Angiogenesis, the formation of new blood vessels, is a critical step for tumor growth and metastasis and an integral component of the pathologic inflammatory response in arthritis and the proliferative retinopathies. The CD13/aminopeptidase N (CD13/APN) metalloprotease is an important regulator of angiogenesis where its expression on activated blood vessels is induced by angiogenic signals. Here, we show that cytokine induction of CD13/APN in endothelial cells is regulated by distinct Ras effector pathways involving Ras/mitogen-activated protein kinase (MAPK) or PI-3K. Signals transduced by activated Ras, Raf, and mitogen-induced extracellular kinase (MEK) stimulate transcription from theCD13/APN proximal promoter. Inhibition of these pathways and extracellular signal–regulated serine/threonine kinase (ERK-2) and PI-3K by expression of dominant-negative proteins or chemical inhibitors prevented induction of CD13/APNtranscription in response to basic fibroblast growth factor (bFGF). We show that Ras-induced signal transduction is required for growth factor–induced angiogenesis, because inhibition of downstream mediators of Ras signaling (MEK or PI-3K) abrogated endothelial cell migration, invasion, and morphogenesis in vitro. Reintroduction of CD13/APN, a shared downstream target of these pathways, overrode the suppressive effect of these inhibitors and restored the function of endothelial cells in migration/invasion and capillary morphogenesis assays. Similarly, inhibition of MEK abrogated cell invasion and the formation of endothelial-lined capillaries in vivo, which was effectively rescued by addition of exogenous CD13/APN protein. These studies provide strong evidence that CD13/APN is an important target of Ras signaling in angiogenesis and is a limiting factor in angiogenic progression.
Introduction
Angiogenesis is functionally defined as the sprouting of new vessels from pre-existing vasculature and consists of several distinct stages including endothelial cell proliferation, migration, invasion, differentiation into capillaries, and eventual maturation into blood vessels1,2 This process is initiated and maintained in part by interactions of specific angiogenic growth factors with their receptors and the subsequent triggering of signaling pathways and gene expression programs essential for angiogenic progression. The activation of fundamentally dormant vascular endothelium is a sequentially regulated process activated by a shift in equilibrium between pro- and antiangiogenic mediators.2,3 A change in the balance of angiogenic inducers to angiogenic inhibitors is capable of initiating an intracellular sequence culminating in new vessel formation. For endothelial cell activation, the Ras pathway is pivotal for coordinating and transducing multiple angiogenic signals.4-10 Accordingly, the downstream targets of Ras inevitably assume major roles in the induction and maintenance of the angiogenic phenotype.
CD13/aminopeptidase N (CD13/APN) is a type II membrane-bound metalloprotease that is expressed on the endothelial cells of angiogenic, but not normal, vasculature.11 Importantly, treatment of animals bearing breast carcinoma xenografts with CD13/APN functional inhibitors significantly impaired tumor growth, indicating that CD13/APN plays a functional role in tumorigenesis.11Further investigation into the mechanisms regulating the activated endothelial cell–specific expression of CD13/APN showed that hypoxia, angiogenic growth factors, and signals mediating capillary network formation potently induce CD13/APNtranscription in primary endothelial cells and tumor xenografts.12 Finally, capillary network formation is significantly inhibited by treatment of endothelial cells with inhibitory anti–CD13/APN monoclonal antibodies or functional inhibitors of CD13/APN, thus identifying this metallopeptidase as essential for later stages of neovascular formation and as an important angiogenic activator.12
The aim of the present study was to investigate the signaling pathways that regulate CD13/APN expression in response to angiogenic stimulation. In this report we provide evidence thatCD13/APN is a transcriptional target of 2 Ras-mediated signaling pathways that have been implicated in the switch from quiescent to activated endothelium: Ras/mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3 kinase (PI-3K).13 Importantly, addition of functional CD13/APN is sufficient to restore arrested endothelial migration and morphogenesis in vitro and cell migration and angiogenesis in vivo resulting from inhibition of Ras/MAPK or PI-3K mediated signaling. Our data indicate that CD13/APN is an important target of Ras signaling pathways in angiogenesis and a necessary factor in angiogenic progression.
Materials and methods
Cell culture
All cell lines except human umbilical vein endothelial cells (HUVECs) were maintained in the indicated medium supplemented with 10% fetal bovine serum, L-glutamine (2 mM), penicillin (100 units/mL), and streptomycin (100 μg/mL) unless otherwise indicated. Kaposi sarcoma tumor endothelial cells (KS1767, passage numbers 5 to 12) and mouse hemangioendothelioma (EOMA) cells were maintained in modified essential medium (MEM) containing the indicated concentration of fetal calf serum (FCS), 5 mML-glutamine, 0.1 mM nonessential amino acids solution, 1 mM sodium pyruvate solution, and 1 × vitamin solution. NIH-3T3 cells were grown in Dulbecco modified Eagle medium (DMEM) containing 25 mM (4.5 g/L) glucose. MS1 and SVR cells were maintained in DMEM containing 4.5 g/L glucose, with 0.75% sodium bicarbonate and 1 mM sodium pyruvate. HUVECs were obtained from Clonetics Corporation (San Diego, CA) and maintained according to the manufacturer's protocol.
Inhibitors, antibodies, and cytokines
All cells were incubated in serum-free medium 18 to 24 hours prior to treatment with chemical inhibitors of signaling pathways for 2 hours. Cells were then washed once with serum-free medium and stimulated with basic fibroblast growth factor (bFGF; 50 ng/mL, R&D Systems, Minneapolis, MN) in medium containing 1% serum for 24 hours. The Ras inhibitor, manumycin A14,15; the mitogen-induced extracellular kinase (MEK) inhibitor, PD09805916,17; p38 kinase inhibitor, SB20358018,19; and PI-3K inhibitors, wortmannin20,21 and LY29400222 23 were obtained from Calbiochem (San Diego, CA) and used at a final concentration of 10 μM, 30 μM, 10 μM, 100 nM, and 300 nM, respectively. MY7 (anti–CD13/APN) antibodies were obtained from Coulter, Miami, FL; Ras (p21), c–Raf-1, phospho– extracellular signal–regulated serine/threonine kinase (ERK), and ERK antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR) analysis and Southern hybridization
cDNA templates for PCR were synthesized by reverse transcriptase (Omniscript RT Kit, Qiagen, Valencia, CA) according to the method recommended by the manufacturer. For CD13/APN mRNA expression levels, a standard PCR was performed (Taq PCR core kit, Qiagen) using 5′-CCT TCA ACC TGG CCA GTG C-3′ (sense primer common to murine and CD13/APN) and 5′-CGT CTT CTC CAG GGC TTG CTC CAG-3′ (antisense primer common to murine and human CD13/APN) as primers. RT-PCR products were run on agarose gels, transferred to nitrocellulose membranes by Southern blotting, and hybridized using the human CD13/APN cDNA probe described in “Northern blot analysis.”
Northern blot analysis
Total RNA was isolated using TRI reagent (Molecular Research Center, Cincinnati, OH). From each sample, 30 μg of total RNA was separated by electrophoresis in a 1% agarose gel containing formaldehyde and transferred to nylon membranes. Hybridization probes (EcoRI fragments [1.8 and 1.6 kb] encompassing the full-length CD13/APNcDNA24) were labeled with [α-32P] deoxycytosine triphosphosphate (dCTP) using a random priming labeling kit (Redi prime II, Amersham Pharmacia Biotech, Arlington Heights, IL). Membranes were hybridized in NorthernMax Prehyb/Hyb buffer (Ambion, Austin, TX). After stripping, the same membrane was subsequently hybridized with human 28S rRNA gene-specific oligonucleotide probe (Clontech Laboratories, Palo Alto, CA) to control for loading and RNA integrity.
