Angiostatin, an inhibitor of angiogenesis, contains 3 to 4 kringle domains that are derived from proteolytic cleavage of plasminogen. The antiangiogenic effects of angiostatin occur, in part, from its inhibition of endothelial cell surface adenosine triphosphate synthase, integrin functions, and pericellular proteolysis. Angiostatin has structural similarities to hepatocyte growth factor (HGF; “scatter factor”), a promoter of angiogenesis, that induces proliferation and migration of both endothelial and smooth muscle cells via its cell surface receptor, c-met. We hypothesized that angiostatin might block HGF-induced signaling in endothelial and smooth muscle cells. Angiostatin inhibited HGF-induced phosphorylation of c-met, Akt, and ERK1/2. Angiostatin also significantly inhibited proliferation of human umbilical vein endothelial cells (HUVECs) induced by HGF. In contrast, angiostatin did not inhibit vascular endothelial growth factor (VEGF)–or basic fibroblast growth factor (bFGF)–induced signaling events or HUVEC proliferation. Angiostatin bound to immobilized truncated c-met produced by A431 cells and could be immunoprecipitated as a complex with soluble c-met. HGF inhibited the binding of 125I-angiostatin to HUVECs. Soluble c-met, produced by several tumor cell lines, could inhibit the antiangiogenic effect of angiostatin. The disruption of HGF/c-met signaling is a novel mechanism for the antiangiogenic effect of angiostatin.
Introduction
Tumor growth and metastasis often depend on the elaboration of new capillary blood vessels, a process called angiogenesis.1 Angiogenesis inhibitors primarily target the proliferation and migration of endothelial cells, providing a complimentary approach, along with direct tumor cytotoxic chemotherapy, for treating cancer.2
Angiostatin is an approximately 38-kDa protein derived from an internal fragment of plasminogen3 that suppresses the growth of experimental tumors in animal models.4 Angiostatin was originally purified from the urine of mice with Lewis lung carcinoma3 and is generated endogenously by a variety of human tumors as well.5,6 The conversion of plasminogen to angiostatin appears to require reduction of disulfide bonds followed by proteolysis with one or more proteinases.7 8
Angiostatin has been observed to inhibit endothelial3 and smooth muscle cell9 proliferation by disrupting the G2/M transition in the cell-cycle progression.10 Angiostatin has also been shown to induce apoptosis of human umbilical vein endothelial cells (HUVECs).11 Angiostatin may have an even more potent inhibitory effect on circulating, bone marrow–derived endothelial progenitor cells than it has on mature endothelial cells.12 However, the molecular mechanisms of the antiangiogenesis effect of angiostatin have not been completely elucidated. Angiostatin binds to the endothelial cell surface F1-F0 adenosine triphosphate (ATP) synthase, where it blocks both the ATP synthase and ATPase activities of this enzyme complex.13,14 The antiangiogenic effects of angiostatin may also be mediated via blockade of αvβ3function15 by inhibiting the activation of plasminogen in the extracellular matrix,16 and by inhibiting migration and tube formation that is stimulated by angiomotin.17
Angiostatin has similarities with hepatocyte growth factor (HGF; “scatter factor”), an angiogenic growth factor that acts as a mitogen and motogen for endothelial and smooth muscle cells. Although they have opposing effects on angiogenesis, both HGF and angiostatin possess kringle motifs18,19 and share significant homology at the amino acid level.19 We have previously shown that angiostatin inhibits HGF-induced smooth muscle cell proliferation and migration, suggesting that angiostatin might competitively inhibit the binding of HGF to its receptor, c-met proto-oncogene.9 In this paper, we examine this hypothesis and demonstrate that angiostatin inhibits signaling events induced by HGF but not by vascular endothelial growth factor (VEGF) or basic fibroblast growth factor (bFGF).
Materials and methods
Angiostatin (K1-3 without N-linked glycosylation) expressed inPichia pastoris, was a gift from Entremed (Rockville, MD). HGF and the alkaline phosphatase–conjugated anti–goat and anti–rabbit IgG were purchased from Sigma (St Louis, MO). Peroxidase-conjugated anti–rabbit IgG and peroxidase-conjugated anti–mouse IgG were from Amersham Pharmacia Biotech (Piscataway, NJ). Antihuman plasminogen and protease inhibitor cocktail were from Calbiochem (San Diego, CA). Recombinant human insulin growth factor-1 (IGF-1) was from Bachem Bio Science (King of Prussia, PA). Monoclonal antibodies to human c-met, specific for the extracellular β-chain (clones DL-21 and DO-24) were purchased from Upstate Biotech (Lake Placid, NY). Rabbit polyclonal antibody against C-terminal c-met was from Assay Design (Ann Arbor, MI). Rabbit polyclonal antibody to phospho–c-met [pYpYpY1230/1234/1235] was purchased from Biosource International (Camarillo, CA). A polyclonal antibody to phospho–VEGFR-2/3, generated by immunizing rabbits with the peptide GLARDIpYKDPDpYVRKGD, was purchased from Oncogene Research Products (Boston, MA). Murine monoclonal (4E2) and rabbit polyclonal antibodies to phospho-Akt (phosphorylated at Ser473) were purchased from Cell Signaling Technology (Beverly, MA). Rabbit polyclonal antibody to total Akt (phosphorylation state–independent) was also from Cell Signaling Technology. A monoclonal antibody to phosphorylated ERK-1 and ERK-2 (E-4, sc-7383) and a polyclonal antibody to a carboxyterminal peptide of actin (I-19, sc-1616) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Centricon-10 concentrators were purchased from Amicon (Beverly, MA). Iodobeads and the Super Signal chemiluminescence kit were purchased from Pierce Chemical (Rockford, IL). Alkaline phosphatase activity was detected using the soluble substrate p-nitrophenylphosphate with diethanolamine buffer from BIORAD (Hercules, CA).
