixed with 1% paraformaldehyde on ice. Cytospin preparations were made and stained with Hoechst 33258 (Molecular Probes, Leiden, The Netherlands) for 3 minutes at room temperature. The percentage of apoptotic cells was determined by examining 200 cells and counting the cells that were characterized by condensed or fragmented nuclei.
Determination of phosphatidyl-serine exposure.
Cells were harvested at the indicated time points, washed, labeled with annexin V–fluorescein isothiocyanate (FITC; Apoptest FITC kit; Nexins Research BV, Kattendijke, The Netherlands) for 30 minutes on ice, and subsequently taken up in 1 μg/mL propidium iodide (PI; Molecular Probes). Annexin V/PI staining was conducted on a FACScan and analyzed using WinMDI software (Becton Dickinson).
Determination of DNA fragmentation (sub-G0 analysis).
Cells were harvested, washed in 1 mM EDTA/PBS (ethylenediaminetetraacetic acid/phosphate-buffered saline), and fixed in 90% ethanol for at least 30 minutes at −20°C. Then the cells were washed, and cellular DNA was stained by treating the cells with 50 μg/mL RNase A (Sigma) and 10 μg/mL PI (Molecular Probes) for 45 minutes at room temperature. Fluorescence intensity was quantified on a per cell basis by flow cytometry (FACScan).
Determination of mitochondrial transmembrane potential.
Mitochondrial dysfunction was assessed by using rhodamine 123 (Rh123; Molecular Probes). Cells were incubated at 37°C for 30 minutes in the presence of 0.1 μg/mL Rh123. Then cells were washed and resuspended in PBS, either with or without 1 μg/mL PI, and Rh123 retention was conducted on a FACScan.
Preparation of whole cell lysates and Western blot analysis
Cultured DCs were harvested, washed, and lysed in Triton X-100 buffer, containing 20 mM Tris (tris(hydroxymethyl)aminomethane)–HCl (pH 7.4), 137 mM NaCl, 10% glycerol, 1% Triton X-100, 2 mM EDTA, 1 mM phenylmethyl sulfonyl fluoride (PMSF), 2 μg/mL leupeptin, 2 μg/mL antipain, 2 μg/mL chymostatin, and 5 U/mL trasylol. The protein concentration was determined by using the bicinchonic acid (BCA) protein assay (Pierce Chemical, Rockford, IL). Each protein sample was separated under reducing conditions on a 12% polyacrylamide sodium dodecyl sulfate (SDS) gel and semi-dry electroblotted on polyvinylidene fluoride (PVDF) membranes (Immobilon-p; Millipore, Bedford, MA). Membranes were incubated with 2% casein in PBS-0.05% Tween 20 for blocking, and then the primary antibody, either mouse-antihuman p27KIP1 (F-8; Santa Cruz Biotechnology, Santa Cruz, CA) or rabbit-antihuman mcl-1 (S-19; Santa Cruz Biotechnology), was added. After incubation with the appropriate secondary antibody (horseradish peroxidase [HRP]–conjugated swine-antirabbit immunoglobulin or goat-antimouse immunoglobulin both from Dako, Glostrup, Denmark) detection was performed with Supersignal (Pierce), and the blots were exposed to Hyperfilm films (Amersham Pharmacia Biotech, United Kingdom). Membranes were stripped by using Restore Stripping Buffer (Pierce) to investigate the expression of several specific proteins within one experiment. Equal protein loading was verified by Coomassie blue staining of the blot, which allows comparison over the whole molecular weight range.
Recombinant adenoviral vectors
Replication-deficient adenoviral vectors containing either human p27KIP1 cDNA (rAd-p27) or the LacZ gene (rAd-LacZ)24 were obtained from Drs I. Naruse and H. Hoshino (Gunma University School of Medicine, Maebashi, Japan). Adenoviral stocks were generated and purified by double CsCl density gradient centrifugation as described previously.26 To remove the CsCl, the virus bands were dialyzed against a Tris buffer (25 mM Tris-Cl, 137 mM NaCl, 5 mM KCl, 0.73 mM Na2HPO4, 0.9 mM CaCl, 0.5 mM MgCl2, pH 7.45). The final dialysis was performed with this Tris buffer containing 5% sucrose, and then virus stocks were stored at −80°C until further use.
Infection of DCs with rAd
Day 6 immature DCs (0.4 × 106) were resuspended in 100 μL PBS and incubated with rAd-LacZ (2.0 × 1010pfu/mL) or rAd-p27 (1.2 × 1010 pfu/mL) (multiplicity of infection [MOI], 1000).27 After 2 hours at 37°C, DCs were washed twice with PBS to remove free adenoviruses. Then cells were resuspended in RPMI 1640 containing 10% ΔFCS and P/S supplemented with 5 ng/mL GM-CSF and 10 ng/mL IL-4 and cultured at 37°C in a 5% CO2 incubator.