Transient transfection of recombinant plasmids and reporter gene assays
A 1004-bp BstXI fragment from the intestinalCD13/APN promoter25 was subcloned upstream of the luciferase gene in the promoterless luciferase reporter vector pGL2 basic (Promega, Madison, WI). Plasmids were transfected into KS1767 cells (3 × 105 cells) using Lipofectamine (Life Technologies, Rockville, MD) following the manufacturer's protocol. KS1767 cells were seeded in medium containing 1% fetal bovine serum 24 hours before transfection and triplicate wells were then transfected with 3 μg of test plasmid and 1 μg of MAP1-SEAP (metallothienein promoter fused to secreted alkaline phosphatase reporter gene) control plasmids. After overnight incubation, fresh growth medium containing 25 μg/mL bFGF was added and cells were harvested 24 hours later. In cotransfection experiments, 2 μg the indicated expression plasmid (detailed in “Details of expression plasmids”) was transfected along with 1 μg CD13/APN promoter and 1 μg MAPI-SEAP. In conditions treated with chemical inhibitors, cells were transfected and incubated in 1% serum overnight, and treated for 6 hours with the indicated concentrations of inhibitors; fresh medium was then added, and cells were harvested the next day (48 hours after transfection). The transfection efficiency for each construct was normalized to the control level of secreted alkaline phosphatase (SEAP) activity26; the reported values were calculated as relative light units (RLUs) per unit of SEAP activity. All experiments were carried out at least 3 times in triplicate.
Details of expression plasmids
Ras and Raf plasmids were purchased from Clontech; MEK and ERK plasmids were the kind gift of Dr Angelika Hoffmeyer. Ras plasmids are as follows: dominant-negative expression construct (Ras-N17 substitutes Ser17 with Asn27,28); constitutively activated Ras expression construct (Ras-L61 substitutes Phe61 with Leu forming the constitutively active GTP-bound form29). Raf plasmids are as follows: dominant negative (Raf-1–C4B lacks the c–Raf-1 kinase domain30) or constitutively activated (Raf-1–BXB lacks the N-terminal negative regulatory domain31,32). MEK plasmids are as follows: constitutively active MEK (MEK-ACT lacks amino acid residues 32 to 51 and substitutes Ser218 with Glu and Ser222 with Asp) or dominant-negative (MEK-DN substitutes Lys97 with Met) expression plasmids.33 ERK plasmids are as follows: dominant-negative ERK-2 expression plasmids; adenosine triphosphate (ATP)–binding site mutants B3 (Lys523Arg) and C3 (Tyr1853Phe).34 35 Expression of plasmid-encoded proteins was tested by functional assays.
Western blot analysis
Whole cell lysates were prepared by lysing the cells in radioimmunoprecipitation assay (RIPA) buffer. Whole cell lysates (25-30 μg) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on a 7.5% polyacrylamide gel and transferred on to nitrocellulose membrane. Immunostaining was carried out as described.36
In vitro endothelial morphogenesis assay
According to the manufacturer's guidelines, 24-well tissue culture plates were coated with 0.2 mL of basement membrane matrix per well and incubated at 37°C for one hour (Matrigel, Becton Dickinson, Bedford, MA). HUVECs were serum starved overnight and 2000 cells were plated on Matrigel (0.2 mL) in a 24-well plate. At 30 minutes after plating, fresh medium containing the indicated concentrations of inhibitors in 1% serum was added and further incubated for 24 hours. Photographs were taken using a Leica (Bannockbern, IL) phase contrast microscope.
Stable expression of CD13/APN in mouse hemangioendothelioma cells
Full-length (3.4 kb) human CD13/APN cDNA was cloned into the EcoRV and NotI site of pCDNA3.1 (+) vector (Invitrogen, Carlsbad, CA) and stably transfected into EOMAs using Lipofectamine (Gibco-BRL, Carlsbad, CA). Neomycin-resistant pools (5 individual dishes) containing pcDNA-CD13 or the parental vector was amplified. The top 5% fraction of CD13/APN-positive cells in each pool was serially sorted 3 times by fluorescence activated cell sorting (FACS). The pool that expressed the highest levels of CD13/APN (EOMA-CD13) was used for in vitro angiogenesis assays and for CD13-high membrane preparations. Control cells (EOMA-pcDNA) contain vector sequences without CD13/APN. FACS analysis indicated that on average, the EOMA-CD13 pool was 20-fold brighter when stained with the MY7 anti–human CD13/APN antibody than the isotype-matched control levels. The EOMA-pcDNA control pool had essentially no CD13+ cells, and the fluorescence detected when stained with MY7 was at or less than that of the isotype-matched control. CD13/APN activity was measured fluorometrically using alanine p-nitroanalide as described.37
Microsomal membrane preparations
Stably transfected EOMA-pcDNA or EOMA-CD13 cells were homogenized and enriched for microsomal peptidase activity as described, with modifications.38-40 Briefly, 2 × 108 EOMA-pcDNA or EOMA-CD13 cells were washed twice in phosphate-buffered saline (PBS) and resuspended in 1 mL of cold 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EGTA (ethylene glycol-bis[β-aminoethyl ether]-N,N,N′,N′-tetra acetic acid), and 10% glycerol. The cells were homogenized with a glass-Teflon homogenizer (12-15 strokes), incubated on ice for 10 minutes, and centrifuged at 10 000g for 10 minutes at 4°C to remove large cellular debris and mitochondria. The supernatant was further centrifuged at 100 000g for one hour at 4°C to pellet microsomal and Golgi membranes. The resulting pellet was resuspended in homogenization buffer containing 1% Triton X-100, rocked for one hour at 4°C, and centrifuged at 100 000gfor 30 minutes. The resulting supernatant contains APN along with other triton-soluble microsomal proteins.39 Triton X-100 was removed from the aqueous solution with Biorad's Biobead SM-2 (Hercules, CA) as recommended and protein fraction stored at −70°C. CD13/APN activity was measured spectrophotometrically using alanine p-nitroanalide as described37 and was 16-fold higher in EOMA-CD13 preparations than those made from EOMA-pcDNA.
