The chemokine receptors (CCRs) CCR4 and CCR10, and the cutaneous lymphocyte antigen (CLA), have each been proposed as critical mediators of skin-specific TH lymphocyte homing in mice and humans. CLA initiates skin homing by mediating E-selectin–dependent tethering and rolling within cutaneous venules, but the specific roles of CCR4 and CCR10 are unclear. We have generated an antihuman CCR10 monoclonal antibody (mAb; 1B5) to illuminate the individual contributions of these molecules. This mAb allows us to compare CCR10, CCR4, and CLA expression within human THpopulations. The mAb 1B5 recognizes functional CCR10 expression, as chemotactic responsiveness to cutaneous T-cell–attracting chemokine (CTACK)/CCL27 (a CCR10 ligand) parallels the staining of TH subsets. We find CCR10 expressed by only a minority (approximately 30%) of blood-borne, skin-homing (CLA+/CCR4+) TH cells. However, essentially all members of the relatively small “effector” (CLA+/CCR4+/CD27−/CCR7−) skin-homing TH population express CCR10. Most skin-infiltrating lymphocytes in allergic delayed-type hypersensitivity (DTH) and bacterial chancroid skin lesions express both CCR4 and CLA, but only about 10% express CCR10. This suggests for the 2 models of TH skin homing studied here that CCR10+ TH cells have no advantage over other CLA+/CCR4+ TH cells in homing to cutaneous sites. We conclude that the skin-homing THcompartment is itself divided into distinct subpopulations, the smaller of which expresses both CCR4 and CCR10, and the larger of which expresses only CCR4. Thus, CCR10 is unlikely to be necessary for cutaneous homing of TH cells in the models studied here. CCR10 may instead play a role in the movement of specialized “effector” cutaneous TH cells to and/or within epidermal microenvironments.
Introduction
Adhesion molecules and tissue-specific homing
Classical studies of tissue-specific homing within the human circulating TH (CD4+) compartment suggest that the memory (CD45RA−) population can be divided into tissue-specific subsets. Each subset traffics through and engages in immunosurveillance of distinct domains of nonlymphoid tissues. For example, memory lymphocytes expressing the α4β7-integrin-heterodimer (an adhesion molecule that binds the mucosal addressin MAdCAM-1) contain antigen specificities associated with the gut1,2 and tend to traffic through intestinal sites.3,4 TH cells that express the cutaneous lymphocyte antigen (CLA) carbohydrate (an adhesion molecule that binds E-selectin) contain specificities for cutaneous antigens5,6 and tend to traffic through cutaneous sites.7,8 CLA-expressing cells are identified by mAb HECA-452 in humans7,8 or by flow cytometry with an E-selectin–immunoglobulin (Ig) chimera in either mice or humans.9
Chemokine receptors and tissue-specific homing
Differential expression of chemokine receptors also correlates with tissue-specific homing. For example, expression of chemokine receptor 9 (CCR9) is associated with a small intestine (but not colon) homing subset of TH cells.10,11 Moreover, CCR7 function is necessary for homing of T cells to secondary lymphoid organs through high endothelial venules (HEVs).12-14
Another chemokine receptor, CCR4, is robustly expressed by most skin-homing (CLA+) TH cells in the circulation, but rarely (and at lower levels on the few positives) by intestinal-homing TH cells.15 CCR4 is also expressed at high levels by skin-infiltrating lymphocytes (isolated directly from skin), but not by gut-infiltrating lymphocytes.10 Additionally, the CCR4 ligand TARC/CCL17 is associated with cutaneous (but not intestinal) endothelial cells.15
CCR4 expression can easily distinguish between cutaneous (CLA+) and intestinal (α4β7+) TH memory cells, but is also expressed by some circulating memory TH cells that lack both CLA and α4β7.15 Some of these CLA−/CCR4+ cells may (like CLA+cells) have cutaneous roles, as vascular cell adhesion molecule-1/integrin interactions appear to be more important than CLA/E-selectin interactions for deep-dermal homing.16However, there also appears to be an additional, less well-understood role for CCR4 in pulmonary leukocyte homing.17-19
The expression of CCR4 by apparently noncutaneous lymphocytes has led to the proposal that CLA and CCR4 must work together to convey skin-specific homing.15 Alternatively, it has been proposed that there may exist another chemokine receptor, with better specificity for cutaneous TH cells than CCR4.
In fact, after the associations among CCR4, TARC, and cutaneous TH homing were discovered, another chemokine, cutaneous T-cell–attracting chemokine (CTACK)/CCL27 (originally cloned as ALP in mice20), was found to be expressed by cutaneous keratinocytes.21 CTACK is not a ligand for CCR4, but like TARC, CTACK preferentially attracts CLA+ THcells from peripheral blood in vitro.21 The receptor for CTACK was identified as CCR10, which has another ligand (MEC, CCL28) expressed within the colon and within secretory tissues, including salivary and mammary glands.22 CCR10 is expressed by a subset of CLA+ TH cells in peripheral blood.23
Thus, CCR4 and CCR10 are both associated with conventionally defined skin homing TH cells from peripheral blood, but the association is imperfect in both cases: CCR4 is present on essentially all skin-homing cells, but also present on other systemic cells. In contrast, CCR10 is absent from non–skin-homing THcells,23 but only present on a subset of skin-homing TH cells.