After 24 hours of culture, DCs were fixed with 0.2% glutaraldehyde/2% formaldehyde in PBS for 5 minutes at 4°C. Fixed cells were incubated in a stain solution containing 5 mM potassium ferrocyanide2+, 5 mM potassium ferrocyanide3+, 2 mM MgCl2, and 1 mg/mL X-gal (Sigma) for 4 hours at 37°C. The percentage of LacZ+ cells (blue cells) was calculated by light microscopy.
Results
Monocytes and DCs differ in their survival mechanisms
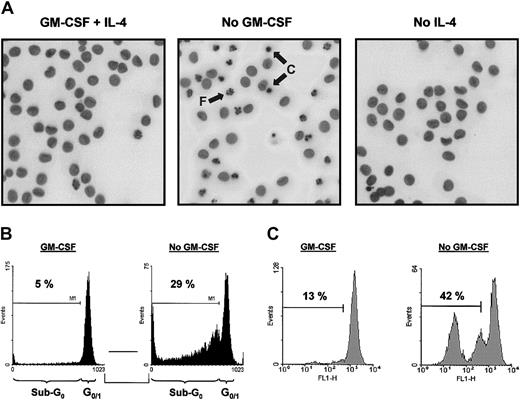
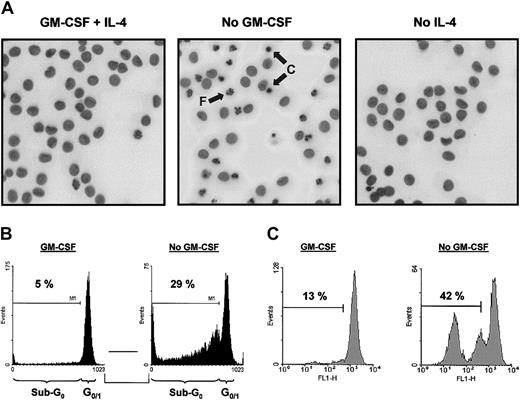
As demonstrated previously, addition of GM-CSF to monocyte cultures increased the yield of functional immature DCs on culture with IL-4.28 To examine whether GM-CSF increases cell recovery by affecting cell survival at the start of culture, ie, an effect on freshly isolated monocytes, or by promoting DC survival, both monocytes and DCs were cultured with or without GM-CSF for 48 hours. GM-CSF withdrawal, but not IL-4 withdrawal, from DC cultures strongly induced apoptosis (Figure 1). GM-CSF withdrawal induced the typical characteristics of apoptosis, including nuclear condensation and fragmentation visualized by Hoechst staining (Figure1A) and DNA fragmentation as demonstrated by an increased sub-G0 fraction after DNA staining with propidium iodide (Figure 1B). The loss of mitochondrial transmembrane potential (ΔΨm) on GM-CSF deprivation, as determined by Rh123 retention, suggested an active role of mitochondria in the induction of apoptosis (Figure 1C).
GM-CSF withdrawal induces apoptosis in DCs.
DCs were cultured as described in “Materials and methods.” At day 6, DCs were harvested, extensively washed, and further cultured in RPMI-10% FCS supplemented with IL-4 (10 ng/mL) plus GM-CSF (5 ng/mL; designated as “GM-CSF” in panels B and C), IL-4 alone (10 ng/mL; designated as “No GM-CSF”), or GM-CSF alone (5 ng/mL; designated as “No IL-4”). After 48 hours of incubation, cells were analyzed for the amount of apoptosis using Hoechst to analyze nuclear morphology (A), PI to detect DNA fragmentation (B), or Rh123 to investigate ΔΨm (C). Photographs (original magnification, × 400) taken from cells stained with Hoechst were inverted and demonstrate condensed (“C”) and fragmented nuclei (“F”) indicated by arrows (A). Data presented are representative of 5 independent experiments with different donors.
GM-CSF withdrawal induces apoptosis in DCs.
DCs were cultured as described in “Materials and methods.” At day 6, DCs were harvested, extensively washed, and further cultured in RPMI-10% FCS supplemented with IL-4 (10 ng/mL) plus GM-CSF (5 ng/mL; designated as “GM-CSF” in panels B and C), IL-4 alone (10 ng/mL; designated as “No GM-CSF”), or GM-CSF alone (5 ng/mL; designated as “No IL-4”). After 48 hours of incubation, cells were analyzed for the amount of apoptosis using Hoechst to analyze nuclear morphology (A), PI to detect DNA fragmentation (B), or Rh123 to investigate ΔΨm (C). Photographs (original magnification, × 400) taken from cells stained with Hoechst were inverted and demonstrate condensed (“C”) and fragmented nuclei (“F”) indicated by arrows (A). Data presented are representative of 5 independent experiments with different donors.