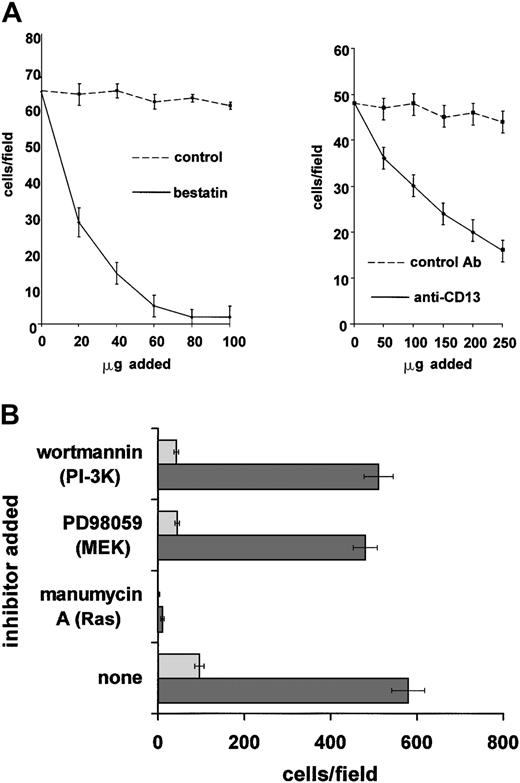
Migration/invasion assays
HUVECs were split 1 × 106 cells per 100-mm dish in 10 mL EGM-2 (Endothelial Growth Medium complete, Clonetics) and incubated for 24 to 48 hours. Cells were then starved for 24 hours in 10 mL EBM-2 (Endothelial Basal Medium, Clonetics; without serum or growth factors). Thawed Matrigel was diluted 1:5 with 1 × PBS and 200 μL was used to coat each invasion chamber (Transwell, Corning, Acton, MA) equipped with an 8-μm pore-size micropore filter; chambers were incubated at 37°C for 1 hour. Meanwhile, cells (HUVEC, EOMA-CD13, EOMA-pcDNA) were trypsinized and washed once with 1 × PBS, resuspended in MEM complete containing 1% FCS. Cells were diluted to 5 × 105cells/mL and 1 mL was inoculated into the upper chamber. Inhibitors were added to appropriate conditions (bestatin [100 μg/mL]), MY7 [100 μL/mL] or PD98059 [30 μM], wortmannin [100 nM], and manumycin A [10 μM]) into the upper chamber and the lower compartment filled with 1% FCS in MEM with or without 10 ng/mL bFGF. After incubation at 37°C for 24 hours, the Matrigel on the filter was removed with a cotton swab and the filter fixed with methanol (medium was removed from the lower chamber; and 600 μL methanol was added per membrane and incubated for 10 minutes). The filter was then stained with crystal violet (600 μL of 0.02% crystal violet solution in 2% ethanol) and incubated for 10 minutes. The membrane was washed several times with 1 ×PBS and the cells that had penetrated the filter were counted under a microscope.
In vivo Matrigel assays
Matrigel (300 μL) was reconstituted with indicated components just before injection as follows: bFGF (100 ng/300 μL), heparin (64 units), PD98059 (30 μM); and control membrane (200 μg) or CD13 membrane preparations (200 μg) were added. In the flank of a Balb/c mouse (Charles River Labs, Wilmington, MA), 300 μL was injected subcutaneously. After 7 days, plugs were harvested and fixed for sectioning and immunohistochemical staining, or for total hemoglobin estimation using Drabkin reagent according to the manufacturer's protocol (Sigma Chemical, St Louis, MO); values are expressed as mean ±SD. Experiments were repeated 3 times.
Results
Identification of signaling intermediates regulating endogenousCD13/APN expression in tumor endothelial cells
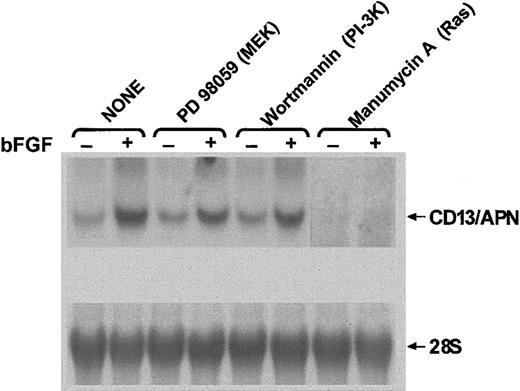
During angiogenesis, the transcription of specific genes is initiated through signaling pathways triggered by angiogenic factors. Numerous pathways have been implicated in transmitting the extracellular signals elicited by growth factors to the nucleus during angiogenesis, the majority of which involve activation of Ras.5-10,41 To investigate the signal transduction pathway(s) involved in regulating CD13/APN expression in response to growth factor signals, we examined the effects of chemical inhibitors of signaling intermediates on endogenous CD13/APNmessage levels in a human endothelial–derived Kaposi sarcoma (KS) cell line (Figure 1). We have previously established that this cell line faithfully recapitulates the transcriptional regulation of CD13/APN seen in primary cells in response to various angiogenic signals.12 Cells treated with chemical inhibitors of components of 2 independent pathways downstream of Ras, MEK (MAPK/ERK kinase; PD98059), or phosphatidylinositol 3-kinase (PI-3K; LY290042) suggest that the bFGF induction of CD13/APN mRNA is partially inhibited when either of these pathways are blocked. Significantly, treatment of cells with manumycin A, a potent inhibitor of the upstream effector of both of these pathways, p21ras, completely abrogated both basal and growth factor–induced levels of CD13/APN, suggesting that Ras plays an important role in the expression ofCD13/APN in the KS cell line.
CD13/APN induction is modulated by inhibitors of signaling intermediates.
KS1767 cells in 1% serum were incubated with (+) or without (−) bFGF and the indicated chemical inhibitors of signal transduction intermediates: PD98059, MEK inhibitor; wortmannin, PI-3K inhibitor; and manumycin A, Ras inhibitor. Total cellularCD13/APN mRNA or control 28S levels were assessed after 24 hours.
CD13/APN induction is modulated by inhibitors of signaling intermediates.
KS1767 cells in 1% serum were incubated with (+) or without (−) bFGF and the indicated chemical inhibitors of signal transduction intermediates: PD98059, MEK inhibitor; wortmannin, PI-3K inhibitor; and manumycin A, Ras inhibitor. Total cellularCD13/APN mRNA or control 28S levels were assessed after 24 hours.
Induction of CD13/APN expression in response to angiogenic growth factors is regulated by the RAS/MAPK pathway
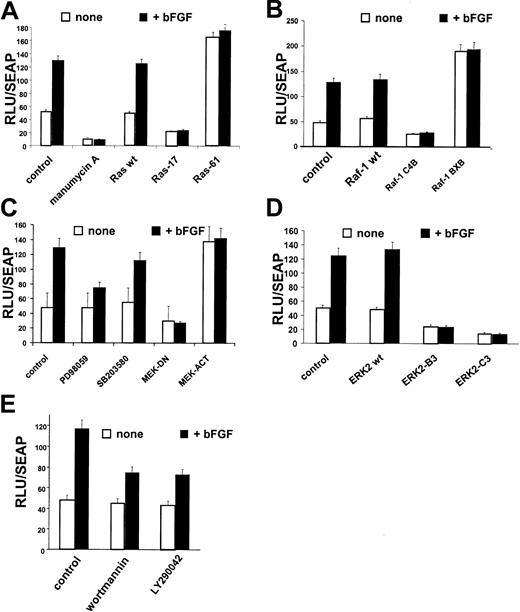
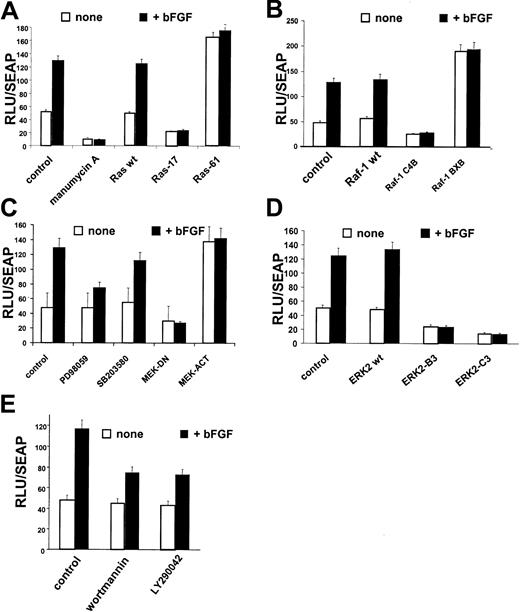
Since chemical inhibitors may affect more than one component of a signaling pathway, we wished to examine the regulation ofCD13/APN expression in response to growth factor signals more precisely. We have shown that the CD13/APN proximal promoter contains the sequences that regulate transcriptional induction in response to angiogenic growth factors, hypoxia, and capillary network formation in primary endothelial cells and the KS cell line in vitro. In addition, the CD13/APNproximal promoter also regulates CD13/APN in response to signals promoting tumor formation in vivo.12 To assess the effect of perturbation of signaling pathways on bFGF-induced CD13/APN transcriptional activity, we transiently transfected KS cells with reporter plasmids containing this endothelial promoter. Treatment of transfected cells with bFGF significantly induced CD13/APNpromoter activity, which is abrogated by inhibition of Ras with manumycin A (Figure 2A). Similar to endogenous CD13/APN expression in these cells, manumycin A inhibited both basal transcription as well as bFGF-mediated induction ofCD13/APN promoter activity. Directed inhibition of Ras activity was accomplished by cotransfection of a dominant-negative expression construct (Ras-1727,28), resulting in no change in luciferase activity upon bFGF addition. Conversely, a constitutively activated Ras expression construct (Ras-6129) raised theCD13/APN promoter activity in uninduced conditions equivalent to growth factor induction. These results confirm that Ras plays a central role in the transcriptional response ofCD13/APN to angiogenic growth factors.
