Plasminogen activator inhibitor type I (PAI-1) antigen concentrations follow a circadian oscillation peaking in the morning. Some individuals show no apparent circadian rhythm, while others show up to a 10-fold variation in PAI-1 over 24 hours. Results from experimental studies suggest that a polymorphism in the promoter of the gene for PAI-1 (4G5G) directly influences the circadian expression of the PAI-1 gene. We studied whether the diurnal variation of PAI-1 antigen differs for the genotypes of the4G5G polymorphism. A population-based, cross-sectional study was performed among 263 subjects selected from the Rotterdam Study, a population-based cohort of 7983 men and women aged 55 years and older. The 4G allele was associated with a more pronounced circadian expression of PAI-1 antigen. Morning PAI-1 antigen concentrations were 79 ng/mL (95% confidence interval [CI], 68-92) in subjects homozygous for 4G, 62 ng/mL (95% CI, 54-72) in heterozygous subjects, and 59 ng/mL (95% CI, 49-71) in subjects homozygous for 5G. While respective PAI-1 antigen concentrations in the afternoon were 40 ng/mL (95% CI, 33-49), 41 ng/mL (95% CI, 37-47), and 40 ng/mL (95% CI, 49-71). These findings suggest that the morning increase in PAI-1 antigen concentration is more pronounced among subjects homozygous for the4G allele compared with the morning increase among the other genotypes. Additionally, these findings show that homozygosity for the 4G allele is associated with increased PAI-1 levels during the morning only.
Introduction
Although acute cardiovascular events occur throughout the day, it has been shown that acute myocardial infarction, sudden cardiac death, silent ischemia, and stroke have a circadian pattern of occurrence with a peak in the morning.1-4 A meta-analysis suggested that 9% of all myocardial infarctions are attributable to the morning excess incidence of cardiac events.5 The morning excess of cardiac events may result in part from the circadian variation of fibrinolytic activity. Plasminogen activator inhibitor type 1 (PAI-1) is the major component of inhibitors of fibrinolytic activity. PAI-1 shows a clear circadian oscillation peaking in the morning.6-8
Recently, a novel transcription factor, CLIF (cyclelike factor), has been identified that regulates the circadian oscillation of PAI-1 gene expression.9 In the PAI-1 promoter, 2 E-box elements (CACGTG) are responsible for the activation of PAI-1 by CLIF; one of the E-boxes is located at −677 to −672. This overlaps with the sequence of the 4G5G polymorphism of the PAI-1 promoter. The 4G5G polymorphism is a common single base-pair polymorphism located 675 bp upstream of the start of transcription of the PAI-1 gene. This polymorphism has been associated with increased plasma PAI-1 concentrations10 and with a slightly increased risk for myocardial infarction.11-13 We examined whether the circadian oscillation pattern of PAI-1 antigen differs for subjects with the different genotypes of the4G5G polymorphism in the PAI-1 gene.
Patients and methods
Population
A cross-sectional study was performed among subjects selected from the Rotterdam Study. The Rotterdam Study is a prospective population-based study of 7983 subjects. The rationale and design of the study have been described elsewhere.14 In short, between March 1990 and July 1993 all subjects aged 55 years and older, living in a suburb of Rotterdam, the Netherlands, were invited to participate. The overall response rate was 78%. The study has been approved by the Medical Ethics Committee of Erasmus University and written informed consent was obtained from all participants. The present study utilizes data from an earlier case-control study that was set up to examine the association of hemostatic and fibrinolytic parameters with cardiovascular diseases.15,16 Cases of myocardial infarction were 150 subjects with a history of myocardial infarction.17 18 Controls were 150 subjects without a history of cardiovascular disease (ie, no history of myocardial infarction, angina pectoris, or stroke; a normal ECG; and no peripheral arterial disease [ankle/arm index > 0.9]) who were randomly drawn from the same 5-year age strata as that of the myocardial infarction cases. We excluded subjects using anticoagulant drugs.