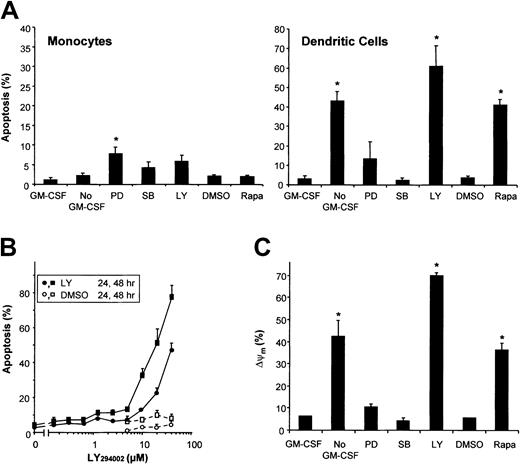
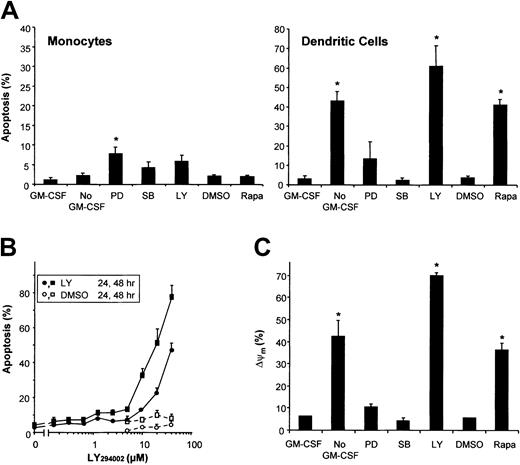
Both mitogen-activated protein kinase (MAPK) and PI3K/mTOR signaling pathways have been suggested to play a role in GM-CSF–induced survival of various hematopoietic cells. To investigate the involvement of the distinct signaling pathways in GM-CSF–mediated survival of DCs, the activation of the MAPK family members extracellular signal–related kinase (ERK) and p38, PI3K, and mTOR was blocked by culturing DCs in the presence of PD98059, SB203580, LY294002, and Rapa, respectively. PD98059 slightly induced apoptosis, whereas inhibition of p38 by SB203580 did not (Figure 2A). Both inhibition of PI3K using LY294002 and inhibition of mTOR using Rapa induced apoptosis comparable to GM-CSF deprivation, demonstrating a critical role for PI3K/mTOR signaling in the regulation of DC survival. Inhibition of PI3K, ERK, or p38 in monocytes slightly induced apoptosis (Figure 2A). As shown previously,15 monocytes were completely insensitive for the proapoptotic activity of Rapa, demonstrating that the longevity of DCs and their precursors, ie, the monocytes, are regulated by different survival mechanisms.
Monocytes and DCs differ in their survival mechanisms.
(A) DCs were cultured in RPMI-10% FCS with the addition of IL-4 and GM-CSF, whereas freshly isolated peripheral blood monocytes were cultured in RPMI-10% FCS alone. After 48 hours of culture, the effects of GM-CSF deprivation (No GM-CSF) or treatment with PD98059 (50 μM), SB203580 (10 μM), LY294002 (20 μM), Rapa (1 μM), or DMSO, were analyzed using Hoechst. Results demonstrate mean ± SD percentage of apoptosis of 3 independent experiments with different donors. * indicates significant apoptotic effect of “No GM-CSF” compared with “GM-CSF” or an inhibitor compared with its solvent “DMSO” (P < .05; Student t test for paired samples). (B) DCs were cultured with IL-4 and GM-CSF in the presence of various concentrations of the PI3K-inhibitor LY294002, or DMSO. Cells were analyzed after 24 hours or 48 hours using Hoechst (n = 2). (C) Day-6 DCs were harvested, extensively washed, and further cultured in RPMI-10% FCS supplemented with IL-4 plus GM-CSF (control), IL-4 alone (No GM-CSF), or IL-4 plus GM-CSF with either Rapa (1 μM), LY294002 (20 μM), PD98059 (50 μM), or SB203580 (10 μM) added. After 48 hours, ΔΨm was investigated using Rh123. The percentage of cells with a decreased ΔΨm are plotted in a histogram. Data are shown as the mean ± SD percentage of apoptosis of duplicate cultures, representative for 3 to 6 independent experiments with different donors. * indicates significant apoptotic effect compared with “GM-CSF” or “DMSO” (P < .05; Student t test for paired samples).
Monocytes and DCs differ in their survival mechanisms.