Cell culture
HUVECs and human aortic smooth muscle cells (HASMCs) were purchased from Clonetics (Walkersville, MD). HUVECs were maintained in endothelial growth medium 2 (EGM-2) containing growth factors, 2% fetal bovine serum (FBS), 50 μg/mL gentamicin, and 50 μg/mL amphotericin. HASMCs were maintained in smooth muscle cell growth medium 2 (SmGM-2) medium containing growth factors, 5% FBS, and 50 μg/mL gentamicin. A431 (human epidermoid carcinoma cells) obtained from American Type Culture Collection (ATCC, Manassas, VA) were grown in Dulbecco modified Eagle medium (DMEM) containing 10% FBS. S180 and A549 were also cultured in DMEM and 10% FBS. MCF-7 and BT474 cells were grown in RPMI and 10% FBS was used. For soluble c-met studies, DMEM or RPMI were used without FBS.
Treatment of cells
HUVECs and HASMCs were seeded in 60-mm tissue culture plates and were grown in complete EGM-2 and SmGM-2, respectively, to 90% confluence, then incubated overnight in SmGM and EGM-2 serum-free medium. The cells were then washed 3 times with phosphate-buffered saline (PBS) and once with serum-free medium, then incubated for the indicated times at 37°C in 1.5 mL of serum-free medium containing the defined concentrations of HGF, VEGF, bFGF, IGF-1, and angiostatin.
Soluble c-met from tumor cell lines
Tumor cell lines were seeded in 100-mm tissue culture dishes and grown in complete medium to near 100% confluency. Cells were washed with PBS and serum-free medium was added to the plate. After 16 hours, the medium was harvested (7.0 mL) and protease inhibitor cocktail was added and centrifuged at 10 000g at 4°C for 1 hour to remove any cell debris. The supernatant was filtered through a 0.22-μm filter and concentrated by using centriprep 10 (cutoff 10 000 kDa) at 3000g until the volume was reduced to 0.5 mL. The concentrated medium (35 μL) was mixed with 35 μL of 2 × sample buffer (reducing or nonreducing) then boiled for 3 minutes and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; 7.5% gels).
Cell proliferation assay
HUVECs (5000 cells per well) were cultured in a 96-well plate in EBM-2 containing 1.0% serum in the presence or absence of HGF (10 ng/mL), bFGF (10 ng/mL), or VEGF (10 ng/mL). Angiostatin, at a final concentration of 3 to 5 μM was added to replicate wells containing growth factors. After 72 hours, 20 μL per well of CellTiter 96 AQueous One cell proliferation assay reagent (Promega, Madison, WI) was added and the plate was incubated for 1 hour at 37°C, 5% CO2. The reaction product (formazan) was then monitored at 490 nm, and the background absorbance of wells containing culture medium only was subtracted. All assays were performed at n = 6.
Immunoprecipitation
Concentrated A431 medium was precleared by adding 50 μL of protein L beads for 2 hours at 4°C; it was then centrifuged and supernatant was collected. Angiostatin (2.5 μg/mL) was added for 2 hours at room temperature with shaking. Anti–c-met clone DO24 (5 μL) or nonimmune IgG was added and incubated overnight at 4°C with shaking. After incubation with primary antibody, 50 μL of protein L beads was added and again incubated for 2 hours at 4°C with shaking. Beads were washed 3 times with ice-cold radioimmunoprecipitation assay (RIPA) buffer and bound protein was extracted by adding 50 μL of 2 × SDS sample buffer and boiling for 5 minutes. Beads were again centrifuged and 50 μL of extracted proteins was loaded onto a polyacrylamide gel (8%). The gel was transferred onto a nitrocellulose membrane and immunoblotting was performed using antiplasminogen to detect angiostatin.
Cell-binding assay
Purified angiostatin (10 μg; Calbiochem) was iodinated using Iodobeads (Pierce Chemical) according to the manufacturer's recommendations. Cell-binding assay was performed essentially as described.20 HUVECs (n = 10 000 in 100 μL) were seeded in a 96-well plate in complete medium. After 24 hours medium was removed and cells were serum starved for 24 hours in medium containing 0.1% serum. Cells were washed 3 times with ice-cold RPMI medium containing 20 mM HEPES (4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid), pH 7.4, 0.1% bovine serum albumin (BSA; binding medium) and incubated at 4°C for 30 minutes. Different concentrations of125I-angiostatin with or without a 100-fold excess of angiostatin were added at a final volume of 100 μL/well and incubated for 1 hour at 4°C. To test the ability of HGF to inhibit125I-angiostatin binding, 125I-angiostatin (5 nM) was incubated with the HUVECs as described in the absence or presence of increasing concentrations of unlabeled HGF (75 nM to 1.0 μM), or with 0.5 μM cold angiostatin. After incubation, cells were washed 5 times with ice-cold binding buffer and extracted with 1% Triton X-100 in PBS, then counted in a γ-counter. Replicates of 6 were performed. Binding studies were graphed and analyzed using GraphPad Prism Version 3.0 (GraphPad Software, San Diego, CA).
Enzyme-linked immunosorbent assay (ELISA)
Concentrated A431 serum-free medium containing soluble c-met (total protein concentration, approximately 4 mg/mL) or Voller buffer (15 mM Na2CO3, 35 mM NaHCO3, 0.2% NaN3, pH 9.6) was added in triplicate wells to a 96-well EIA/RIA plate (100 μL) and allowed to adsorb overnight at 4°C. The plates were washed 3 times with PBS between each step. To block the plates, PBS containing 1% nonfat dry milk was added for one hour at room temperature. For a sandwich ELISA, 96-well enzyme immunoassay/radioimmunoassay (EIA/RIA) plates were incubated overnight with 100 μL of a 1:100 dilution of a monoclonal antibody to the extracellular β-chain of c-met in Voller buffer. The plates were blocked and concentrated soluble c-met receptor was added. Angiostatin at concentrations of 0 to 5 μM in PBS/1% milk was incubated on the plate for one hour at room temperature. Antihuman plasminogen at 1:250 dilution was added for one hour at room temperature. Alkaline phosphatase–conjugated secondary antibodies (1:250 of anti–rabbit IgG) were incubated for one hour at room temperature. Alkaline phosphatase activity was detected in 1 M diethanolamine (pH 9.8), 0.5 mM MgCl2, and 12 mM p-nitrophenyl phosphate. Plates were read at OD405 (optical density at 405 nm) in an ELISA plate reader. The Vmax (maximum velocity) was determined using SOFTMAX Pro software (version 2.1.1; Molecular Devices, Sunnyvale, CA).