Peripheral blood TH lymphocytes lacking CD27 and CCR7
In addition to the tissue-specific divisions among THsubsets, there exist other axes of subdivision. For example, a relatively small subset of TH cells does not express the tumor necrosis factor–receptor family member CD2724-26 and/or the chemokine receptor CCR7.24,27 Such cells are found within the CLA+/α4β7−, CLA−/α4β7+, and CLA−/α4β7− memory compartments, but not within the naive (CD45RA+) compartment (virtually all naive cells express both CD27 and CCR724). The THphenotypes that lack these markers have been called “effector-memory,” because they are enriched in recently seen antigen specificities, and they respond more quickly to antigen than most other memory TH cells.25-27 Absence of CCR7 would preclude entry of such cells into lymphoid organs via HEVs through the known mechanisms,13,14,27 suggesting that such cells might home through only those nonlymphoid tissues to which they have become dedicated. Indeed, effector-memory cells are found within uninflamed or inflamed nonlymphoid tissues, often side-by-side with cells that continue to express both CD27 and CCR7.24 The relationship between effector phenotype and trafficking phenotype has not yet been thoroughly assessed.
In order to understand the interesting differences between CCR10 and CCR4 expression among human TH subpopulations, we have generated a monoclonal antibody that recognizes functional expression of CCR10 by human lymphocytes. We have performed extensive analyses to compare and contrast CCR10 versus CCR4 expression by THsubsets associated with cutaneous homing. We have examined expression (and functionality) of these receptors by several distinct subsets of peripheral blood TH memory cells, including those classified as “effector” TH cells. Furthermore, we have compared expression of these receptors by TH lymphocytes directly isolated from inflamed cutaneous sites.
Patients, materials, and methods
Generation of mAbs against CCR10
The murine pre-B lymphoma cell line L1/2 was transfected with an expression vector (pcDNA3.1) containing the human CCR10 DNA sequence, as previously described.28 Transfected pools were chemotaxed to CTACK/CCL27 and/or to MEC/CCL28, and clones with the best chemotactic potential were selected (L1/2 cells expressing human CCRs 1 through 9 were created in the same manner). CCR10-specific monoclonal antibody 1B5 was generated by immunizing C57Bl/6 mice intraperitoneally with 107 CCR10 L1/2 cell transfectants every 2 weeks for 5 to 6 times. The final immunization was performed intravenously 4 days prior to extraction of the spleen for fusion with the SP2/O cell line as described.24CCR10-specific antibody-secreting hybridoma cell clones were identified by performing immunofluorescence staining of wild-type (WT) and CCR10 L1/2 cells with cell supernatants followed by a fluorescently-labeled antimouse secondary antibody. Fluorescent cells were detected by flow cytometry. Antibody-secreting clones were isolated by multiple rounds of subcloning. For screening the CCR10 mAb against L1/2 cells transfected with other CCRs (Figure 1), each transfectant was stained with a specific mAb to its respective receptor, to ensure high expression levels of the transfected gene (not shown).
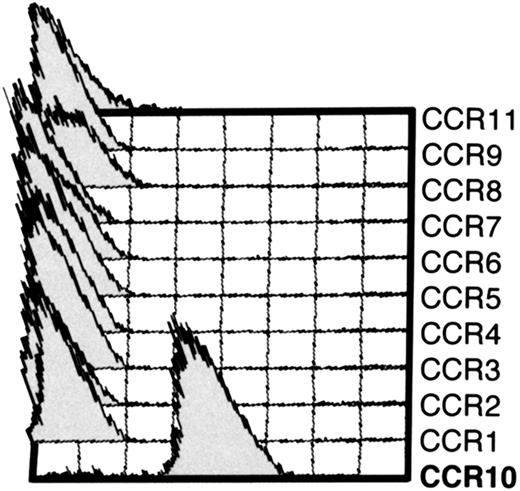
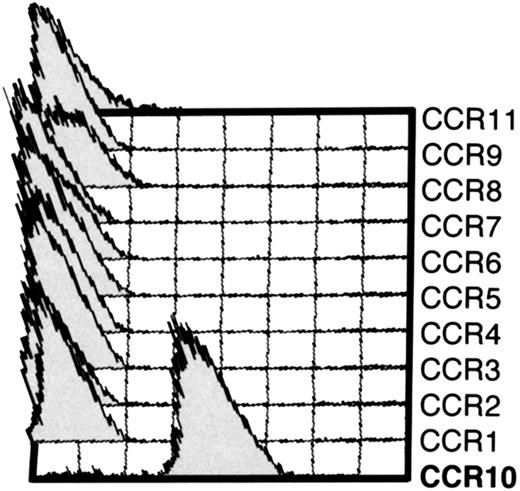
mAb 1B5 recognizes CCR10-expressing L1/2 cell transfectants but not other CC chemokine receptor transfectants.
Cytometry histograms showing the indicated L1/2 CC chemokine receptor transfectants (CCR1-CCR11/GusB) stained with anti-CCR10 mAb 1B5. Each cell line was stained with a mAb to its corresponding receptor to ensure high expression (not shown). Except for CCR10 cells, 1B5 staining was indistinguishable from the isotype-matched control (IgG2a) for all other transfectants (not shown). 1B5 also recognized CCR10 expressed by CHO cells (not shown).
mAb 1B5 recognizes CCR10-expressing L1/2 cell transfectants but not other CC chemokine receptor transfectants.