Ras, Raf-1, MEK, ERK-2, and PI-3K regulate
CD13/APN induction. KS1767 cells were incubated in 1% serum 24 hours before transient transfection with 1 μg reporter plasmids containing the CD13/APN promoter and 2 μg of the indicated expression plasmids (for details, see “Materials and methods”), followed by stimulation with bFGF and/or addition of chemical inhibitors of specific pathway intermediates at concentrations detailed in “Materials and methods.” The MAP1-SEAP plasmid (1 μg) was cotransfected in each condition to normalize for transfection efficiency. Results are shown as fold activation at 48 hours after transfection over the activity of the promoterless vector plasmid transfected in parallel. (A) Manumycin A, Ras inhibitor; Ras-17, dominant-negative expression plasmid; and Ras-61, constitutively active Ras expression plasmid. (B) Raf-1–wt, wild-type Raf expression plasmid; Raf-1–C4B, dominant-negative Raf expression plasmid; and Raf-1–BXB, constitutively activated Raf-1 expression plasmid. (C) PD98059, MEK inhibitor; SB203580, p38 kinase inhibitor; MEK-DN, dominant-negative expression plasmid; and MEK-ACT, constitutively active MEK expression plasmid. (D) ERK2-B3, ERK2-C3–dominant-negative ERK2 expression plasmids. (E) Wortmannin and LY290042, PI-3K inhibitors. Error bars indicate standard deviations of at least 3 replicates.
Ras, Raf-1, MEK, ERK-2, and PI-3K regulate
CD13/APN induction. KS1767 cells were incubated in 1% serum 24 hours before transient transfection with 1 μg reporter plasmids containing the CD13/APN promoter and 2 μg of the indicated expression plasmids (for details, see “Materials and methods”), followed by stimulation with bFGF and/or addition of chemical inhibitors of specific pathway intermediates at concentrations detailed in “Materials and methods.” The MAP1-SEAP plasmid (1 μg) was cotransfected in each condition to normalize for transfection efficiency. Results are shown as fold activation at 48 hours after transfection over the activity of the promoterless vector plasmid transfected in parallel. (A) Manumycin A, Ras inhibitor; Ras-17, dominant-negative expression plasmid; and Ras-61, constitutively active Ras expression plasmid. (B) Raf-1–wt, wild-type Raf expression plasmid; Raf-1–C4B, dominant-negative Raf expression plasmid; and Raf-1–BXB, constitutively activated Raf-1 expression plasmid. (C) PD98059, MEK inhibitor; SB203580, p38 kinase inhibitor; MEK-DN, dominant-negative expression plasmid; and MEK-ACT, constitutively active MEK expression plasmid. (D) ERK2-B3, ERK2-C3–dominant-negative ERK2 expression plasmids. (E) Wortmannin and LY290042, PI-3K inhibitors. Error bars indicate standard deviations of at least 3 replicates.
Similar analysis of the downstream components of the Ras pathway that have been implicated in angiogenic growth factor signaling5-10 further delineated intermediates that regulate the response of the CD13/APN proximal promoter to bFGF treatment. Cotransfection of the reporter plasmid with wild-type (Raf-1–wt), dominant-negative (Raf-1–C4B30), or constitutively activated (Raf-1–BXB) Raf-1 expression plasmids31,32 indicated that this important mediator of angiogenic signaling is also involved in CD13/APN induction (Figure 2B). Expression of constitutively active MEK (MEK-ACT) or dominant-negative (MEK-DN) expression plasmids33 (Figure2C) in KS cells affected CD13/APN transcription in a similar fashion. Furthermore, treatment of transfected cells with the MEK inhibitor, PD98059, but not the p38 kinase inhibitor, SB203580, partially inhibited bFGF-induced, but not basalCD13/APN promoter activity (Figure 2C), suggesting a role for MEK in the CD13/APN response to angiogenic stimulation. Finally, the participation of the MEK downstream mediator, ERK in this response, was evaluated using wild-type ERK-2 (ERK2-wt) or dominant-negative ERK-2 expression plasmids (ERK2-B3; ERK2-C3,34 35 Figure 2D). While wild-type ERK-2 had no effect on bFGF induction of CD13/APNpromoter activity, expression of 2 independent dominant-negative forms of ERK-2 significantly inhibited both the basal and inducedCD13/APN transcription levels. Taken together, these studies establish that the Ras/MAPK pathway is involved in the up-regulation ofCD13/APN promoter activity in response to bFGF triggered signaling.
PI-3K signals CD13/APN induction
A distinct Ras effector pathway involving the phosphatidylinositol-3 kinase (PI-3K) has been implicated in tumor angiogenesis and angiogenic growth-factor signaling.7,13,42 43 To test the importance of this kinase activity modulating CD13/APN angiogenic expression, we transiently transfected CD13/APN promoter constructs into tumor endothelial cells treated with the PI-3K inhibitors wortmannin or LY29004 and assayed luciferase activity. Both inhibitors suppressed bFGF-induced CD13/APN promoter activity (Figure 2E) suggesting that PI-3K also regulates CD13/APN expression in response to growth factors.