Clinical investigation
Information on current health status, medical history, drug use, and smoking behavior was obtained by a questionnaire. The home interview was followed by 2 visits to the research center, between 8 am and 4 pm. Patients were not asked to fast or to refrain from smoking. During these visits several cardiovascular risk indicators were determined. Sitting blood pressure was measured at the right upper arm with a random zero sphygmomanometer.
Laboratory investigations
Blood sampling and storage have been described elsewhere.19 Blood samples were collected during the first visit to the research center between 8 am and 4pm, using CTAD vacutainers (0.11M citrate, 15 mM theophylline, 3.7 mM adenosine, and 0.198 mM dipyridamole; Diatube H, Becton and Dickinson, Mylan, Cedex, France).20 Serum total and high-density lipoprotein (HDL) cholesterol were determined with an automated enzymatic procedure. PAI-1 antigen concentration was measured in CTAD plasma using the Innotest PAI-1 (Innogenetics, Zwijndrecht, Belgium).21 Genomic DNA was isolated from blood cells and PAI-1 genotype was determined by allele-specific PCR amplification.22
Statistical analysis
The distribution of the plasma concentration of PAI-1 antigen was positively skewed, therefore statistical analysis was carried out on log-transformed values, but the presented mean values and 95% confidence intervals (CIs) have been transformed back into the original scale. For each genotype, mean PAI-1 antigen concentration for subjects who visited the research center before noon was compared with that in subjects who visited the center after noon. Linear regression analysis with log-transformed PAI-1 concentrations as dependent variable was used to compare PAI-1 levels between subjects visiting the research center before or after noon, adjusted for systolic blood pressure, serum HDL cholesterol, and body mass index. Back transformation of the regression coefficients has been performed by taking the exponent of the coefficient and the exponent of upper and lower values of the confidence interval. The result is a multiplicative factor.
This is a relatively small study with relatively little power to test for interaction. We noticed that the morning/afternoon difference of heterozygous subjects was similar to that of subjects homozygous for5G. We therefore decided to combine these 2 groups. Interaction between the effect of homozygosity for the 4Gallele and the diurnal effect were tested with the product of an indicator variable for 4G4G and an indicator variable for visit before noon, together with both indicators as independent variables in a linear regression model.
Results
Characteristics of the study population
After exclusion of those with missing data on either the4G5G polymorphism (n = 22) or PAI-1 antigen concentration (n = 29), or time of blood collection (n = 4), the study population consisted of 263 subjects (132 cases of myocardial infarction and 131 control subjects). We compared the observed distribution of genotypes among the subjects without cardiovascular disease with that expected for a population according to a Hardy Weinberg equilibrium. The frequency of the 4G allele was 53% and that of the5G allele was 47%, and the distribution of genotypes was in Hardy-Weinberg equilibrium; 39 control subjects (30%) were found to be homozygous for the 4G allele, whereas 62 (47%) control subjects were heterozygous, and 30 (23%) control subjects were homozygous for the 5G allele.
Table 1 presents characteristics of the study population according to time of blood collection.
4G5G genotypes and PAI-1 levels
Homozygosity for the 4G allele was associated with increased levels of PAI-1 antigen. In subjects homozygous for the4G allele PAI-1 antigen concentration was 62 ng/mL (95% CI, 54-70), in those heterozygous it was 50 ng/mL (95% CI, 46-56), and in those homozygous for the 5G allele it was 50 ng/mL (95% CI, 43-58).