Cytometry histograms showing the indicated L1/2 CC chemokine receptor transfectants (CCR1-CCR11/GusB) stained with anti-CCR10 mAb 1B5. Each cell line was stained with a mAb to its corresponding receptor to ensure high expression (not shown). Except for CCR10 cells, 1B5 staining was indistinguishable from the isotype-matched control (IgG2a) for all other transfectants (not shown). 1B5 also recognized CCR10 expressed by CHO cells (not shown).
Peripheral blood
Skin inflammation
Delayed-type hypersensitivity (DTH) responses toCandida extract, and subsequent isolation of lymphocytes from vacuum-blister fluid were performed as described previously.10,24 Experimental human chanchroid was induced by inoculation of healthy, HIV-seronegative volunteers at 3 sites withHaemophilus ducreyi 35000HP (a human passaged isolate of 35000). Sites that evolved into pustules were biopsied 7 to 14 days after inoculation. In some cases, multiple sites from one subject were biopsied and then pooled. Isolation of lymphocytes from biopsied skin using EDTA (ethylenediaminetetraacetic acid) was performed as described previously.10,24 Informed consent was obtained from the subjects in accordance with the guidelines for human experimentation of the US Department of Health and Human Services and the institutional review board of Indiana University–Purdue University at Indianapolis under grants AI31494 and AI27863 to S.M.S. and M01RR00750 to the GCRC at IU. Enrollment procedures, exclusion criteria, preparation of the bacteria, inoculation procedures, and clinical outcomes are described in detail elsewhere.31-33
Six-color flow immunocytometry
Immunostaining was performed with a 5-step method. Cells were stained first with unconjugated mAb (or its isotype-matched control); followed by polyclonal second-stage Abs; followed by blocking with 3 mg/mL mouse IgG (technical grade; Sigma) and 0.3 mg/mL mouse IgM (technical grade; Sigma); followed by directly-conjugated mAbs; followed by streptavidin conjugate. (1) The first fluorescent color used (color 1) was: CLA–fluorescein isothiocyanate (FITC; HECA-452; BD/Pharmingen, San Diego, CA) or CD27-FITC (M-T271; BD/Pharmingen); (2) color 2: CD27-phycoerythrin (PE; M-T271, BD/Pharmingen) or β7-Integrin-PE (FIB-504; BD/Pharmingen); (3) color 3: CD45RA-PE-TR (2H4; Beckman-Coulter, Brea, CA); (4) color 4: IgG2a isotype control (UPC-10; Sigma), CCR4 (2B10;15 Millennium Pharmaceuticals), or CCR10 (1B4; Millennium Pharmaceuticals); followed by goat anti–mouse IgG2a(γa)-biotin (Southern Biotechnology, Birmingham, AL); followed by Streptavidin-PE-Cy7 (Cedarlane Labs, Ontario, CA); (5) color 5: CCR7 (3D9;24 Millennium Pharmaceuticals); followed by goat anti–mouse IgM(μ)-Cy5 (Jackson Immunoresearch, West Grove, PA); and (6) color 6: CD4-allophycocyanin (APC)–Cy7 (S3.5; Caltag, Burlingame, CA).
Data on stained cells were acquired on dual-laser MoFlo cytometer (Cytomation, Ft. Collins, CO) configured for 6 colors: (1) FITC, (2) PE, (3) PE-TR, (4) PE-Cy7, (5) Cy5, (6) APC-Cy7. Raw data were compensated and analyzed using Summit 3.0 software (Cytomation).
Chemotaxis
Chemotaxis was performed as described.12 15For each chemokine for each donor, 2 chemotaxis wells of the following concentrations were set up: 1000 nM, 300 nM, 100 nM, and 30 nM; data are shown in Figure 4 for the optimal concentrations. Recombinant stromal cell–derived factor-1α (SDF-1α; PeproTech), recombinant TARC (R&D Systems, Minneapolis, MN) and synthetic CTACK (Gryphon Sciences, South San Francisco, CA) were used for all experiments shown. Input and migrated cells were stained with a 5-color protocol: CLA-FITC, CD27-PE (M-T271, BD-Pharmingen), CD45RA-PETR, CD4-biotin (13B8.2, Beckman-Coulter) + streptavidin-PE-Cy7, and CCR7 (7H12, Millennium Pharmaceuticals) + goat anti–mouse IgG (H&L)-Cy5 (Jackson Immunoresearch).
Results
Generation of a mAb to human CCR10
A monoclonal antibody against human CCR10 was developed by immunizing mice with L1/2 cells (a murine pre-B lymphoma) transfected with human CCR10 mRNA in a mammalian expression vector.24 The resulting mAb, 1B5, recognized CCR10-transfected (but not WT) L1/2 cells (Figure1). 1B5 did not recognize L1/2 cells transfected with any of the other known CC chemokine receptors (CCR1 through 9 plus CCR11/GusB) (Figure 1).
Expression of CCR10 by peripheral blood THcells
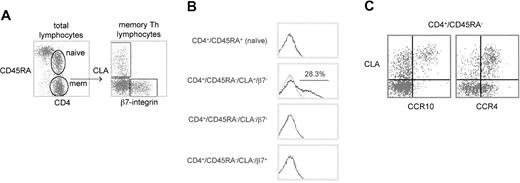
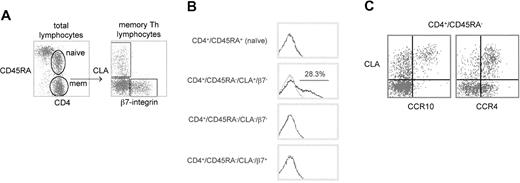
We first examined the CCR10 expression of naive TH and the 3 peripheral blood memory TH subsets classically defined by the homing receptors CLA or α4β7-integrin34 35 (Figure2A). Among the memory TH populations, CCR10 expression was restricted to the CLA+/α4β7− “skin homing” subpopulation (Figure 2B). Interestingly, however, CCR10 was not expressed by approximately 70% of the CLA+TH population. CCR10 was absent from naive THcells (Figure 2B).