The MEK and PI-3K pathways converge on ERK
The ERK proteins are activated enzymatically through tyrosine and threonine phosphorylation by their upstream activator kinase, MEK, in response to growth factor stimulation.44-46 In addition, there is evidence for a role for PI-3K in ERK activation.42 To determine if bFGF-activated PI-3K stimulates ERK through MEK in endothelial cells, we tested the capacity of inhibitors of these kinases to affect ERK phosphorylation in response to bFGF. Treatment of KS cells with bFGF significantly activated ERK (Figure 3A). However, while bFGF-induced ERK phosphorylation is completely blocked upon inhibition of Ras (treatment with manumycin A), this induction is only partially reduced when either MEK or PI-3K is inhibited (treatment with PD98059 or LY29004, respectively), suggesting that in endothelial cells these pathways converge on ERK, but that PI-3K activates ERK independently of MEK (Figure 3A). Finally, to determine if activation of these pathways was solely responsible for ERK phosphorylation in response to bFGF, we treated KS cells with the MEK and PI-3K inhibitors in combination and assessed the levels of phospho-ERK (pERK). The complete absence of detectable pERK under these conditions indicates that ERK is activated solely by MEK or PI-3K–dependent mechanisms in tumor endothelial cells upon angiogenic stimulation by bFGF (Figure 3B). Thus, during angiogenesis, the Ras/MAPK and PI-3K pathways converge to activate ERK, which in turn results in the transcriptional induction ofCD13/APN in angiogenic blood vessels.
The PI-3K and Ras/MAPK pathways converge on ERK.
KS1767 cells in 1% serum were incubated with the indicated individual signaling inhibitors in the presence (+) or absence (−) of bFGF stimulation and lysates were assayed for levels of total ERK (ERK1/2) or activated ERK (pERK) by Western blot analysis. (B) Inhibition of both pathways abrogates ERK phosphorylation in response to bFGF. Lysates from cells incubated in the presence of both inhibitors were assayed for levels of total ERK (ERK1/2) or control protein.
The PI-3K and Ras/MAPK pathways converge on ERK.
KS1767 cells in 1% serum were incubated with the indicated individual signaling inhibitors in the presence (+) or absence (−) of bFGF stimulation and lysates were assayed for levels of total ERK (ERK1/2) or activated ERK (pERK) by Western blot analysis. (B) Inhibition of both pathways abrogates ERK phosphorylation in response to bFGF. Lysates from cells incubated in the presence of both inhibitors were assayed for levels of total ERK (ERK1/2) or control protein.
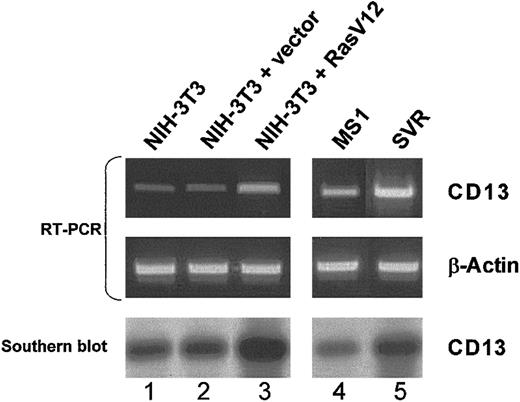
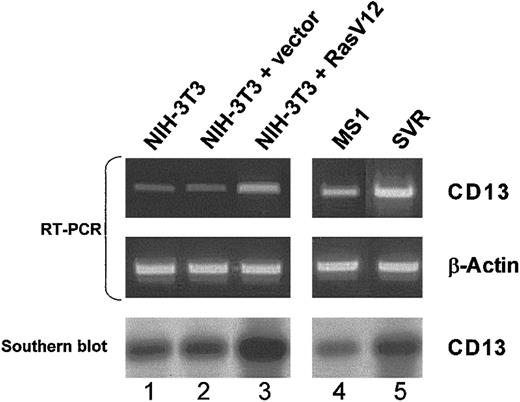
To assess the effect of Ras activation on endogenousCD13/APN gene expression, we obtained sets of matched cell lines transformed by constitutively active Ras expression constructs (Ras-V12) or vector controls (Figure 4). In either fibroblast13,47 or endothelial13cells, Southern hybridization of RT-PCR products demonstrated that expression of activated Ras leads to significant up-regulation of endogenous CD13/APN mRNA. Since the activation of H-Ras drives the switch of normally quiescent endothelial cells to a highly tumorigenic state,1 13 these data are consistent withCD13/APN as a target of Ras signaling during the process of tumor angiogenesis.
Expression of activated Ras induces endogenous CD13/APN expression in endothelial cells and fibroblasts.
Matched NIH-3T3 fibroblast (lanes 1-3) or murine endothelial MS1 (lanes 4-5) cell lines stably transfected with either empty vector control plasmids (lane 2: NIH-3T3 + vector, and lane 4: MS1) or activated Ras-V12 expression constructs (lane 3: NIH-3T3 + Ras V12, and lane 5: SVR) were assessed by semiquantitative RT-PCR for CD13 or control β-actin levels. Identity of the RT-PCR products was verified by Southern blot analysis probed with CD13/APN cDNA (bottom panel). Lane 1 shows untransfected parental NIH-3T3 fibroblasts.
Expression of activated Ras induces endogenous CD13/APN expression in endothelial cells and fibroblasts.
Matched NIH-3T3 fibroblast (lanes 1-3) or murine endothelial MS1 (lanes 4-5) cell lines stably transfected with either empty vector control plasmids (lane 2: NIH-3T3 + vector, and lane 4: MS1) or activated Ras-V12 expression constructs (lane 3: NIH-3T3 + Ras V12, and lane 5: SVR) were assessed by semiquantitative RT-PCR for CD13 or control β-actin levels. Identity of the RT-PCR products was verified by Southern blot analysis probed with CD13/APN cDNA (bottom panel). Lane 1 shows untransfected parental NIH-3T3 fibroblasts.