(A) DCs were cultured in RPMI-10% FCS with the addition of IL-4 and GM-CSF, whereas freshly isolated peripheral blood monocytes were cultured in RPMI-10% FCS alone. After 48 hours of culture, the effects of GM-CSF deprivation (No GM-CSF) or treatment with PD98059 (50 μM), SB203580 (10 μM), LY294002 (20 μM), Rapa (1 μM), or DMSO, were analyzed using Hoechst. Results demonstrate mean ± SD percentage of apoptosis of 3 independent experiments with different donors. * indicates significant apoptotic effect of “No GM-CSF” compared with “GM-CSF” or an inhibitor compared with its solvent “DMSO” (P < .05; Student t test for paired samples). (B) DCs were cultured with IL-4 and GM-CSF in the presence of various concentrations of the PI3K-inhibitor LY294002, or DMSO. Cells were analyzed after 24 hours or 48 hours using Hoechst (n = 2). (C) Day-6 DCs were harvested, extensively washed, and further cultured in RPMI-10% FCS supplemented with IL-4 plus GM-CSF (control), IL-4 alone (No GM-CSF), or IL-4 plus GM-CSF with either Rapa (1 μM), LY294002 (20 μM), PD98059 (50 μM), or SB203580 (10 μM) added. After 48 hours, ΔΨm was investigated using Rh123. The percentage of cells with a decreased ΔΨm are plotted in a histogram. Data are shown as the mean ± SD percentage of apoptosis of duplicate cultures, representative for 3 to 6 independent experiments with different donors. * indicates significant apoptotic effect compared with “GM-CSF” or “DMSO” (P < .05; Student t test for paired samples).
DC survival requires GM-CSF signaling via PI3K and mTOR
Like treatment of DCs with Rapa to inhibit mTOR,15blockade of PI3K in DCs by addition of LY294002 time- and dose-dependently induced apoptosis that was characterized by nuclear condensation and fragmentation (Figure 2B) and DNA fragmentation (data not shown). In addition to the data obtained from GM-CSF deprivation experiments, both LY294002 and Rapa demonstrated loss of ΔΨm associated with the percentage of apoptosis induced (Figure 2C). In line with the induction of apoptosis, inhibition of ERK by PD98059 only slightly induced loss of ΔΨm, and inhibition of p38 did not significantly change ΔΨm(Figure 2C).
Rapa increases the expression of p27KIP1
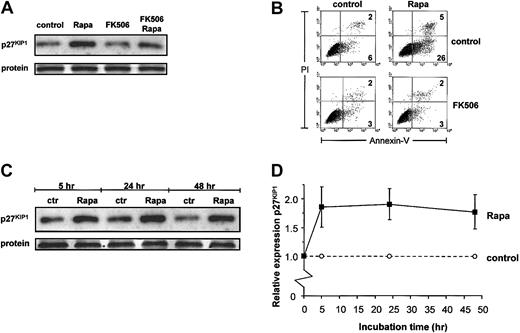
Because mTOR is the most downstream target in GM-CSF–driven survival of DCs as determined so far, and because Rapa is clinically used as an immunosuppressive drug, we further explored the mechanism used by Rapa to specifically induce apoptosis in DCs. In T lymphocytes, Rapa exerts its effects via the up-regulation of the cell cycle inhibitor p27KIP1. An increased expression of p27KIP1 in nonproliferating eosinophils has been shown to correlate with the induction of apoptosis.23 Therefore, we investigated whether Rapa or GM-CSF signaling could influence p27KIP1 protein expression in monocyte-derived DCs, which are also nonproliferating cells. A 48-hour incubation in the presence of Rapa strongly increased the expression of p27KIP1(Figure 3A). When DCs were simultaneously treated with FK506, which antagonizes the apoptotic effect of Rapa (Figure 3B), the Rapa-induced p27KIP1 expression also could be inhibited (Figure 3A). Although Rapa required more than 24 hours to induce strong apoptosis,15 the effect on the expression of p27KIP1 was observed relatively early. Treatment with Rapa for 5 hours already increased the expression of p27KIP1that was not further increased by an extended incubation period in the presence of the drug (Figure 3C-D).
Rapa increases expression of p27KIP1 protein.
(A-B) DCs were cultured with IL-4 and GM-CSF in the absence or presence of Rapa (10−7 M) and either with or without addition of FK506 (5 × 10−6 M) for 48 hours. Part of the cells was used to prepare whole cell lysates. Equal amounts of protein (20 μg/lane) were loaded, and the levels of p27KIP1 were determined (A). Apoptosis was detected in remaining cells by flow cytometry, using annexin V–FITC and PI staining (B). Data shown are representative for 3 independent experiments performed with different donors. (C-D) DCs were cultured in IL-4 and GM-CSF with or without addition of 1 μM Rapa. Cells were harvested after 5 hours, 24 hours, or 48 hours of incubation. Whole cell lysates (20 μg /lane) were loaded, and the levels of p27KIP1 were determined by Western blot analysis (C). The relative expression of p27KIP1 was determined by using Stratagene-EagleSight (La Jolla, CA) and represents the ratio of each band from Rapa-treated DCs to that of untreated DCs. Results are expressed as mean ratio (± SD) of 4 independent experiments (D).