Western blotting of cell lysates and conditioned medium
Cell lysates were prepared by adding 300 μL ice-cold RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA [ethylenediaminetetraacetic acid], 1% nonidet P-40 [NP-40] and 0.25% Na-deoxycholate) containing protease and phosphatase inhibitors cocktail. Cells were incubated for 30 minutes on ice containing RIPA buffer. The lysates were cleared by centrifugation and protein concentration of the supernatant was determined by using BioRad Dc Protein assay reagent. Electrophoresis was performed in reducing and nonreducing Laemmli buffer using SDS-polyacrylamide gel. After electrophoresis, proteins were transferred onto nitrocellulose membrane, then blocked in 5% dried milk or 3% BSA in TBST (10 mM Tris, pH 7.5, 100 mM NaCl, 0.1% Tween20) for 1 hour at room temperature. The membranes were washed twice in TBST for 10 minutes, then probed overnight at 4°C with primary antibody (0.5 to 1 μg/mL) in 5% nonfat milk or 3% BSA in TBST. The membranes were washed twice in TBST and incubated in secondary antibody labeled with horseradish peroxidase for 1 hour at room temperature, then washed twice in TBST for 10 minutes and developed for enhanced chemiluminescence.
In some cases, immunoblots were stripped by incubating the blot at 50°C for 30 minutes in a buffer containing 62.5 mM Tris-HCl, pH 6.7, containing 2% SDS and 100 mM 2-mercaptoethanol. The blot was then washed twice with TBST for 5 minutes and blocked with 5% milk for 30 minutes at room temperature followed by a washing twice with TBST for 5 minutes, then re-probing with a primary antibody as described.
Results
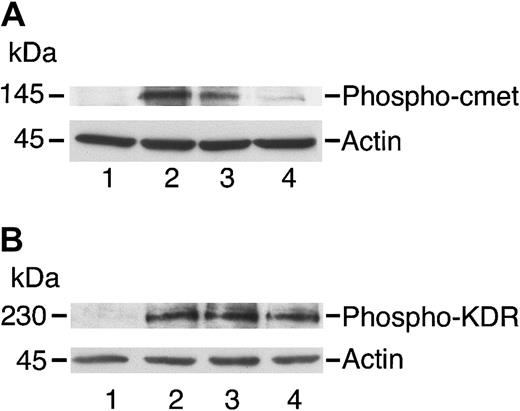
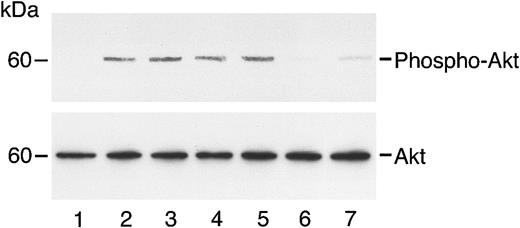
c-met was detected in immunoblots from HUVECs and HASMCs, as well as from A431 cells, which are known to overexpress c-met (Figure1; Galvani et al21). HGF induced the phosphorylation of c-met, a process inhibited by angiostatin (Figure 2A). In contrast, angiostatin did not inhibit the phosphorylation of KDR (flk-1; Figure2B). By itself, angiostatin at 5 μM did not induce phosphorylation of c-met, KDR, Akt, or ERK1/2 (data not shown). Angiostatin also blocked the phosphorylation of the survival signal, Akt, in response to HGF. This effect was demonstrated in endothelial (Figure3) and smooth muscle (Figure4) cells and appeared to be competitive, since higher levels of HGF were able to reverse inhibition by angiostatin (Figure 5). Angiostatin failed to inhibit phospho-Akt generation in response to VEGF, bFGF (Figure 6A-B), or IGF-1 (data not shown). Plasminogen did not inhibit HGF-induced AKT phosphorylation in HUVECs (Figure 7). Angiostatin inhibited ERK1 and ERK2 phosphorylation by HGF (Figure8A) but not by VEGF or bFGF (Figure8B).
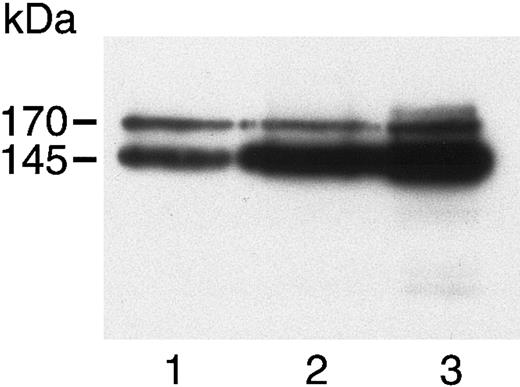
c-met in HASMCs, HUVECs, and A431 cells.
Cell lysates of HASMCs (lane 1), HUVECs (lane 2), and A431 cells (lane 3) were separated by 7.5% SDS-PAGE, then immunoblotted using a polyclonal antibody to the c-terminus of the c-met β chain. A 170-kDa precursor and 145-kDa mature β chain were visualized in all 3 cells.
c-met in HASMCs, HUVECs, and A431 cells.