CD13/APN rescues in vitro capillary morphogenesis in the presence of Ras/MAPK or PI-3K inhibitors
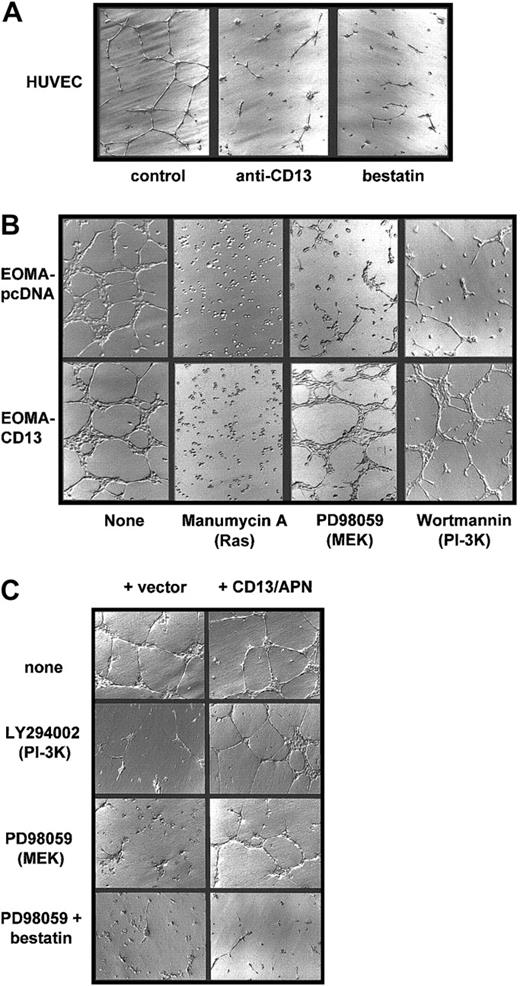
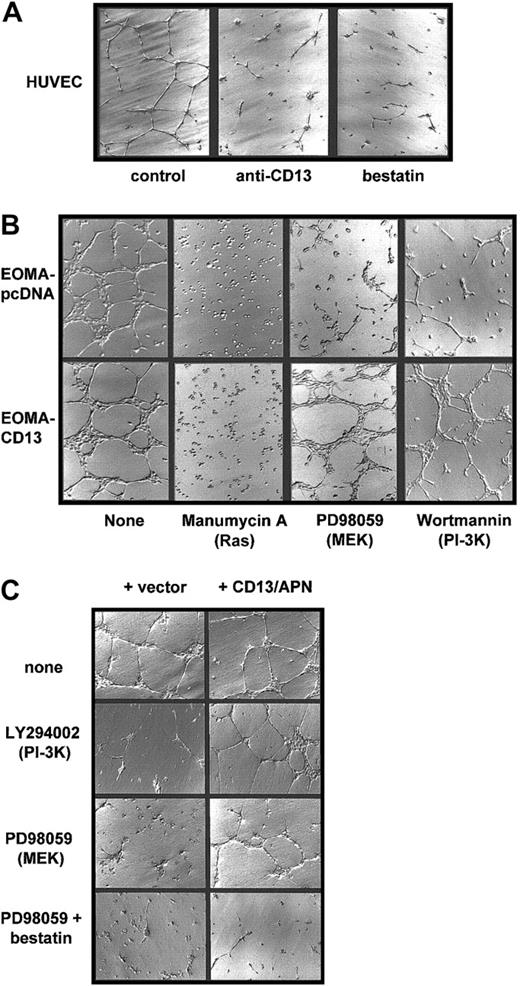
One approach to determine the functional significance of the transcriptional targets associated with a signaling pathway is by restoration of a loss of function with a downstream component. We therefore tested whether constitutive expression of CD13/APN, a downstream target of MEK and PI-3K, could rescue inhibitor-induced loss of endothelial function using paired cell lines differing only by the amount of CD13/APN on their cell surface. We stably transfected an endothelial cell line that normally expresses undetectable levels of CD13/APN (EOMA)12 with a plasmid expressing the humanCD13/APN cDNA driven by the cytomegalovirus promoter. Therefore, in this cell line (EOMA-CD13), CD13/APN expression is high (20-fold more than controls by FACS analysis), constitutive, and uncoupled from its normal dependence on signal transduced induction. As we have previously shown,12 treatment of primary endothelial cells (HUVECs) on Matrigel with the CD13/APN functional antagonists, bestatin or the MY7 monoclonal antibody, prevents these cells from forming characteristic organized networks, reiterating the requirement for functional CD13/APN in endothelial morphogenesis (Figure 5A). Similarly, when we plated the engineered EOMA cell lines on Matrigel in the presence of the Ras, MEK, or PI-3K inhibitors (Figure 5B), network formation by the vector control cells was clearly suppressed. However, the cells constitutively expressing CD13/APN are fully capable of network formation under these same conditions, indicating that an increase in functional CD13/APN is sufficient to rescue endothelial function in the absence of Ras-MAPK or PI-3K signaling. In contrast, cells plated in the presence of manumycin A neither adhered nor differentiated regardless of the level of CD13/APN expression, suggesting that the activation of Ras triggers additional gene expression programs necessary for cell adhesion that likely function upstream of CD13/APN in angiogenic progression.
CD13/APN rescues capillary morphogenesis despite MEK or PI-3K inhibition.
(A) CD13/APN is required for capillary morphogenesis. HUVECs were plated on Matrigel containing the indicated CD13/APN functional antagonists or isotype-matched control antibody (control) and photographed after 18 to 24 hours. (B) Increased cell surface CD13/APN can rescue inhibited morphogenesis. EOMA engineered to express high levels of CD13/APN (20-fold increase, EOMA-CD13, bottom row) or vector control cells (EOMA-pcDNA, top row) were plated on Matrigel in the presence of the indicated Ras, MEK, or PI-3K inhibitors. (C) Transmembrane expression of CD13/APN is not required for rescue. HUVECs were plated on Matrigel containing microsomal membrane preparations of CD13-high cells (+ CD13, right column) or vector control cells (left column) in the absence (top row) or presence of the MEK inhibitor PD98059 (middle row) or both PD98059 and the aminopeptidase inhibitor bestatin (bottom row). Original magnification A-C, × 10.
CD13/APN rescues capillary morphogenesis despite MEK or PI-3K inhibition.
(A) CD13/APN is required for capillary morphogenesis. HUVECs were plated on Matrigel containing the indicated CD13/APN functional antagonists or isotype-matched control antibody (control) and photographed after 18 to 24 hours. (B) Increased cell surface CD13/APN can rescue inhibited morphogenesis. EOMA engineered to express high levels of CD13/APN (20-fold increase, EOMA-CD13, bottom row) or vector control cells (EOMA-pcDNA, top row) were plated on Matrigel in the presence of the indicated Ras, MEK, or PI-3K inhibitors. (C) Transmembrane expression of CD13/APN is not required for rescue. HUVECs were plated on Matrigel containing microsomal membrane preparations of CD13-high cells (+ CD13, right column) or vector control cells (left column) in the absence (top row) or presence of the MEK inhibitor PD98059 (middle row) or both PD98059 and the aminopeptidase inhibitor bestatin (bottom row). Original magnification A-C, × 10.
The fact that an increase in CD13/APN on the endothelial cell surface restores endothelial morphogenesis in the absence of PI-3K or Ras/MAPK signal transduction raises the possibility that CD13/APN is itself a component of a cell-surface signaling complex. To address this question, we prepared soluble microsomal membrane preparations from either CD13/APN high EOMA-CD13 cells or vector control EOMA-pcDNA cells as a source of exogenous CD13/APN. CD13/APN-high preparations contained 16-fold higher aminopeptidase activity (as determined by a spectrophotometric functional assay37) than preparations from control cells. Addition of only CD13/APN-high membrane preparations to HUVECs plated on Matrigel in the presence of PI-3K or MEK inhibitors was able to efficiently rescue capillary network formation (Figure 5C), indicating that CD13/APN functions extracellularly, perhaps by proteolytic cleavage of bioactive peptides in the intercellular space. Moreover, transmembrane expression of CD13/APN on the endothelial cell surface is not obligatory for its contribution to angiogenic progression. Additionally, morphogenic rescue by CD13/APN of HUVECs treated with both the MEK inhibitor and CD13-high membrane preparations is prevented by adding the aminopeptidase inhibitor bestatin to Matrigel (Figure 5C, bottom row), verifying that aminopeptidase activity is essential for endothelial rescue by the membrane preparations.