Rapa increases expression of p27KIP1 protein.
(A-B) DCs were cultured with IL-4 and GM-CSF in the absence or presence of Rapa (10−7 M) and either with or without addition of FK506 (5 × 10−6 M) for 48 hours. Part of the cells was used to prepare whole cell lysates. Equal amounts of protein (20 μg/lane) were loaded, and the levels of p27KIP1 were determined (A). Apoptosis was detected in remaining cells by flow cytometry, using annexin V–FITC and PI staining (B). Data shown are representative for 3 independent experiments performed with different donors. (C-D) DCs were cultured in IL-4 and GM-CSF with or without addition of 1 μM Rapa. Cells were harvested after 5 hours, 24 hours, or 48 hours of incubation. Whole cell lysates (20 μg /lane) were loaded, and the levels of p27KIP1 were determined by Western blot analysis (C). The relative expression of p27KIP1 was determined by using Stratagene-EagleSight (La Jolla, CA) and represents the ratio of each band from Rapa-treated DCs to that of untreated DCs. Results are expressed as mean ratio (± SD) of 4 independent experiments (D).
p27KIP1 expression is increased after GM-CSF withdrawal or PI3K inhibition
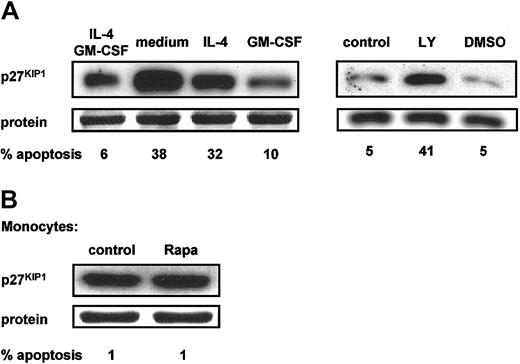
To support the hypotheses that Rapa-induced apoptosis is mediated by an increased p27KIP1 expression, we analyzed the effect of cytokine withdrawal and PI3K inhibition on the expression of p27KIP1. All culture conditions that lead to apoptosis of DCs, including cultures without GM-CSF or with the PI3K inhibitor LY294002, were accompanied by an increased p27KIP1expression, whereas no changes were observed in conditions without apoptosis, such as IL-4 withdrawal or DMSO (Figure4A).
p27KIP1 protein levels correlate with the induction of apoptosis.
(A) Day-6 DCs were harvested, extensively washed, and further cultured in RPMI-10% FCS in the presence or absence of IL-4 and GM-CSF (left panel) or in the presence of both IL-4 and GM-CSF with or without LY294002 (20 μM) or DMSO (right panel). Data demonstrated in left and right panels are derived from different experiments with different donors. (B) Freshly isolated peripheral blood monocytes were cultured in RPMI-10% FCS with or without addition of Rapa (1 μM).
p27KIP1 protein levels correlate with the induction of apoptosis.
(A) Day-6 DCs were harvested, extensively washed, and further cultured in RPMI-10% FCS in the presence or absence of IL-4 and GM-CSF (left panel) or in the presence of both IL-4 and GM-CSF with or without LY294002 (20 μM) or DMSO (right panel). Data demonstrated in left and right panels are derived from different experiments with different donors. (B) Freshly isolated peripheral blood monocytes were cultured in RPMI-10% FCS with or without addition of Rapa (1 μM).
p27KIP1 gene transduction induces apoptosis in DCs
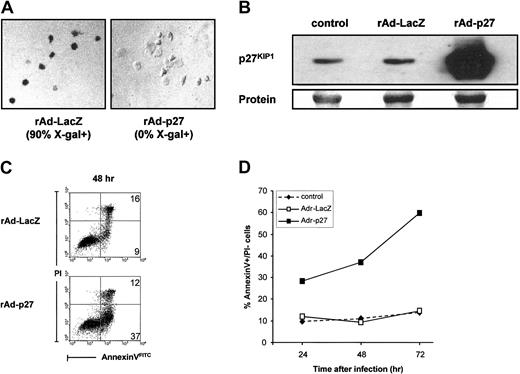
To determine whether up-regulation of p27KIP1 has a causative role in Rapa-induced apoptosis of monocyte-derived DCs, DCs were infected with recombinant adenoviruses (rAd) containing human p27KIP1 cDNA. Infection of DCs with rAd-LacZ served as a control and showed that transduction efficiencies of above 90% were achieved at an MOI of 1000 (Figure 5A). Expression of p27KIP1 protein after infection with the adenoviral vectors was measured by Western blot analysis. A strong overexpression of p27KIP1 was detected at 48 hours after rAd-p27 infection (Figure 5B), which was not further increased after 72 hours. Expression of p27KIP1 protein in DCs infected with rAd-LacZ was not different from control levels.