CCR10 expression by peripheral blood memory TH subsets defined by memory markers and tissue-specific adhesion molecules.
(A) Cytometry plots showing gated populations from 5-color experiment analyzed in panel B. Left plot: CD45RA versus CD4 (on lymphocyte scatter gate) shows gating of naive TH(CD4+/CD45RA+) and memory TH(CD4+/CD45RA−). Right plot: expression of the adhesion molecules CLA and β7-integrin by memory THcells, showing cutaneous (CLA+/β7−), intestinal (CLA−/β7+), and undefined (CLA−/β7−). (B) CCR10 expression by each of the populations defined in A on a representative healthy blood donor. CCR10 staining (black line) overlaid on isotype-matched control staining (gray line). (C) Cytometry plots showing CLA versus CCR10 (left plot) or CLA versus CCR4 (right plot) in the memory TH lymphocyte gate for a representative healthy donor.
CCR10 expression by peripheral blood memory TH subsets defined by memory markers and tissue-specific adhesion molecules.
(A) Cytometry plots showing gated populations from 5-color experiment analyzed in panel B. Left plot: CD45RA versus CD4 (on lymphocyte scatter gate) shows gating of naive TH(CD4+/CD45RA+) and memory TH(CD4+/CD45RA−). Right plot: expression of the adhesion molecules CLA and β7-integrin by memory THcells, showing cutaneous (CLA+/β7−), intestinal (CLA−/β7+), and undefined (CLA−/β7−). (B) CCR10 expression by each of the populations defined in A on a representative healthy blood donor. CCR10 staining (black line) overlaid on isotype-matched control staining (gray line). (C) Cytometry plots showing CLA versus CCR10 (left plot) or CLA versus CCR4 (right plot) in the memory TH lymphocyte gate for a representative healthy donor.
Two-color plots of CCR10 or CCR4 versus CLA are shown for the memory TH-gated population in Figure 2C. CCR4 stains the vast majority of CLA+ TH cells with equal intensity and also stains a subset of CLA− TH cells (Figure 2C, right panel). In contrast, CCR10 appears to preferentially stain only those TH cells in the brightest one half to one third of the CLA+ distribution (Figure 2C, left panel).
Characterization of the CLAhi-enriched memory TH subset that expresses CCR10, and comparison with CCR4 expression
Another previously identified axis of subdivision within the peripheral blood CLA+ TH memory compartment (as well as the other TH memory compartments) involves CD27 and CCR7 expression.24-27 We therefore asked whether the observed patterns of CCR10 expression correlate with CD27 and/or CCR7 expression by using 6-color flow cytometry.
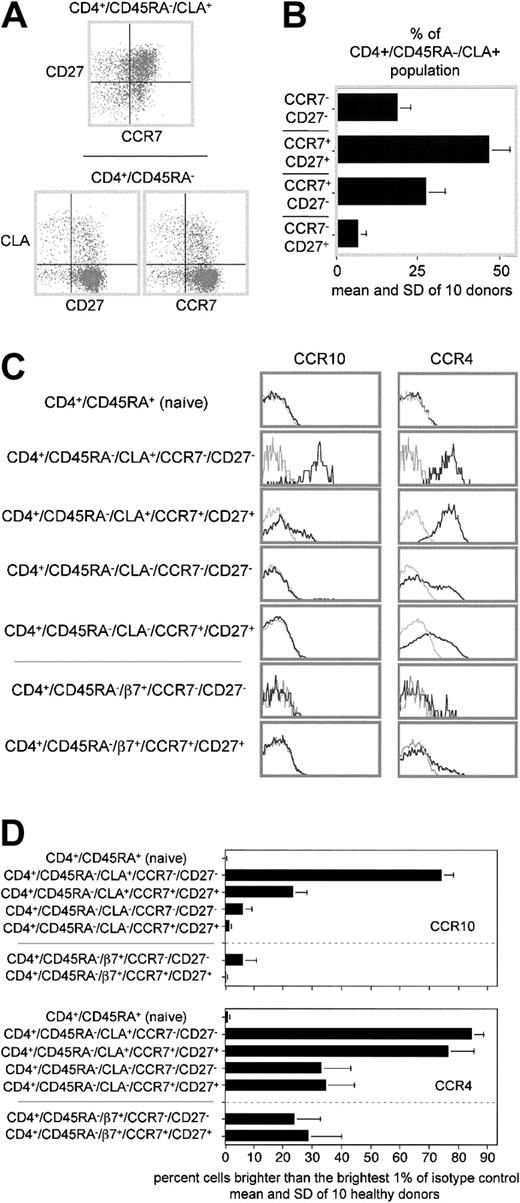
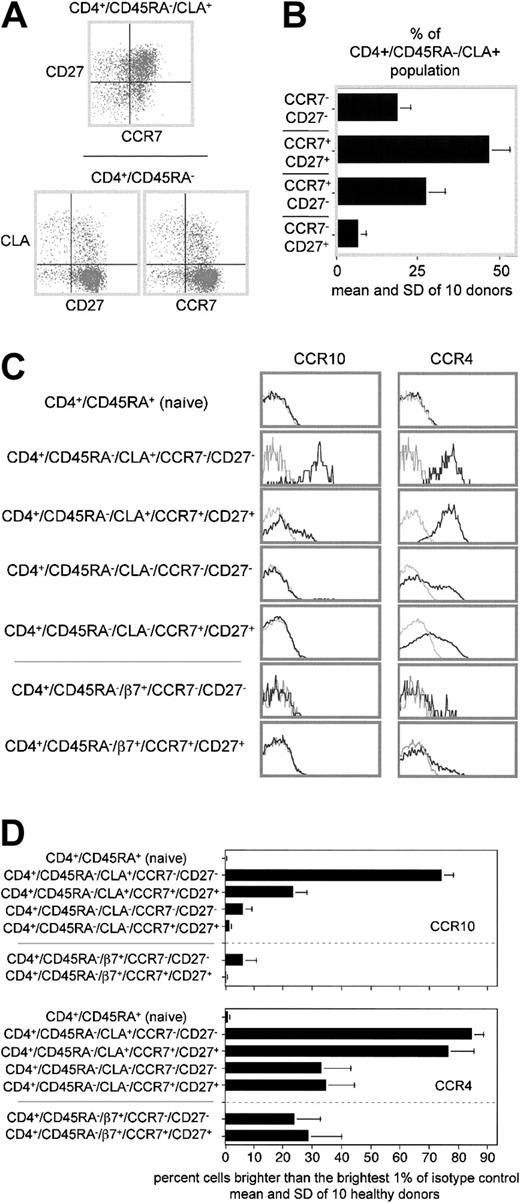