Cell lysates of HASMCs (lane 1), HUVECs (lane 2), and A431 cells (lane 3) were separated by 7.5% SDS-PAGE, then immunoblotted using a polyclonal antibody to the c-terminus of the c-met β chain. A 170-kDa precursor and 145-kDa mature β chain were visualized in all 3 cells.
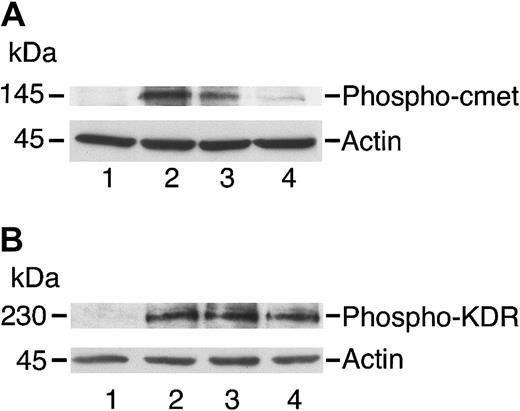
Angiostatin inhibits phosphorylation of c-met by HGF in HUVECs.
HUVECs were cultured in serum-free medium as described in “Materials and methods,” then stimulated with HGF (10 ng/mL; panel A) or with VEGF (10 ng/mL; panel B) for 5 minutes prior to preparation of cell lysates. The lysates were separated by SDS-PAGE and subjected to immunoblotting. (A) Immunoblotting for Phospho–c-met: lane 1 indicates control cells; lane 2, cell lysates after stimulation with HGF (10 ng/mL); lane 3, cells treated with HGF and angiostatin (1 μM); and lane 4, cells treated with HGF and angiostatin (3 μM). (B) Immunoblotting for Phospho-KDR: lane 1 indicates control cells; lane 2, cell lysates after stimulation with VEGF (10 ng/mL); lane 3, cells treated with VEGF and angiostatin (1 μM); and lane 4, cells treated with VEGF and angiostatin (3 μM). Angiostatin inhibited the phosphorylation of c-met induced by HGF but did not inhibit KDR phosphorylation induced by VEGF. Blots were stripped and re-probed for actin as a loading control.
Angiostatin inhibits phosphorylation of c-met by HGF in HUVECs.
HUVECs were cultured in serum-free medium as described in “Materials and methods,” then stimulated with HGF (10 ng/mL; panel A) or with VEGF (10 ng/mL; panel B) for 5 minutes prior to preparation of cell lysates. The lysates were separated by SDS-PAGE and subjected to immunoblotting. (A) Immunoblotting for Phospho–c-met: lane 1 indicates control cells; lane 2, cell lysates after stimulation with HGF (10 ng/mL); lane 3, cells treated with HGF and angiostatin (1 μM); and lane 4, cells treated with HGF and angiostatin (3 μM). (B) Immunoblotting for Phospho-KDR: lane 1 indicates control cells; lane 2, cell lysates after stimulation with VEGF (10 ng/mL); lane 3, cells treated with VEGF and angiostatin (1 μM); and lane 4, cells treated with VEGF and angiostatin (3 μM). Angiostatin inhibited the phosphorylation of c-met induced by HGF but did not inhibit KDR phosphorylation induced by VEGF. Blots were stripped and re-probed for actin as a loading control.
Angiostatin inhibits HGF-induced phosphorylation of Akt in HUVECs.
HUVECs in serum-free medium were unstimulated (lane 1) or treated with HGF (10 ng/mL) in the absence (lane 2) or presence (lanes 3-7) of angiostatin. Angiostatin concentrations were 0.5 μM (lane 3), 0.75 μM (lane 4), 1.0 μM (lane 5), 2 μM (lane 6), or 3 μM (lane 7). After a 5-minute incubation, cell lysates were prepared and immunoblotting for phospho-AKT was performed as described. The blot was stripped and re-probed for total Akt.
Angiostatin inhibits HGF-induced phosphorylation of Akt in HUVECs.
HUVECs in serum-free medium were unstimulated (lane 1) or treated with HGF (10 ng/mL) in the absence (lane 2) or presence (lanes 3-7) of angiostatin. Angiostatin concentrations were 0.5 μM (lane 3), 0.75 μM (lane 4), 1.0 μM (lane 5), 2 μM (lane 6), or 3 μM (lane 7). After a 5-minute incubation, cell lysates were prepared and immunoblotting for phospho-AKT was performed as described. The blot was stripped and re-probed for total Akt.
Angiostatin inhibits HGF-induced phosphorylation of Akt in HASMCs.
HASMCs in serum-free medium were unstimulated (lane 1) or treated with HGF (10 ng/mL) in the absence (lane 2) or presence (lanes 3-6) of angiostatin. Angiostatin concentrations were 0.5 μM (lane 3), 0.75 μM (lane 4), 1.0 μM (lane 5), or 3 μM (lane 6). After a 5-minute incubation, cell lysates were prepared and immunoblotting for phospho-AKT was performed as described. The blot was stripped and re-probed for total Akt.
Angiostatin inhibits HGF-induced phosphorylation of Akt in HASMCs.
HASMCs in serum-free medium were unstimulated (lane 1) or treated with HGF (10 ng/mL) in the absence (lane 2) or presence (lanes 3-6) of angiostatin. Angiostatin concentrations were 0.5 μM (lane 3), 0.75 μM (lane 4), 1.0 μM (lane 5), or 3 μM (lane 6). After a 5-minute incubation, cell lysates were prepared and immunoblotting for phospho-AKT was performed as described. The blot was stripped and re-probed for total Akt.
Reversal of angiostatin inhibition of AKT phosphorylation with excess HGF.
HUVECs in serum-free medium were untreated (lane 1) or treated with angiostatin (3 μM) and increasing concentrations of HGF: 0.5 ng/mL (lane 2), 10 ng/mL (lane 3), 25 ng/mL (lane 4), 50 ng/mL (lane 5), and 100 ng/mL (lane 6). After a 5-minute incubation, cell lysates were prepared and immunoblotting for phospho-AKT was performed as described. The blot was stripped and re-probed for total Akt.