Overexpression of p27KIP1 induces apoptosis in DCs.
(A) Evaluation transduction efficiency of rAd-LacZ or rAd-p27–infected immature DCs using X-gal. (B) DCs were infected with rAd-LacZ or rAd-p27. After 48 hours of culture in the presence of IL-4 and GM-CSF, infected DCs and control DCs were harvested for the preparation of whole cell lysates. Then equal amounts of protein (20 μg/lane) were loaded, and the levels of p27KIP1 were determined. Data shown are representative for 3 independent experiments with different donors. (C) DCs were infected with rAd-LacZ or rAd-p27 and subsequently cultured in IL-4 and GM-CSF. After 48 hours of culture, annexin V–FITC/PI staining was performed to determine the percentage of apoptotic cells. Data shown are representative for 2 independent experiments with different donors. (D) DCs were left untreated or infected with rAd-LacZ or rAd-p27 and subsequently cultured in IL-4 and GM-CSF. Cells were harvested after 24, 48, and 72 hours of culture. Annexin V–FITC/PI stainings were performed to determine the percentage of annexin V+/PI− (early apoptotic) cells (n = 2).
Overexpression of p27KIP1 induces apoptosis in DCs.
(A) Evaluation transduction efficiency of rAd-LacZ or rAd-p27–infected immature DCs using X-gal. (B) DCs were infected with rAd-LacZ or rAd-p27. After 48 hours of culture in the presence of IL-4 and GM-CSF, infected DCs and control DCs were harvested for the preparation of whole cell lysates. Then equal amounts of protein (20 μg/lane) were loaded, and the levels of p27KIP1 were determined. Data shown are representative for 3 independent experiments with different donors. (C) DCs were infected with rAd-LacZ or rAd-p27 and subsequently cultured in IL-4 and GM-CSF. After 48 hours of culture, annexin V–FITC/PI staining was performed to determine the percentage of apoptotic cells. Data shown are representative for 2 independent experiments with different donors. (D) DCs were left untreated or infected with rAd-LacZ or rAd-p27 and subsequently cultured in IL-4 and GM-CSF. Cells were harvested after 24, 48, and 72 hours of culture. Annexin V–FITC/PI stainings were performed to determine the percentage of annexin V+/PI− (early apoptotic) cells (n = 2).
Cells were harvested after 48 hours of incubation. Whole cell lysates were prepared, equal amounts of protein (20 μg/lane) were loaded, and the levels of p27KIP1 were determined. Data shown are representative for 3 independent experiments with different donors.
In the presence of GM-CSF, DCs infected with rAd-p27 went into apoptosis as demonstrated by an increased annexin V binding within the PI− population (Figure 5C) and nuclear condensation and fragmentation (data not shown). Kinetic experiments showed that p27KIP1-induced apoptosis of DCs was already present 24 hours after infection, which further increased in time (Figure 5D). No differences were found in annexin V binding and nuclear morphology between rAd-LacZ–infected DCs and control DC cultures.
Increased expression of p27KIP1 is responsible for down-regulation of mcl-1
As demonstrated earlier, apoptosis of DCs induced as a consequence of a disruptive GM-CSF/PI3K/mTOR signaling pathway is associated with loss of mitochondrial integrity. The bcl-2 family of proteins, containing both antiapoptotic and proapoptotic members, controls mitochondrial permeability and thus plays a critical role in the regulation of apoptosis. mcl-1 is an antiapoptotic protein, which is thought to be an important bcl-2 family member in GM-CSF–mediated survival of hematopoietic cells.29
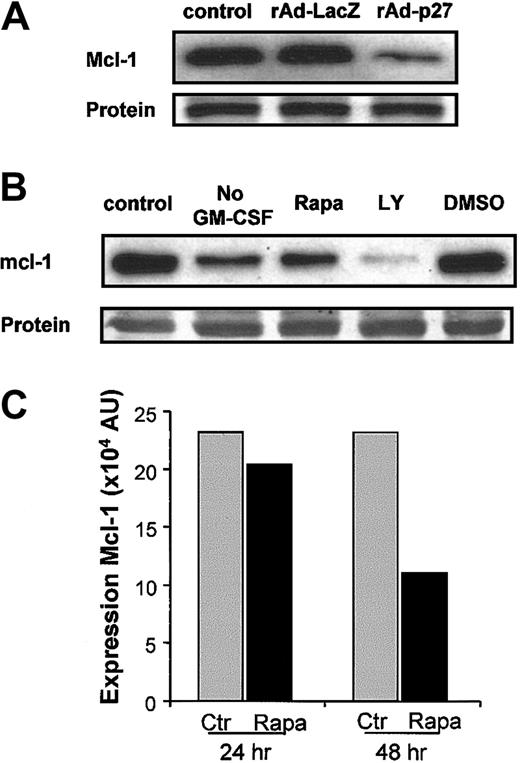
Western blot analysis of lysates prepared after rAd-p27 infection demonstrated that increased expression of p27KIP1 directly causes down-regulation of mcl-1 protein expression in DCs (Figure6A). The correlation between the down-regulation of mcl-1 and the interference in the GM-CSF signaling pathway at the level of PI3K and mTOR finally leading to apoptosis was further examined. Addition of either LY294002 or Rapa to DC cultures, as well as deprivation of GM-CSF, induced a down-regulation of mcl-1 that correlated with the percentage of apoptosis induced, thereby demonstrating that expression of mcl-1 is tightly regulated via PI3K and mTOR in response to GM-CSF (Figure 6B).