The upper panel in Figure3A shows that peripheral blood CLA+ TH cells are spread among all 4 quadrants of a CD27 versus CCR7 plot. However, plots of CLA versus CD27 or CCR7 distinguish the individual subsets more clearly (Figure 3A, lower plots). The latter type of plot was used to enumerate CLA+ subsets displaying the 4 permutations of CD27 and CCR7 expression for 10 healthy donors (Figure 3B). Interestingly, the CD27− and CCR7− subsets of the CLA+ TH populations were enriched for the brightest one third to one half of the CLA expressers, with striking similarity to the CLA pattern observed for CCR10 expressers in Figure2C.
Subpopulations of peripheral blood CLA+ (and other) memory TH cells express different levels of CCR10.
(A) CLA+ “cutaneous” TH cells can be further divided into distinct subpopulations based on CD27 and CCR7 expression. Top: CD27 versus CCR7 expression on CLA+ memory TH cells. Bottom: CLA versus CD27 (left plot) or CLA versus CCR7 (right plot) for a representative healthy donor. (B) Bar graph showing proportion of CLA+ memory TH cells expressing the 4 possible permutations of CD27 and CCR7 expression. Mean and SD of 10 healthy donors shown. (C) Cytometry plots of CCR10 or CCR4 expression by adhesion receptor–defined memory subsets further divided by CD27 and CCR7 expression on representative donor blood. CCR10 staining (black lines) overlaid on isotype-matched control staining (gray lines). Naive, CLA+, and CLA−plots are all from the same 6-color stain. The β7+ and β7− populations are from a separate 6-color stain of the same donor. (D) Statistical analysis of CCR10 and CCR4 expression on 10 healthy donors by subsets gated as in B. The difference in CCR10 expression between each of the 2 CLA+ populations and all other populations is significant (P < .05). There is no significant difference in CCR4 expression between the 2 CLA+ populations.
Subpopulations of peripheral blood CLA+ (and other) memory TH cells express different levels of CCR10.
(A) CLA+ “cutaneous” TH cells can be further divided into distinct subpopulations based on CD27 and CCR7 expression. Top: CD27 versus CCR7 expression on CLA+ memory TH cells. Bottom: CLA versus CD27 (left plot) or CLA versus CCR7 (right plot) for a representative healthy donor. (B) Bar graph showing proportion of CLA+ memory TH cells expressing the 4 possible permutations of CD27 and CCR7 expression. Mean and SD of 10 healthy donors shown. (C) Cytometry plots of CCR10 or CCR4 expression by adhesion receptor–defined memory subsets further divided by CD27 and CCR7 expression on representative donor blood. CCR10 staining (black lines) overlaid on isotype-matched control staining (gray lines). Naive, CLA+, and CLA−plots are all from the same 6-color stain. The β7+ and β7− populations are from a separate 6-color stain of the same donor. (D) Statistical analysis of CCR10 and CCR4 expression on 10 healthy donors by subsets gated as in B. The difference in CCR10 expression between each of the 2 CLA+ populations and all other populations is significant (P < .05). There is no significant difference in CCR4 expression between the 2 CLA+ populations.