Reversal of angiostatin inhibition of AKT phosphorylation with excess HGF.
HUVECs in serum-free medium were untreated (lane 1) or treated with angiostatin (3 μM) and increasing concentrations of HGF: 0.5 ng/mL (lane 2), 10 ng/mL (lane 3), 25 ng/mL (lane 4), 50 ng/mL (lane 5), and 100 ng/mL (lane 6). After a 5-minute incubation, cell lysates were prepared and immunoblotting for phospho-AKT was performed as described. The blot was stripped and re-probed for total Akt.
Angiostatin does not inhibit VEGF- or bFGF-induced phosphorylation of Akt.
HUVECs (A) or HASMCs (B) in serum-free medium were unstimulated (lane 1) or treated with VEGF (50 ng/mL) in the absence (lane 2) or presence (lane 3) of angiostatin (ANG; 5 μM). Similarly, treatment with bFGF, 25 ng/mL (lane 4) resulted in AKT phosphorylation that was not inhibited by angiostatin, 5 μM (lane 5).
Angiostatin does not inhibit VEGF- or bFGF-induced phosphorylation of Akt.
HUVECs (A) or HASMCs (B) in serum-free medium were unstimulated (lane 1) or treated with VEGF (50 ng/mL) in the absence (lane 2) or presence (lane 3) of angiostatin (ANG; 5 μM). Similarly, treatment with bFGF, 25 ng/mL (lane 4) resulted in AKT phosphorylation that was not inhibited by angiostatin, 5 μM (lane 5).
Plasminogen does not inhibit HGF-induced phosphorylation of Akt.
HUVECs in serum-free medium were unstimulated or treated with HGF for 5 minutes with or without plasminogen at varying concentrations. Lane 1 is control (unstimulated cells); lane 2, HGF (10 ng/mL); lanes 3-5, HGF (10 ng/mL) plus plasminogen, at 0.5μM (lane 3), 1.0 μM (lane 4), and 3 μM (lane 5); lane 6, plasminogen at 3 μM without HGF; lane 7, HGF (10 ng/mL) and angiostatin (3 μM). Cell lysates were prepared for immunoblotting to detect phospho-AKT, then total AKT (after stripping).
Plasminogen does not inhibit HGF-induced phosphorylation of Akt.
HUVECs in serum-free medium were unstimulated or treated with HGF for 5 minutes with or without plasminogen at varying concentrations. Lane 1 is control (unstimulated cells); lane 2, HGF (10 ng/mL); lanes 3-5, HGF (10 ng/mL) plus plasminogen, at 0.5μM (lane 3), 1.0 μM (lane 4), and 3 μM (lane 5); lane 6, plasminogen at 3 μM without HGF; lane 7, HGF (10 ng/mL) and angiostatin (3 μM). Cell lysates were prepared for immunoblotting to detect phospho-AKT, then total AKT (after stripping).
Angiostatin inhibits ERK1/2 phosphorylation induced by HGF but not by VEGF or bFGF.
(A) HUVECs in serum-free medium were stimulated with HGF (10 ng/mL) for 5 minutes with or without angiostatin at varying concentrations. Lane 1 is control (unstimulated cells); lane 2, HGF (10 ng/mL); lanes 3-6, HGF (10 ng/mL) plus angiostatin, at 0.5 μM (lane 3), 1.0 μM (lane 4), 2.0 μM (lane 5), and 3.0 μM (lane 6). (B) HUVECs in serum-free medium were unstimulated (lane 1) or treated with VEGF (50 ng/mL) in the absence (lane 2) or presence (lane 3) of angiostatin (ANG; 5 μM). Similarly, treatment with bFGF, 25 ng/mL (lane 4), resulted in ERK1/2 phosphorylation that was not inhibited by angiostatin (5 μM, lane 5).
Angiostatin inhibits ERK1/2 phosphorylation induced by HGF but not by VEGF or bFGF.
(A) HUVECs in serum-free medium were stimulated with HGF (10 ng/mL) for 5 minutes with or without angiostatin at varying concentrations. Lane 1 is control (unstimulated cells); lane 2, HGF (10 ng/mL); lanes 3-6, HGF (10 ng/mL) plus angiostatin, at 0.5 μM (lane 3), 1.0 μM (lane 4), 2.0 μM (lane 5), and 3.0 μM (lane 6). (B) HUVECs in serum-free medium were unstimulated (lane 1) or treated with VEGF (50 ng/mL) in the absence (lane 2) or presence (lane 3) of angiostatin (ANG; 5 μM). Similarly, treatment with bFGF, 25 ng/mL (lane 4), resulted in ERK1/2 phosphorylation that was not inhibited by angiostatin (5 μM, lane 5).
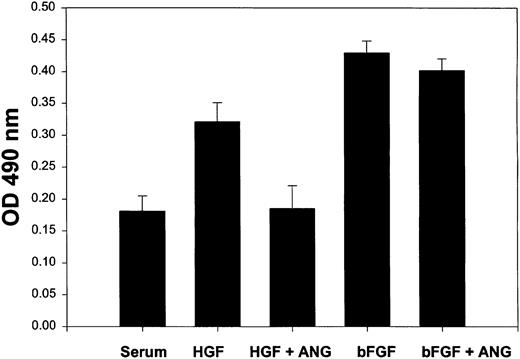
Angiostatin significantly inhibited HGF-induced proliferation of HUVECs (Figure 9). Although there was a trend toward inhibition of bFGF-induced proliferation, this effect was not statistically significant. Angiostatin did not significantly inhibit VEGF-induced proliferation (not shown).