Overexpression of p27KIP1 as well as disruptive GM-CSF/PI3K/mTOR signaling down-regulate mcl-1 protein expression.
(A) DCs were left untreated or infected with rAd-LacZ or rAd-p27. After 48 hours of culture in the presence of IL-4 and GM-CSF, DCs were harvested for the preparation of whole cell lysates. Then equal amounts of protein (25 μg/lane) were loaded, and the levels of mcl-1 were determined. Data shown are representative for 2 independent experiments with different donors. (B) DCs were cultured either in IL-4 (No GM-CSF) alone or IL-4 and GM-CSF. Cultures containing IL-4 and GM-CSF were supplemented with or without 10 μM LY294002, 1 μM Rapa, or DMSO. Equal amounts of protein (20 μg whole cell lysate/lane) were loaded, and the levels of mcl-1 were determined. Data shown are representative for 3 independent experiments performed with different donors. (C) Quantification of mcl-1 expression in control and Rapa-treated DCs after either 24 hours or 48 hours of culture as described in “Materials and methods.” Data shown are representative for 4 independent experiments performed with different donors.
Overexpression of p27KIP1 as well as disruptive GM-CSF/PI3K/mTOR signaling down-regulate mcl-1 protein expression.
(A) DCs were left untreated or infected with rAd-LacZ or rAd-p27. After 48 hours of culture in the presence of IL-4 and GM-CSF, DCs were harvested for the preparation of whole cell lysates. Then equal amounts of protein (25 μg/lane) were loaded, and the levels of mcl-1 were determined. Data shown are representative for 2 independent experiments with different donors. (B) DCs were cultured either in IL-4 (No GM-CSF) alone or IL-4 and GM-CSF. Cultures containing IL-4 and GM-CSF were supplemented with or without 10 μM LY294002, 1 μM Rapa, or DMSO. Equal amounts of protein (20 μg whole cell lysate/lane) were loaded, and the levels of mcl-1 were determined. Data shown are representative for 3 independent experiments performed with different donors. (C) Quantification of mcl-1 expression in control and Rapa-treated DCs after either 24 hours or 48 hours of culture as described in “Materials and methods.” Data shown are representative for 4 independent experiments performed with different donors.
A more detailed analysis of the effect of Rapa on the expression of mcl-1 in DCs was performed. The Rapa-induced reduction of mcl-1 protein levels was already observed within 24 hours of incubation, as demonstrated by Western blot analysis, but became more pronounced after 48 hours of treatment, finally resulting in a 2-fold reduction in mcl-1 protein levels (Figure 6C).
Discussion
In the present study we show that defective GM-CSF signaling in human nonproliferating DCs, either because of selective inhibition of PI3K or mTOR, or GM-CSF deprivation, caused an increased expression of the cyclin-dependent kinase (CDK) inhibitor p27KIP1 prior to a decreased expression of the antiapoptotic protein mcl-1 and apoptosis. Overexpression of p27KIP1 in DCs by using adenoviral gene transduction revealed that both mcl-1 expression and cell survival are directly regulated by p27KIP1.
By examining the mechanism used by Rapa for the induction of apoptosis in DCs, we found that the PI3K/mTOR pathway is a critical signaling route for GM-CSF–driven survival of monocyte-derived DCs. GM-CSF is a potent growth factor for a variety of hematopoietic cells. On binding to its heterodimeric receptor, GM-CSF activates several signaling pathways, including the Jak-Stat (Janus kinase and signal transducer and activator of transcription) and Ras pathways,30,31 resulting in both mitogenic and antiapoptotic signals. Although downstream from Ras, both Raf/MAPK and PI3K/mTOR pathways have been suggested to play a role in cytokine-driven survival of hematopoietic cells,31,32 we found that for monocyte-derived DCs only the PI3K/mTOR pathway is absolutely necessary. In line with a previous study on lipopolysaccharide (LPS)–stimulated monocyte-derived DCs,33 which also demonstrate a cell death–inducing effect of LY294002 but not of PD98059 or SB203580, we observed only a limited role for ERK activation in DC survival. Although our data suggest that Rapa specifically counteracts the antiapoptotic activity of GM-CSF that results in an increased expression of p27KIP1, the fact that Rapa further increases apoptosis on GM-CSF withdrawal (data not shown) might suggest additional modes of action of Rapa.