For simplicity, we have chosen to focus on the 2 most extreme phenotypes identified here for more detailed analysis: the CD27−/CCR7− “double negatives” (which meet all immunophenotypic criteria for being “effector” TH cells), and CD27+/CCR7+“double positives” (which meet none of the criteria for being “effector” TH cells). Figure 3C-D compares the CCR4 and CCR10 expression patterns of CD27/CCR7–double positive and –double negative subsets of CLA+, CLA− and α4β7+ memory TH populations, as well as the naive population (note that the CLA− memory population also contains the α4β7+ population as a subset). Figure 3C shows CCR10 staining (black lines, left column) or CCR4 staining (black lines, right column) overlaid on isotype-matched controls for the same gated populations from a representative donor (gray lines, both columns). Figure 3D shows the mean CCR4 and CCR10 expression (and SD) for the same populations by 10 healthy blood donors. Although CCR4 is expressed by the same proportion of cells and (at equal levels) by both double-positive and double-negative CLA+ populations, CCR10 is expressed by a much greater proportion (and at much greater levels) by double-negative cells. (Note that CCR10 and CCR4 expression is enumerated in Figure 3D by counting those cells brighter than the brightest 1% of the isotype control. This results in an underestimate of CCR4 expression by the CLA+ populations [and CCR10 by the “effector” cutaneous population], as it is quite clear that the entire stained population is shifted with respect to the control-stained population. Also, this method does not consider the mean expression of receptor by positive cells, as exemplified by the apparently modest difference in CCR4 expression between the CLA− and α4β7+populations in Figure 3D, which in truth have a dramatically different mean expression as seen in Figure 3C.) In fact, CCR10 is expressed by virtually all double-negative CLA+TH cells. The CD27+/CCR7− and CD27−/CCR7+ populations (not shown) expressed intermediate CCR10 levels (D.S. and J.J.C., unpublished data, May 2002).
Responsiveness of peripheral blood TH subsets to the CCR10 ligand CTACK
Whenever possible, it is important to confirm chemokine receptor flow cytometry results through an independent method. We therefore set out to determine whether CCR10 expression patterns (determined by 1B5) correlate with functional responses to CTACK/CCL27 (a CCR10 ligand) in standard chemotaxis assays. Each of the populations examined express very similar amounts of the SDF-1 receptor CXCR4 (J.J.C., unpublished data, November 2000). This fact allows normalization of their CTACK-mediated migration to that of SDF-1, to correct for potential intrinsic differences in migration rates among the various populations (Figure4, upper scale). Migration is also plotted (on the same graphs) as percent of input (Figure 4, lower scale) with comparable outcome. Figure 4clearly demonstrates that the double-negative CLA+TH population responds significantly better to CTACK than any of the other populations tested (including the double-positive CLA+ TH population, Figure 4, left panel). There was no significant difference in TARC responsiveness between double-positive and double-negative populations (Figure 4, right panel).
Chemotaxis of TH subsets to CCR10 and CCR4 ligands.
Peripheral blood lymphocytes were attracted to optimal concentrations of CTACK or TARC through 5-μm pores, and 5-color immunostaining was performed. Migration is expressed as percent of input (lower scale) and percent of response to optimal concentration of SDF-1α for each subset (upper scale). Background migration for each subset (ranging from 0.5%-3.0%) has been subtracted. Mean and SEM are shown for CD4+ subsets from 3 healthy donors. For each donor, migration was performed in duplicate for medium alone, SDF-1α, CTACK, and TARC. The difference in CTACK responsiveness between CD27+/CCR7+ and CD27−/CCR7− cutaneous TH cells was significant using the Mann-Whitney rank-sum test (P < .05). The difference in TARC responsiveness between these same 2 populations was not significant.
Chemotaxis of TH subsets to CCR10 and CCR4 ligands.
Peripheral blood lymphocytes were attracted to optimal concentrations of CTACK or TARC through 5-μm pores, and 5-color immunostaining was performed. Migration is expressed as percent of input (lower scale) and percent of response to optimal concentration of SDF-1α for each subset (upper scale). Background migration for each subset (ranging from 0.5%-3.0%) has been subtracted. Mean and SEM are shown for CD4+ subsets from 3 healthy donors. For each donor, migration was performed in duplicate for medium alone, SDF-1α, CTACK, and TARC. The difference in CTACK responsiveness between CD27+/CCR7+ and CD27−/CCR7− cutaneous TH cells was significant using the Mann-Whitney rank-sum test (P < .05). The difference in TARC responsiveness between these same 2 populations was not significant.
CCR10 expression by tissue-infiltrating lymphocytes from inflamed skin
The data indicate that while CCR4 is expressed by most CLA+ TH cells in blood, CCR10 is expressed only by a minority subset. We therefore asked whether the CCR10+subset might have an advantage over other CLA+/CCR4+ TH cells in its ability to home to inflamed cutaneous sites.
We chose to analyze the homing phenotypes of TH cells directly isolated from 2 different types of cutaneous lesions. The first was a widely studied DTH model, in which cutaneous inflammation is induced by intradermal injection of a sterile extract ofCandida albicans.10 The second was an experimental cutaneous bacterial infection of human volunteers withH ducreyi. H ducreyi causes chancroid, a sexually transmitted genital ulcer disease that facilitates efficiency of HIV transmission by disruption of epithelial barriers and infiltration of TH (CD4+) cells into the lesions.36
Essentially all of the TH cells isolated from DTH skin were of the CD45RAlo/neg memory phenotype (Figure5A). TH cells from donor-matched peripheral blood contained 40% to 50% CD45RAhi naive cells (not shown), suggesting that the skin-derived samples were not significantly contaminated with peripheral blood lymphocytes. The number of cells expressing CLA was greatly enriched with respect to peripheral blood memory THcells, but the ratio of CD27/CCR7 double positives to double negatives did not significantly differ from that of peripheral blood memory CLA+ TH cells (Figure 5B-C). CCR4 was expressed by skin-infiltrating TH cells at greatly enriched levels, but, interestingly, CCR10 was not (Figure 5D-E).
CCR10 expression is rare among skin-infiltrating lymphocytes isolated from Candida-induced DTH responses.