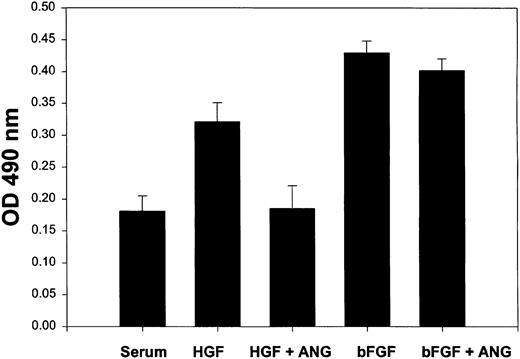
Angiostatin selectively inhibits HGF-induced proliferation of HUVECs.
HUVECs (5000 cells/well) were cultured in 96-well plates in serum in the presence or absence of HGF (10 ng/mL) or bFGF (10 ng/mL). Angiostatin was added to some wells at 3 μM. After 72 hours of culture, a metabolic cell proliferation assay was performed with monitoring of formazan production at 490 nm. Each assay was performed in replicates of 6. Angiostatin significantly (P < .01) inhibited HGF-induced proliferation, but did not inhibit bFGF-induced proliferation. Angiostatin also failed to significantly reduce VEGF-induced proliferation (data not shown). Error bars indicate ± 1 SD.
Angiostatin selectively inhibits HGF-induced proliferation of HUVECs.
HUVECs (5000 cells/well) were cultured in 96-well plates in serum in the presence or absence of HGF (10 ng/mL) or bFGF (10 ng/mL). Angiostatin was added to some wells at 3 μM. After 72 hours of culture, a metabolic cell proliferation assay was performed with monitoring of formazan production at 490 nm. Each assay was performed in replicates of 6. Angiostatin significantly (P < .01) inhibited HGF-induced proliferation, but did not inhibit bFGF-induced proliferation. Angiostatin also failed to significantly reduce VEGF-induced proliferation (data not shown). Error bars indicate ± 1 SD.
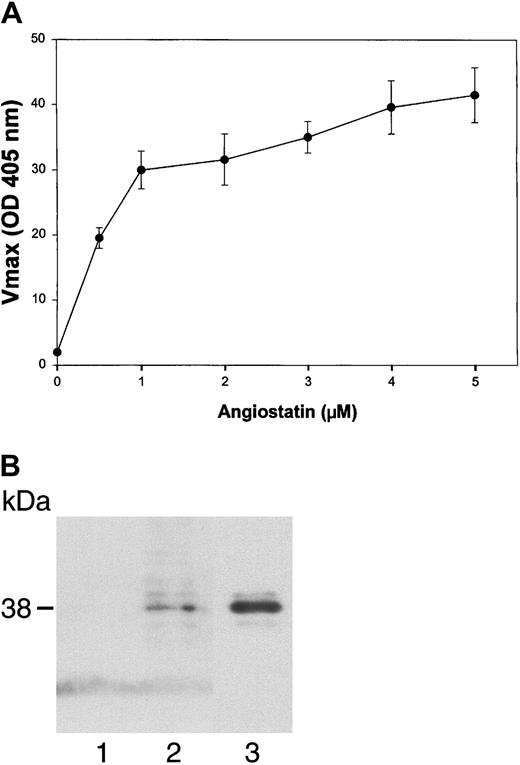
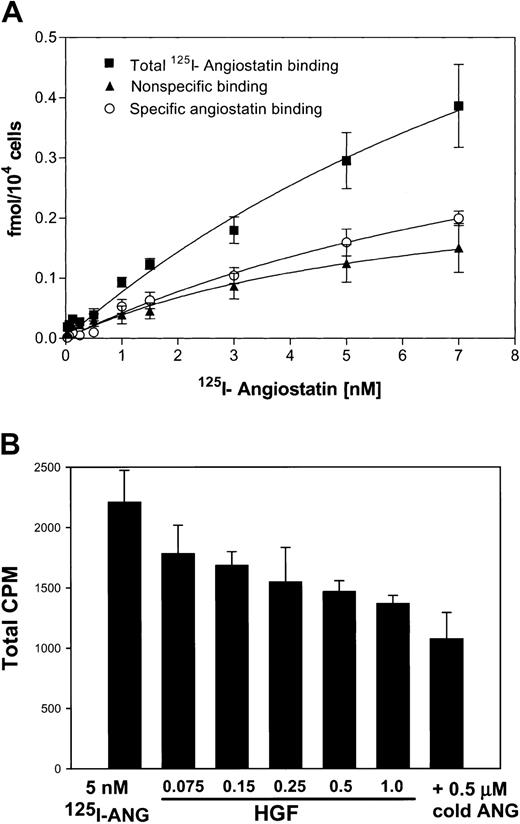
To test the ability of angiostatin, in the absence of HGF, to bind to truncated c-met, we performed an ELISA with medium containing truncated c-met or Voller buffer to control for nonspecific binding. Angiostatin (0-5 μM) binding was detected with a polyclonal antibody to plasminogen. Angiostatin bound to truncated c-met with a dissociation constant (Kd) of approximately 0.75 μM (Figure 10A). Similar results were obtained with sandwich ELISA, in which soluble c-met was captured with an antibody to the β-chain (not shown). Furthermore, angiostatin coimmunoprecipitated with soluble c-met derived from A431 cells (Figure10B). 125I-angiostatin bound to HUVECs with a Kd of approximately 5 nM and to 12 044 sites per cell (Figure 11A). HGF was able to inhibit 125I-angiostatin binding to HUVECs (Figure11B).