p27KIP1 is an inhibitor of cell cycle progression, exerting its effect through interaction with cyclin-CDK complexes and arresting cells in G0/1.22 p27KIP1 is present at relatively high levels in quiescent cells and is down-regulated by mitogenic stimulation21,34,35; the latter process being blocked by Rapa.21 It is important to note that a cell cycle inhibitor can also play a decisive role in a nonproliferating cell type such as DCs or, as shown, nonproliferating eosinophils.23 However, although both in eosinophils and in the murine IL-3–dependent cell line Ba/F3 apoptosis was linked to increased p27KIP1 and interference with the PI3K pathway, apoptosis could not be induced by Rapa.23 This finding further underlines the specificity of Rapa toward DC apoptosis. It has been shown that p27KIP1-deficient mice demonstrate an increased survival of bone marrow–derived stem cells when cultured ex vivo compared with wild-type mice,23 but the immune status has not been specifically investigated.36 The mechanism by which p27KIP1 can regulate cell survival is not known. In search of a potential mechanism by which p27KIP1 could modulate GM-CSF–driven DC survival, we focused on the regulation of mcl-1.
mcl-1, initially cloned as a gene differentially expressed in human ML-1 myeloid leukemia cells,37 shows extensive homology to bcl-2. Like bcl-2 and bcl-xL, mcl-1 heterodimerizes with bax38 and thus plays an important role in the prevention of apoptosis. Although mcl-1 and bcl-2 show strong homology, their distribution, expression levels, and regulation of apoptosis are independently regulated.39-41 mcl-1 is thought to be an important protein in IL-3 and GM-CSF–mediated survival of hematopoietic cells,29,42 a finding supported by the fact that mcl-1 transgenic mice possess an enhanced viability in a wide range of hematopoietic cell types.43
We demonstrate that the protein level of mcl-1 in DCs is tightly regulated by GM-CSF signaling via PI3K and mTOR. Despite the large decrease in the level of mcl-1 protein following treatment with Rapa or LY294002, no significant decrease in the level of mcl-1 mRNA was observed (data not shown). This finding is consistent with previous work demonstrating that GM-CSF–mediated mcl-1 expression is regulated at the translational level by a PI3K-controlled pathway.44LY294002-treated human monocyte-derived macrophages45showed also a marked decrease of mcl-1 protein expression, but they appear to have different PI3K/mcl-1 survival mechanisms than monocyte-derived DCs, because monocytes and macrophages do not undergo Rapa-induced apoptosis (Figure 2).15 In addition, we showed that monocytes do not increase their p27KIP1expression on Rapa treatment, whereas DCs clearly do. Therefore, alternative survival mechanisms might be present in monocytes/macrophages that regulate the expression of mcl-1 independently of mTOR and p27KIP1.
In conclusion, given their central role in the immune system, DCs are important targets for both immunosuppressive or immunostimulatory therapies.46-50 Understanding the survival program of DCs will provide the opportunity to either increase immune responses by prolonged DC survival or to terminate these responses by specific depletion of the cells. Rapa, which has been introduced recently as an effective drug to prevent allograft rejection, might partially exert its immunosuppressive effect by virtue of its proapoptotic effect on DCs. Our finding that the survival of monocyte-derived DCs is specifically regulated by the GM-CSF signaling pathway via PI3K/mTOR and involves the regulation of p27KIP1 and mcl-1 might provide additional tools to control immune responses.
We thank Drs I. Naruse and H. Hoshino (Gunma University School of Medicine, Maebashi, Japan) for providing the adenoviral vectors containing either p27KIP1 or LacZ, and M. J. W. E. Rabelink (Department of Molecular Cell Biology), Dr L. T. C. Peltenburg (Department of Clinical Oncology), and N. Schlagwein (Department of Nephrology; all from LUMC, Leiden, The Netherlands) for technical assistance.
C. van K. is a fellow of the Royal Netherlands Academy of Arts and Sciences (KNAW).
Prepublished online as Blood First Edition Paper, September 26, 2002; DOI 10.1182/blood-2002-06-1688.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andrea M. Woltman, Leiden University Medical Center, Department of Nephrology, C3-P, Albinusdreef 2, 2333 ZA Leiden, The Netherlands; e-mail: a.m.woltman@lumc.nl.