CD13/APN rescues in vitro endothelial invasion in the presence of Ras/MAPK or PI-3K inhibitors
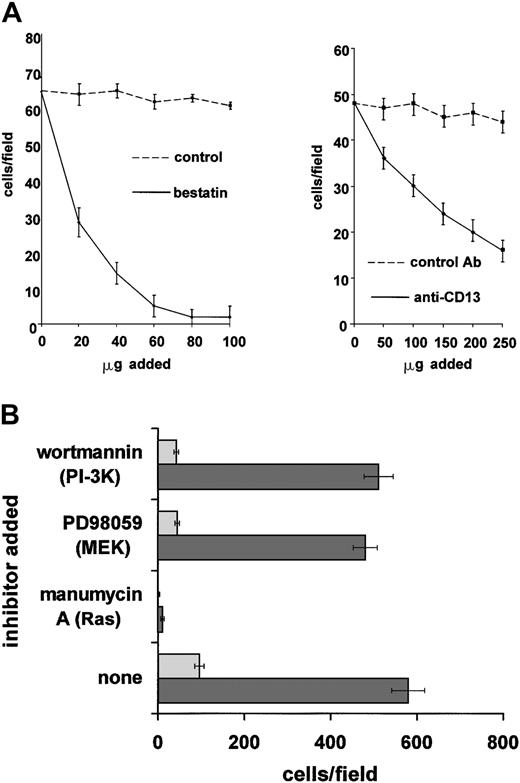
Since endothelial cell migration and invasion also involve MEK and PI-3K signaling4,13,48 49 it is possible that CD13/APN may play a role in these stages of angiogenesis. Inhibition of CD13/APN activity with either the aminopeptidase inhibitor bestatin or the CD13/APN monoclonal antibody MY7 significantly inhibited primary endothelial cell invasion through Matrigel toward a bFGF angiogenic stimulus, indicating that CD13/APN participates in these endothelial cell functions (Figure 6A). Furthermore, as we observed in the capillary network assay, interrupting signal transduction pathways with MEK or PI-3K inhibitors reduced the ability of stably transfected vector control EOMA cells to invade the barrier (Figure 6B). However, constitutive expression of CD13/APN protein on stable transfectants enhanced the number of cells invading the Matrigel by 6-fold, which was unaffected by the presence of either MEK or PI-3K inhibitors. By contrast, CD13/APN expression was incapable of overcoming the Ras (manumycin A) block, consistent with results in the capillary network assay. Therefore, CD13/APN appears to be a functional downstream target of MEK and PI-3K pathways in the migration and/or invasion phase of angiogenesis as measured in this assay.
CD13/APN rescues endothelial invasion in the presence of Ras/MAPK or PI-3K inhibition.
(A) CD13/APN is required for migration/invasion. Primary endothelial cells (HUVEC) in 1% serum in the presence or absence of the indicated CD13/APN functional antagonists or vehicle or isotype-matched control antibodies were plated in the top chamber of Matrigel-coated transwell plates and bFGF angiogenic stimulus was placed in the bottom chamber. The number of cells invading and migrating through the Matrigel barrier in response to bFGF was counted after 24 hours. (B) Increased cell surface CD13/APN enhances endothelial invasion and can overcome inhibited migration/invasion. EOMA engineered to express high (20-fold) levels of CD13/APN (EOMA-CD13, ▪) or vector control cells (EOMA-pcDNA, ░) were plated in the top chamber of Matrigel-coated transwell plates in the presence of the indicated Ras, MEK, or PI-3K inhibitors, and bFGF angiogenic stimulus was placed in the bottom chamber. The number of cells invading and migrating through the Matrigel barrier in response to bFGF was counted after 24 hours. Error bars in each panel indicate standard deviation of results of at least 3 replicates.
CD13/APN rescues endothelial invasion in the presence of Ras/MAPK or PI-3K inhibition.
(A) CD13/APN is required for migration/invasion. Primary endothelial cells (HUVEC) in 1% serum in the presence or absence of the indicated CD13/APN functional antagonists or vehicle or isotype-matched control antibodies were plated in the top chamber of Matrigel-coated transwell plates and bFGF angiogenic stimulus was placed in the bottom chamber. The number of cells invading and migrating through the Matrigel barrier in response to bFGF was counted after 24 hours. (B) Increased cell surface CD13/APN enhances endothelial invasion and can overcome inhibited migration/invasion. EOMA engineered to express high (20-fold) levels of CD13/APN (EOMA-CD13, ▪) or vector control cells (EOMA-pcDNA, ░) were plated in the top chamber of Matrigel-coated transwell plates in the presence of the indicated Ras, MEK, or PI-3K inhibitors, and bFGF angiogenic stimulus was placed in the bottom chamber. The number of cells invading and migrating through the Matrigel barrier in response to bFGF was counted after 24 hours. Error bars in each panel indicate standard deviation of results of at least 3 replicates.
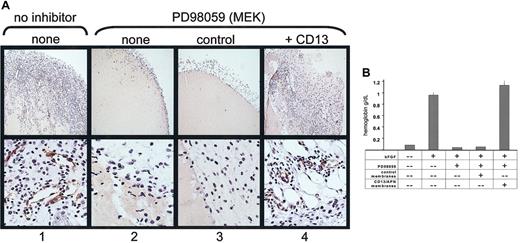
CD13/APN rescues angiogenesis in vivo
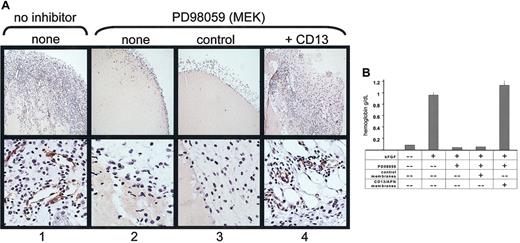
We have shown that functional CD13/APN is necessary for tumorigenesis in vivo.11 To determine if functional CD13/APN activity is also a target of signaling pathways during angiogenesis in vivo, we inhibited bFGF-induced angiogenesis in the murine Matrigel plug assay50 using the MEK inhibitor PD98059, and added functional CD13/APN-high or control membrane preparations to the injected matrix. As predicted from our in vitro data, PD98059 completely inhibited neovessel formation in the Matrigel plug as quantitated by hemoglobin content (> 10-fold reduction to less than unstimulated conditions) as well as formation of CD31+ endothelial-lined tubules and infiltration of cells into the matrix (Figure 7A). However, addition of active CD13/APN-containing membrane preparations to PD98059-containing Matrigel completely restored cell infiltration, tubule formation, and the corresponding increase in hemoglobin concentration of the matrix plug to levels equivalent with uninhibited controls (Figure 7B). Importantly, addition of CD13/APN-negative control membrane preparations had no effect on these parameters, indicating that other endothelial proteins present in these membrane preparations are unable to rescue angiogenesis in the absence of CD13/APN. Therefore, CD13/APN is sufficient to rescue angiogenesis when key signaling pathways are interrupted, and is most likely an essential target of Ras signaling pathways.1,2 51
CD13/APN rescues angiogenesis in vivo.
Matrigel containing bFGF, heparin, and either the vehicle control (column 1) or the MEK inhibitor PD98059 (columns 2-4) plus functional CD13/APN-high (column 4) or control EOMA (column 3) membrane preparations were injected subcutaneously into mice. Plugs were harvested after 7 days and (A) examined for CD31+ (brown color, anti-CD31) endothelial-lined tubules and infiltration of inflammatory cells (top panel, original magnification × 10; bottom panel, original magnification × 40); or (B) angiogenesis quantitated by hemoglobin content.
CD13/APN rescues angiogenesis in vivo.
Matrigel containing bFGF, heparin, and either the vehicle control (column 1) or the MEK inhibitor PD98059 (columns 2-4) plus functional CD13/APN-high (column 4) or control EOMA (column 3) membrane preparations were injected subcutaneously into mice. Plugs were harvested after 7 days and (A) examined for CD31+ (brown color, anti-CD31) endothelial-lined tubules and infiltration of inflammatory cells (top panel, original magnification × 10; bottom panel, original magnification × 40); or (B) angiogenesis quantitated by hemoglobin content.