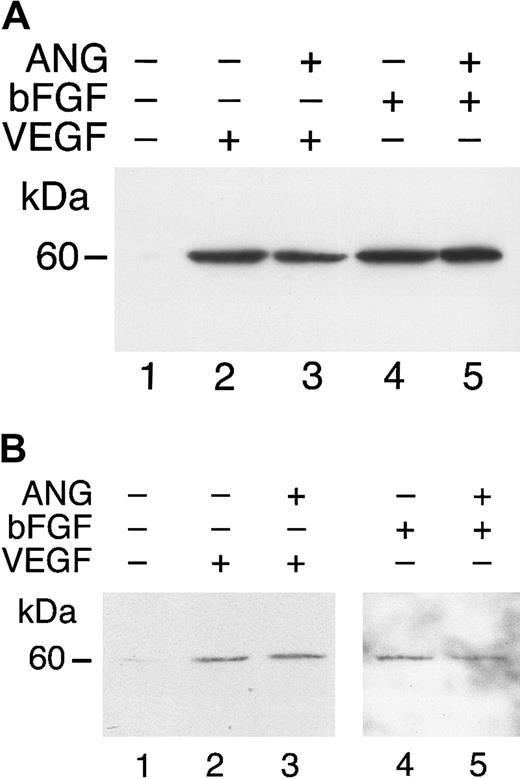
Angiostatin binds to soluble c-met.
(A) Media containing the soluble, truncated c-met receptor derived from A431 cells were adsorbed to an EIA/RIA plate. Angiostatin was added in concentrations of 0 to 5 μM and bound angiostatin was detected with a polyclonal antibody to plasminogen followed by an alkaline phosphatase–conjugated anti–rabbit IgG (solid line). Antibody detection was quantified using p-nitrophenylphosphate and measuring the Vmax at OD405. At each concentration of angiostatin, Voller buffer was used to show nonspecific binding of angiostatin to the plate, which was subtracted from total binding to obtain specific binding (displayed in this graph). Replicates of n = 3 were performed. The error bars represent ± 1 SD. (B) Coimmunoprecipitation. A431 medium containing soluble c-met was incubated with angiostatin, then immunoprecipitated with nonimmune mouse IgG (lane 1) or with anti–c-met extracellular domain (lane 2). Immunoblotting was performed with antiplasminogen. Lane 3: angiostatin (positive control). Angiostatin was immunoprecipitated by anti–c-met, demonstrating a soluble c-met/angiostatin complex.
Angiostatin binds to soluble c-met.
(A) Media containing the soluble, truncated c-met receptor derived from A431 cells were adsorbed to an EIA/RIA plate. Angiostatin was added in concentrations of 0 to 5 μM and bound angiostatin was detected with a polyclonal antibody to plasminogen followed by an alkaline phosphatase–conjugated anti–rabbit IgG (solid line). Antibody detection was quantified using p-nitrophenylphosphate and measuring the Vmax at OD405. At each concentration of angiostatin, Voller buffer was used to show nonspecific binding of angiostatin to the plate, which was subtracted from total binding to obtain specific binding (displayed in this graph). Replicates of n = 3 were performed. The error bars represent ± 1 SD. (B) Coimmunoprecipitation. A431 medium containing soluble c-met was incubated with angiostatin, then immunoprecipitated with nonimmune mouse IgG (lane 1) or with anti–c-met extracellular domain (lane 2). Immunoblotting was performed with antiplasminogen. Lane 3: angiostatin (positive control). Angiostatin was immunoprecipitated by anti–c-met, demonstrating a soluble c-met/angiostatin complex.
Binding of 125I-angiostatin to HUVECs with inhibition by HGF.
(A) HUVECs were cultured in 96-well plates, then incubated with125I-angiostatin in the absence or presence of 100-fold molar excess of unlabled angiostatin as described in “Materials and methods.” Total, nonspecific, and specific binding are shown. (B)125I-angiostatin (5 nM) was incubated with HUVECs in the absence or presence of increasing concentrations of HGF (75 nM-1.0 μM) or with 0.5 μM cold angiostatin. HGF inhibits125I-angiostatin binding to HUVECs. Error bars indicate ± 1 SD.
Binding of 125I-angiostatin to HUVECs with inhibition by HGF.
(A) HUVECs were cultured in 96-well plates, then incubated with125I-angiostatin in the absence or presence of 100-fold molar excess of unlabled angiostatin as described in “Materials and methods.” Total, nonspecific, and specific binding are shown. (B)125I-angiostatin (5 nM) was incubated with HUVECs in the absence or presence of increasing concentrations of HGF (75 nM-1.0 μM) or with 0.5 μM cold angiostatin. HGF inhibits125I-angiostatin binding to HUVECs. Error bars indicate ± 1 SD.
We examined the production of soluble c-met by 5 tumor cell lines. A431 and A549 cells produced soluble c-met, but MCF-7, S180, and BT474 cells did not (Figure 12).
Soluble c-met is produced by some tumor cell lines.
Medium from A431 cells (lane 1), MCF-7 cells (lane 2), S180 cells (lane 3), BT474 cells (lane 4), and A549 cells (lane 5) was concentrated and immunoblotted using an antibody to the β chain of c-met. Only A431 and A549 cells produce soluble c-met.
Soluble c-met is produced by some tumor cell lines.
Medium from A431 cells (lane 1), MCF-7 cells (lane 2), S180 cells (lane 3), BT474 cells (lane 4), and A549 cells (lane 5) was concentrated and immunoblotted using an antibody to the β chain of c-met. Only A431 and A549 cells produce soluble c-met.
Discussion
In this paper, we have shown that angiostatin inhibits endothelial and smooth muscle cell responses to HGF, while not affecting VEGF- or bFGF-induced signaling events. Angiostatin inhibited autophosphorylation of the HGF receptor (c-met), as well as downstream events including the phosphorylation of Akt and ERK1/2. The inhibition appeared to be competitive, since high concentrations of HGF could overcome the inhibition by angiostatin. HGF competed with125I-angiostatin for binding to HUVECs. Finally, angiostatin inhibited endothelial cell proliferation that was induced by HGF, but not proliferation that was induced by VEGF or bFGF. Plasminogen did not inhibit HGF-induced AKT phosphorylation, indicating that this property is unique to angiostatin.