(A-C) Two-color cytometry plots of lymphocytes from DTH skin. Panel A is gated only on the lymphocyte scatter gate. Panels B and C are gated on CD4+ cells. (D-E) Single-color cytometry plots of CCR10 or CCR4 expression on the same CD4+ cells. CCR10 or CCR4 staining (gray lines) overlaid on isotype-matched control staining (gray lines). Results shown are from a single, typical DTH experiment.
CCR10 expression is rare among skin-infiltrating lymphocytes isolated from Candida-induced DTH responses.
(A-C) Two-color cytometry plots of lymphocytes from DTH skin. Panel A is gated only on the lymphocyte scatter gate. Panels B and C are gated on CD4+ cells. (D-E) Single-color cytometry plots of CCR10 or CCR4 expression on the same CD4+ cells. CCR10 or CCR4 staining (gray lines) overlaid on isotype-matched control staining (gray lines). Results shown are from a single, typical DTH experiment.
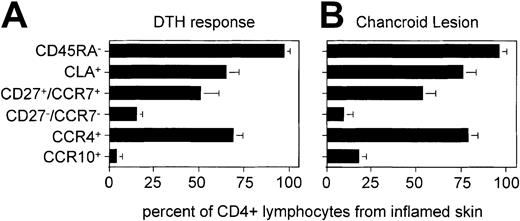
The identical flow cytometry was performed on a total of 3 DTH and 3H ducreyi lesions, and the data presented as bar graphs (Figure6). For both types of lesion, the ratio of memory to naive cells was significantly different from that of the peripheral blood TH population from matched donors (not shown). The proportion of CLA+ and CCR4+ THcells was significantly increased with respect to the peripheral blood memory population. In contrast, the ratio of CD27/CCR7 double positives to double negatives was not significantly different from that of peripheral blood memory TH cells. Most importantly for the present study, the proportion of CCR10+ cells was not significantly enriched with respect to peripheral blood CLA+ memory TH cells.
Analysis of TH phenotype from 2 different types of cutaneous lesion.
(A) Mean and SD from 3 separate DTH reactions as shown in Figure5. (B) Similar analysis of lymphocytes derived from biopsies of chancroid pustules from cutaneous H ducreyi infections of 3 individual patients. For each cutaneous lymphocyte donor, peripheral blood was compared with the database of 10 healthy donors presented in Figures 2 and 3. The composition of peripheral blood (for each subset shown in this figure) was not significantly different from this database using the Mann-Whitney rank-sum test (not shown). The difference between peripheral blood and skin-infiltrating cells was significant for the CD45RA− (P < .05), CLA+ (P < .05), and CCR4+(P < .05) populations. The difference between peripheral blood and skin-infiltrating cells was not significant for the CD27+/CCR7+, CD27−/CCR7−, or CCR10+populations.
Analysis of TH phenotype from 2 different types of cutaneous lesion.
(A) Mean and SD from 3 separate DTH reactions as shown in Figure5. (B) Similar analysis of lymphocytes derived from biopsies of chancroid pustules from cutaneous H ducreyi infections of 3 individual patients. For each cutaneous lymphocyte donor, peripheral blood was compared with the database of 10 healthy donors presented in Figures 2 and 3. The composition of peripheral blood (for each subset shown in this figure) was not significantly different from this database using the Mann-Whitney rank-sum test (not shown). The difference between peripheral blood and skin-infiltrating cells was significant for the CD45RA− (P < .05), CLA+ (P < .05), and CCR4+(P < .05) populations. The difference between peripheral blood and skin-infiltrating cells was not significant for the CD27+/CCR7+, CD27−/CCR7−, or CCR10+populations.
Discussion
We have performed a detailed analysis of functional CCR4 and CCR10 expression by peripheral blood– and skin-derived human THcells. These studies are part of an ongoing effort to understand the role of chemokines and their receptors in cutaneous (and other tissue-specific) lymphocyte homing.
Peripheral blood contains a heterogeneous mixture of TH(and other) lymphocytes, many with distinct trafficking patterns.34 35 Therefore, within a given tissue, the specific enrichment of cells bearing a particular homing molecule suggests the molecule's potential importance in homing to that tissue.
We confirm that lymphocytes expressing CLA and CCR4 are greatly enriched within the cutaneous lesions studied here, which is not the case for other tissues.10 15 CCR10 is slightly enriched in skin when compared with the total circulating memory THpool. However, CCR10 is not enriched in cutaneous sites when compared with CLA+ memory T cells from peripheral blood. Thus CLA+/CCR4+ TH cells that express CCR10 have no apparent advantage over their CCR10−counterparts in homing to inflamed cutaneous sites.
One should consider the caveat that cell-surface CCR10 might indeed be necessary for skin infiltration, but becomes down-regulated upon entry. This is clearly not the case for CCR4, which is expressed at comparable levels by both CLA+ blood TH cells and skin-infiltrating TH cells.10 Further, we demonstrated that CCR10 expression is greatly enriched on cells lacking CD27 and/or CCR7 in peripheral blood (Figure 3C-D), and that the CD27/CCR7 phenotype of cutaneous-infiltrating cells is not significantly different from that of peripheral blood CLA+memory TH cells. Therefore, as the CD27/CCR7-negatives are not enriched in cutaneous sites, there is no reason to propose that CCR10 expression has been underrepresented by this assay.
Why are there 2 “cutaneous” chemokine receptors?