Discussion
The regulation of angiogenesis is a complex process involving the acquisition and integration of external signals, the modulation of the expression of numerous genes, and the proteolytic processing of multiple substrates. We have recently identified a novel role for the CD13/APN cell surface peptidase as a potent angiogenic regulator, where functional CD13/APN is required for tumor growth in vivo11and endothelial migration and capillary network formation in vitro.12 Furthermore, we demonstrated that the exclusive expression of CD13/APN on activated endothelial cells is precisely controlled by angiogenic signals present in the tumor microenvironment.12 In the present study, we have investigated growth factor–mediated signaling mechanisms controlling the expression of CD13/APN in resting endothelial cells. Importantly, these studies show that under conditions where CD13/APN up-regulation is inhibited, exogenous expression of CD13/APN can rescue angiogenic progression arrested by inhibition of key angiogenic signaling pathways. While the precise role of CD13/APN in neovascularization is under investigation, these observations identify CD13/APN as a critical downstream target of angiogenic growth factor signal transduction and a limiting factor in angiogenic progression. Understanding the signaling cascades regulating CD13/APNexpression will allow the identification of new targets for antiangiogenic therapy and clarify the mechanisms of effective anticancer drugs whose modes of action are not well defined.
Our study showed that activated Ras is critical for the induction ofCD13/APN transcription in endothelial cells treated with bFGF. The central role of Ras in signal transduction, tumorigenesis, and angiogenesis is well established.5-10,13,52,53 Understandably, the majority of studies have focused on the tumorigenic potential of activated Ras and its central role in cell transformation. These studies have shown Ras to be so critical that Ras growth signaling pathways are postulated to be corrupted in the majority of human tumors.53 In addition to this important role in cell transformation, aberrant Ras signaling in tumor cells elicits angiogenic factors that in turn perturb surrounding, normally quiescent, stromal cells by altering their expression of key angiogenic modulators. Hence, the balance between proangiogenic and antiangiogenic regulators is tipped and angiogenesis is initiated.2,3 We have previously shown that CD13/APN is transcriptionally inactive in the quiescent endothelial cells of normal vessels but is highly up-regulated in response to angiogenic signals present in the tumor microenvironment.12 Our present study shows that this up-regulation occurs via activation of Ras and its downstream mediators, and that CD13/APN is required for new vessel formation, thereby identifying CD13/APN as a critical target in this essential signaling pathway in angiogenic progression.
Further investigation of the downstream mediators of bFGF induction ofCD13/APN showed that the MAP kinase and PI-3K pathways act in concert to relay signals to the nucleus. Activation of these pathways individually by specific stimuli results in the transduction of signals controlling diverse cellular functions including cell-cycle progression, differentiation, and cell migration, and each have been implicated previously in bFGF signaling (reviewed in Cross and Claesson-Welsh54). Similarly to induction ofCD13/APN transcription by bFGF, both phorbol myristate acetate (PMA)–7 and transforming growth factor (TGFβ1)55–induced capillary network formation are also controlled by parallel pathways involving PI-3K and MEK. The activation of both intermediates was found to be necessary for PMA-induced HUVEC network formation and, like CD13/APN induction, the elicited signals converged on ERK/MAPK. Alternatively, TGFβ1 promotes endothelial morphogenesis and cell survival through an autocrine mechanism involving TGFα and the epidermal growth factor (EGF) receptor and the resultant parallel activation of PI-3K and MEK essential for network formation.55 In agreement with these studies, we also find that inhibition of either PI-3K or MEK activity abrogates cellular network formation, and furthermore, that both these pathways contribute to the maximal induction of CD13/APNtranscription. Significantly, expression of exogenous CD13/APN can compensate for the loss of either pathway and rescue network formation in vitro and angiogenesis in vivo, thus defining CD13/APN as an important target for these pathways in angiogenesis. Interestingly, PMA treatment of endothelial cells also induces CD13/APN expression (S.V.B. and L.H.S., unpublished data, January 2001); and it is likely that CD13/APN is a downstream target of PMA-induced network formation as well. Likewise, CD13/APN may be causally involved in TGFβ1-induced angiogenesis since TGFβ1 treatment of corneal endothelial cells increases bFGF synthesis,56 which could up-regulate CD13/APN transcription.
Our data also indicate that CD13/APN is not the sole target of Ras activation in angiogenesis, since expression of CD13/APN is not sufficient to overcome the inhibition of capillary network formation in the presence of the Ras inhibitor manumycin A. These data may reflect the fact that CD13/APN is operative after the early steps of angiogenesis have already been initiated. We have shown that CD13/APN has no apparent role in endothelial proliferation, but it is strictly required for in vitro capillary network formation and in vivo angiogenesis.12 Signals transmitted by activated Ras are critical for cell proliferation and cell-cycle progression, so these essential early stages of angiogenesis may not be rescued by a regulator that acts further downstream. Clinical trials of vascular endothelial growth factor (VEGF) inhibitors have shown that tumor progression often recurs despite this treatment, suggesting that steps subsequent to growth factor signaling are also important to angiogenic regulation.57 58
While these experiments clearly identify CD13/APN as a regulator of angiogenesis, the mechanism by which it does so remains to be determined. Certain clues, however, can be derived from CD13/APN's structure and functional activities. For example, although CD13/APN is a large-cell surface molecule, its intracellular domain consists of only 9 amino acids.59 This fact, together with our experiments demonstrating that transmembrane CD13/APN expression is not obligatory to rescue endothelial morphogenesis (Figures 6-7), argues that CD13/APN itself does not act as a signaling molecule but more likely acts in its capacity as a cell-surface peptidase. CD13/APN sequentially trims single amino acids from the amino terminus of small peptides in the intercellular space and hence, its function in a particular tissue is dictated by the substrates available in the immediate environment. Previous studies have shown that CD13/APN participates in antigen processing and presentation60,61; scavenging of amino acids and catabolism of regulatory peptides62,63; degradation of vasoactive and neuroactive peptides64-68; and tumor invasion and metastasis.69 70 Because proteolytic processing is a critical aspect of angiogenesis, it is intriguing to postulate that the sequential breakdown of extracellular matrix proteins or other angiogenic proteins may generate bioactive peptides that can positively or negatively regulate angiogenesis. In this scenario, CD13/APN could either activate a small proactivating molecule, or inactivate a small molecule inhibitor of angiogenesis, thereby allowing unimpeded angiogenesis. Given our observation that CD13/APN can sustain angiogenesis in the absence of MEK or PI-3K signaling, it would appear that its putative peptide substrate is a significant regulator of angiogenesis and could be an attractive target for antiangiogenic therapy.
We would like to thank Dr Richard Ashmun for flow cytometric data analysis; John Zacher for photomicrography; Drs John Hood, Angelika Hoffmeyer, Catriona McKay, Shrikanth Hegde, Renata Pasqualini, and Wadih Arap for technical assistance and helpful discussions; Dr Martine Roussel for Ras-transformed NIH-3T3 cells; and Drs David Shapiro, Timothy Hla, and Kevin Claffey for critical reading of the manuscript.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood- 2002-05-1422.
Supported by National Institutes of Health (NIH) grant R01-CA85714 (L.H.S.), by the National Cancer Institute Cancer Center Support (CORE) grant CA-21765, and by the American Lebanese Syrian Associated Charities (ALSAC), St Jude Children's Research Hospital.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Linda Shapiro, Center for Vascular Biology MC3501, University of Connecticut Health Center, 263 Farmington Ave, Farmington, CT 06030; e-mail:lshapiro@neuron.uchc.edu.