Our finding of a specific effect of angiostatin on signal transduction by HGF is consistent with previous reports. Claesson-Welsh et al11 found that angiostatin did not block the binding of125I-VEGF to its receptor on bovine adrenal cortex capillary endothelial cells. Furthermore, angiostatin had no effect on the bFGF-induced tyrosine phosphorylation of the adapter protein Shb, on bFGF-induced p42 MAPK (ERK1) phosphorylation, or on bFGF-induced tyrosine phosphorylation of an unidentified 140-kDa protein.11
Our results are also similar to those obtained by Kuba et al who studied NK4, a derivative of HGF composed of the N-terminal hairpin and 4 kringle domains of HGF.22 NK4 has 47% amino acid homology with angiostatin.22 NK4 inhibited the HGF-induced phosphorylation of c-met but not the phosphorylation of KDR induced by VEGF. Furthermore, NK4 inhibited HGF-induced ERK1/2 activation, but did not inhibit the phosphorylation of ERK1/2 in response to bFGF or VEGF.22
The antiangiogenic effects of NK4 could not be ascribed solely to antagonism of HGF activities, however, because NK4 also blocked proliferation and migration induced by bFGF and VEGF, as well as HGF.22 Similarly, angiostatin inhibited migration of cells toward bFGF and VEGF,11 an effect that appears to be largely due to interference with integrin function. Tarui et al reported that bovine aortic endothelial cells adhered to angiostatin in an integrin-dependent manner, with the predominant receptor being αvβ3.15 Other integrins that interacted with angiostatin to a lesser extent were α9β1 and α4β1. In contrast, plasminogen, which does not inhibit angiogenesis, did not bind to αvβ3. Since angiostatin did not induce strong spreading or stress fiber formation, it has been hypothesized that angiostatin competes with other αvβ3 ligands for this integrin, thereby producing an antiangiogenic effect.15 The inhibition of αvβ3 could contribute to the antiangiogenic effects of angiostatin that are independent of the HGF/c-met pathway since endothelial cell survival during angiogenesis is dependent on αvβ323and antagonists of αvβ3 are useful as inhibitors of angiogenesis.24
The inhibition of Akt phosphorylation by angiostatin is not solely a marker for the inhibition of HGF binding to c-met; instead, a reduction in phospho-Akt could directly contribute to the disruption of angiogenesis. Akt is a serine/threonine kinase that is rapidly activated as a downstream effector of phosphatidylinositol 3 (PI3) kinase in response to a variety of cytokines and growth factors, including HGF.25 Akt plays multiple roles in cellular homeostasis including the regulation of glucose metabolism,26 the activation of eNOS,27 and the suppression of apoptosis.28 The antiapoptotic effect of Akt may occur through the phosphorylation of the apoptosis-inducing proteins BAD,29,30 caspase-9,31 and FKHRL1.32 The activation of Akt is also essential for the induction of a survival pathway involving ligation of αvβ3 (or α5β1) and resulting in the up-regulation of bcl-2 expression.33The activation of the PI3K-Akt axis by both integrins and growth factor receptors emphasizes the importance of this signal pathway for cell survival. Finally, Akt promotes cell-cycle progression into the S-phase by repressing the expression of p27kip1, an inhibitor of the cyclin E/cdk2 complex.34
At first thought, the finding that angiostatin inhibits HGF but not VEGF or bFGF signaling might appear to be an inadequate explanation for the inhibition of angiogenesis by angiostatin. Several factors need to be considered in this analysis, however. First, it has been demonstrated that amplification and signaling cross-talk between angiogenic growth factors are common. For example, HGF in cell culture35 and when injected in vivo36 induces the expression of VEGF. The induction of VEGF expression by HGF depends on the activation of Akt, which up-regulates HIF1α transcription and stabilizes the protein against degradation.37 HIF1α is a transcription factor that promotes VEGF transcription. The importance of Akt activation in VEGF expression is emphasized by a study demonstrating that overexpression of a myristylated form of Akt induces angiogenesis by increasing endothelial cell expression of VEGF.38 Furthermore, HGF and VEGF act synergistically to induce endothelial cell morphogenesis into tubelike structures in 3-D collagen gels.39 Therefore, loss of HGF signaling could reduce VEGF expression or its angiogenic potency. The second consideration is to re-emphasize that the inhibition of integrin function, ATP synthase activity, pericellular plasmin generation, and angiomotin function also contribute to the antiangiogenic activity of angiostatin.
We found that angiostatin binds to soluble c-met derived from A431 epidermoid carcinoma cells. Soluble c-met was also detected in the medium of A549 lung carcinoma cells, but not in the medium of MCF-7, S180, or BT474 breast carcinoma cells. Soluble c-met is also produced by GTL-16 gastric carcinoma cells40 and by LoVo colon carcinoma cells41 We have also previously demonstrated that soluble c-met is released from cultured HUVECs and HASMCs, and can bind to the ligand HGF.41 These findings suggest that the ability of angiostatin to block the HGF/c-met interaction as well as its other antiangiogenic actions could be abrogated in the presence of high plasma levels of soluble c-met. Therefore, an important clinical implication of this work is that tumors that produce high levels of soluble c-met may be relatively resistant to the antiangiogenic effects of angiostatin, while others that do not produce soluble c-met may be more sensitive. Finally, it is also possible that angiostatin exerts effects directly at the surface of tumor cells that express c-met.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-02-0582.
Supported by the National Institutes of Health (NIH) grant CA 81233 (D.C.S.). The DNA Core Synthesis Laboratory of the Comprehensive Cancer Center and Wake Forest University School of Medicine was supported in part by National Cancer Institute grant CA 12197.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David C. Sane, Section of Cardiology, Wake Forest University School of Medicine, Medical Center Blvd, Winston-Salem, NC 27157-1045; e-mail:dsane@wfubmc.edu.