Both CCR10 and CCR4 have qualities that suggest a role in TH homing to cutaneous sites. However, our data suggest that, unlike CCR4, CCR10 is not a necessary component of cutaneous homing for most TH cells. Within the skin-homing TH population, CCR10 is associated with the “effector” phenotype. Virtually all those cells displaying the most extreme effector phenotype (ie, CD27/CCR7 double negatives) express CCR10.
In vitro studies make it clear that effector TH cells are functionally distinct from conventional memory THcells.25-27 However, it is not clear how such cells actually contribute to in vivo immune responses. It is possible that such effector TH cells play a regulatory role in coordinating immune responses for the tissues through which they traffic. For example, CLA+ cutaneous “effector” TH cells may coordinate the responses of conventional cutaneous CLA+ memory TH cells, either positively or negatively. Part of this role may involve coordinating the traffic of conventional TH cells through the skin, perhaps by regulation of TARC/CCR4 interactions. If this scenario is proved true, effector TH cells would require a TARC/CCR4-independent mechanism for skin entry. CTACK/CCR10 interactions could provide such a mechanism.
This hypothesis would predict that cells entering the skin in the absence of TARC/CCR4 interactions (ie, in CCR4-deficient mice, as discussed in “Murine models”37) would be greatly enriched in effector TH cells. The potential effect that blocking CTACK/CCR10 interactions would have on cutaneous inflammation is less straightforward to predict. If effector cells tend to positively regulate the intensity of skin inflammation, such blocking might be expected to dampen the response. If, in contrast, effector cells tend to negatively regulate inflammation, blocking of CCR10 might actually exacerbate skin inflammation.
CCR10 and other types of skin inflammation
Recent immunohistochemistry studies suggest that CCR10 is expressed by many cells within allergic dermatitis and psoriatic lesions.23 However, it is not clear (from this type of data) what proportion of TH cells express CCR10 within these lesions. Therefore, it is not clear that these findings differ significantly from our own. However, if CCR10 is indeed expressed by many TH cells in such lesions (as suggested by Homey et al23), then CCR10 may play a more pervasive role in homing to allergic dermatitis and psoriatic sites than played in the DTH and bacterial skin lesions studied here. In such a case, one would expect the allergic dermatitis and psoriatic sites to have more CD27/CCR7 double-negative cells than DTH and bacterial skin lesions.
Murine models
Two recent murine studies of dinitrofluorobenzene (DNFB)–induced DTH responses have demonstrated an inhibitory effect of anti-CTACK polyclonal antibodies on cutaneous lymphocyte homing. In the first model (on the C57Bl/6 background37), treatment with anti-CTACK inhibited cutaneous lymphocyte homing in CCR4-deficient but not WT mice. This implies that signaling through both CCR4 and CCR10 must be blocked to influence cutaneous homing through the chemokine system. In the second model (on the Balb/c background23), treatment with very large amounts of anti-CTACK polyclonal Ab alone influenced cutaneous THhoming in WT mice. Such classical murine DNFB-induced models of atopic dermatitis, however, raise concerns when used to study cutaneous TH homing in this particular case: although large numbers of TH cells do indeed enter such lesions, skin swelling itself (as measured by changes in ear thickness after application of DNFB) is not T-cell–dependent. (We have demonstrated that the classical DNFB-induced ear thickness procedure [used in both of the murine studies under discussion] produces swelling in all T-cell–deficient mouse strains tested, including homozygous nude, scid, and Rag-1– or Rag-2–deficient strains. In such experiments, the increases in ear thickness were indistinguishable from those induced in WT mice of the same genetic background [J.J.C., unpublished data, June 2002]). Thus, antibodies to CTACK must have a not-yet-understood influence on some component of the intrinsic immune system to explain their effects on T-cell–independent swelling.
Therefore, the observed influence of anti-CTACK on cutaneous lymphocyte homing should not necessarily be interpreted as having a direct effect on T-cell trafficking. It is equally plausible that the observed reduction of T-cell infiltrates within DNFB-treated skin is secondary to anti-CTACK–mediated prevention of the inflammatory response itself, through effects on the intrinsic immune system. Further studies will be required to resolve this issue, such as reproducing the finding in strictly T-cell–dependent models of cutaneous inflammation.
Although this manuscript addresses only the putative skin-homing TH aspects of CCR10/CTACK interactions, it should be noted that another CCR10 ligand (MEC/CCL28) is expressed by exocrine tissues.22 This suggests that CCR10 may have other roles in leukocyte trafficking yet to be discovered.
Conclusions
Our data support the notion that CLA and CCR4 are intimately associated with the process by which most memory TH cells specifically enter the skin. We suggest that CCR10 may be part of an alternative cutaneous homing pathway utilized primarily by the less numerous “effector” subset of the TH lymphocyte pool.
We thank Nasim Kassam and Meghan Wells from Millennium Pharmaceuticals for invaluable technical assistance. We thank Drs L. Silberstein, L. Jopling, M. Wurbel, and E. Baekkevold for critical reading of the manuscript and useful discussions of the data. We also thank Rob Hromas for instigating the Spinola Lab/Campbell Lab collaboration.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-07-2348.
Supported by National Institute of Allergy and Infectious Diseases (NIAID) grants AI46784 (J.J.C.), and AI31494 and AI27863 (S.M.S.), and M01RR00750 to the General Clinical Research Center (GCRC) at Indiana University (IU). T.L.H. was supported by T32AI07367 from NIAID.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
James J. Campbell, Children's Hospital, Bader 401, 300 Longwood Ave, Boston, MA 02115; e-mail:james.campbell@tch.harvard.edu.