Diurnal pattern of PAI-1
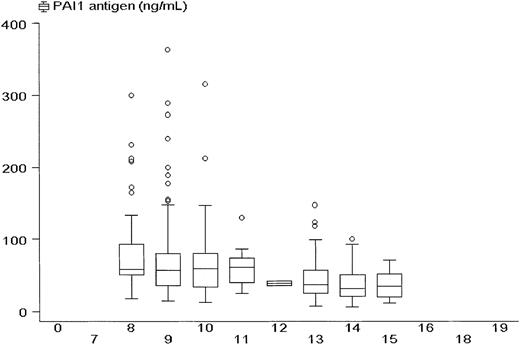
PAI-1 antigen levels showed a diurnal pattern with higher values in the morning. Figure 1 presents PAI-1 levels according to the time of blood collection. Between 8 and 9am, 28 subjects had a median PAI-1 of 63 ng/mL; between 9 and 10 am, 69 subjects had a median PAI-1 of 64 ng/mL; between 10 and 11 am, 44 subjects had a median PAI-1 of 65 ng/mL; between 11 am and noon, 5 subjects had a median PAI-1 of 62 ng/mL; between noon and 1 pm, blood was drawn in one subject with a PAI-1 of 43 ng/mL; between 1 and 2 pm, 58 subjects had a median PAI-1 of 40 ng/mL; between 2 and 3 pm, 44 subjects had a median PAI-1 of 38 ng/mL; between 3 and 4 pm, 14 subjects had a median PAI-1 of 36 ng/mL. The figure illustrates that high peaks of PAI-1 antigen are predominantly found during the morning hours.
PAI-1 antigen concentrations according to the time of day.
Box and whisker plot showing median values, interquartile ranges (box), and extreme values (whiskers) of plasma PAI-1 antigen concentrations among all 263 subjects according to the time their blood was collected.
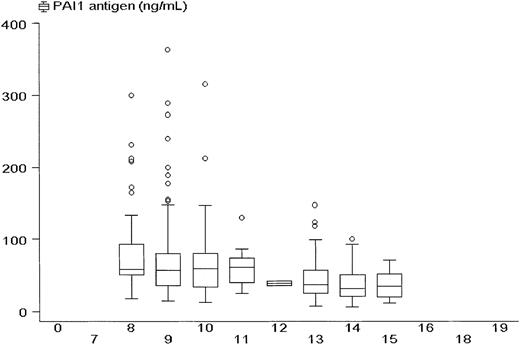
PAI-1 antigen concentrations according to the time of day.
Box and whisker plot showing median values, interquartile ranges (box), and extreme values (whiskers) of plasma PAI-1 antigen concentrations among all 263 subjects according to the time their blood was collected.
Diurnal pattern of PAI and 4G5G genotypes
Differences in PAI-1 expression levels according to time of blood collection appeared to depend somewhat on genotype (test for interaction, P = .057), and for this reason separate analyses were conducted according to genotype. In subjects with the4G4G genotype the morning/afternoon difference was larger than in the other genotypes (Table 2). Results from univariate linear regression analyses suggest that PAI-1 antigen was 1.95 (95% CI, 1.54-2.51) times higher in the morning among subjects homozygous for 4G; for heterozygous subjects it was 1.50 (95% CI, 1.23-1.82) times higher in the morning; and for subjects homozygous for 5G it was 1.47 (95% CI, 1.08-1.97) times higher in the morning. Similar statistically significant differences between morning and afternoon PAI-1 levels were found after adjustment for the confounders (potential confounders are presented in Table 1). After adjustment for systolic blood pressure, HDL cholesterol, and a history of myocardial infarction, PAI-1 antigen was 1.92 (95% CI, 1.61-2.41) times higher in the morning among subjects homozygous for the 4G allele; 1.58 (95% CI, 1.32-1.90) times higher in the morning among heterozygous subjects; and 1.65 (95% CI, 1.26-2.16) times higher in the morning among those homozygous for5G.
History of myocardial infarction and diurnal PAI-1
There was no statistically significant difference between the morning effect on PAI-1 among subjects with a history of myocardial infarction compared with those without a history of cardiovascular diseases. PAI-1 antigen was 1.54 (95% CI, 1.28-1.85) times higher in the morning among subjects with a history of myocardial infarction; it was 1.75 (95% CI, 1.43-2.13) times higher in the morning among subjects without a history of cardiovascular diseases.
Discussion
We assessed whether the circadian oscillation pattern of PAI-1 antigen differs for the genotypes for the 4G5G polymorphism in the PAI-1 gene. Homozygosity for the 4G allele was associated with increased PAI-1 levels only during the morning hours. All 3 genotypes showed higher PAI-1 antigen concentrations in the morning, but this morning effect was more pronounced among subjects homozygous for the 4G allele (test for interaction,P= .057).
We believe that our findings provide a valid estimate of the effect of the 4G allele of the 4G5G polymorphism on the circadian oscillation pattern of PAI-1 antigen. PAI-1 antigen levels were measured in a selected group of subjects who participated in a large population-based cohort study. Half of the study population had a history of myocardial infarction and the other half did not have a history of cardiovascular diseases. The proportion of patients with a history of myocardial infarction was slightly higher in the morning, which of course explains higher PAI-1 levels in the morning.23 However, the fact that roughly half the studied population had a history of myocardial infarction does not totally account for the observed differences in PAI-1. History of myocardial infarction was adjusted for in the models, and this adjustment did not qualitatively alter the conclusion. Moreover, the morning effect among subjects with myocardial infarction was not more pronounced than among patients without myocardial infarction. Increased PAI-1 levels have been associated with increased blood pressure, increased body mass index, and decreased HDL cholesterol.16 24 Systolic blood pressure and HDL cholesterol were also related to time of blood collection in our study. Therefore, these factors may also be an alternate explanation for our findings. Yet, adjustment for these factors did not notably change our findings.
This study is the first to show an association between the4G5G polymorphism and the circadian pattern of plasma PAI-1 antigen concentrations. Experimental work had already suggested that the 4G5G polymorphism might influence the circadian oscillation of PAI-1 antigen.9 The observation that the4G5G polymorphism is associated with the circadian pattern of PAI-1 supports the view that the 4G5G polymorphism may affect the circadian expression of PAI-1.
Our findings shed an interesting light on the current knowledge about PAI-1 as a risk factor for myocardial infarction. The role of PAI-1 as a risk factor for cardiovascular diseases has been questioned.23 The finding that the 4G allele is associated with increased plasma levels of PAI-1 in the morning confirms that the 4G5G polymorphism in the PAI-1 gene is of functional importance in regulating the expression of the PAI-1 gene. Additionally, homozygosity for the 4G allele is associated with a slightly increased risk for myocardial infarction.12 And, subjects with 1 or 2 4Galleles had a faster progression from first anginal symptoms to acute coronary syndromes than 5G5G subjects.25 The present study suggests that the morning increase in PAI-1 is more pronounced in 4G4G subjects. The morning peak corresponds to the diurnal variation of the time of onset of myocardial infarction. It is tempting to suggest that the 4G4G genotype, maybe through the morning increase in PAI-1, plays a role in the excess number of cases of myocardial infarction in the morning. This would confirm a role of PAI-1 as a risk factor for myocardial infarction.
In conclusion, our findings support the view that the 4G5Gpolymorphism in the promoter of the gene for PAI-1 may affect the circadian expression of the PAI-1 gene.
We are grateful to the participants of the Rotterdam Study. We thank all field workers; laboratory technicians in the Ommoord Research center; and G. J. Derksen and the clinical assay unit of the Gaubius Laboratory of TNO-PG for their enthusiasm and skillful contributions to the data collection.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-07-2181.
Supported by grants from the Netherlands Heart Foundation (no. 92.398) and the Dutch Thrombosis Foundation (no. 91.004). The Rotterdam Study is supported in part by the NESTOR program for geriatric research (Ministry of Health and Ministry of Education), the Netherlands Heart Foundation, the Netherlands Organisation for Scientific Research (NWO), the Rotterdam Medical Research Foundation (ROMERES), and the Municipality of Rotterdam.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
J. G. van der Bom, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Internal address D01.335, PO Box 85500, 3508 GA Utrecht, the Netherlands; e-mail: j.g.vanderbom@jc.azu.nl.