A novel approach to treat bleeding episodes in patients with Glanzmann thrombasthenia (GT) and perhaps also in patients receiving αIIbβ3 inhibitors is the administration of recombinant factor VIIa (rFVIIa). The mechanism of action of rFVIIa in these patients is, however, still unclear. We studied the effect of rFVIIa-mediated thrombin formation on adhesion of αIIbβ3-deficient platelets under flow conditions. Adhesion of αIIbβ3-deficient platelets to the extracellular matrix (ECM) of stimulated human umbilical vein endothelial cells or to collagen type III was studied using a model system with washed platelets and red cells. When αIIbβ3-deficient platelets were perfused over the surface at arterial shear rate for 5 minutes, a low surface coverage was observed (GT platelets, mean ± SEM, 37.5% ± 5.0%; normal platelets preincubated with an RGD-containing peptide, 7.4% ± 2.1%). When rFVIIa, together with factors X and II, was added to the perfusate, platelet deposition significantly increased (GT platelets, mean ± SEM, 67.0% ± 4.3%; normal platelets preincubated with an RGD-containing peptide, 48.2% ± 2.9%). The same effect was observed when normal platelets were pretreated with the commercially available anti-αIIbβ3 drugs abciximab, eptifibatide, or tirofiban. It was shown that tissue factor–independent thrombin generation (presumably induced by binding of rFVIIa to adhered platelets) was responsible for the increase in platelet deposition. In conclusion, defective adhesion of αIIbβ3-deficient platelets to ECM can be restored by tissue factor–independent rFVIIa-mediated thrombin formation. The enhanced generation of platelet procoagulant surface facilitates fibrin formation, so that lack of platelet aggregate formation might be compensated for.
Introduction
Platelets play a crucial role in hemostasis and in thrombotic processes. On vessel wall injury, platelets adhere to the subendothelium through the interaction of glycoprotein (GP) Ib with von Willebrand factor (VWF) bound to subendothelial collagen. Stable adhesion is subsequently accomplished by binding of platelet integrin receptors such as αIIbβ3, α2β1, α5β1, α6β1, and αvβ3to their ligands present in the subendothelium. When stable adhesion is accomplished, secretion and activation of the platelets occurs, followed by aggregation through bridging of VWF or fibrinogen to αIIbβ3 on 2 different platelets.1
The importance of αIIbβ3 in platelet functioning is demonstrated by the bleeding tendency of patients with Glanzmann thrombasthenia (GT), who have a congenital qualitative or quantitative defect in this receptor.2 Also, inhibition of αIIbβ3 by antiplatelet drugs such as abciximab, eptifibatide, and tirofiban is of proven benefit to patients suffering from acute coronary artery disease.3 Although these αIIbβ3 antagonists are highly effective in preventing reocclusion after thrombolysis, stenting, or angioplasty, administration of these compounds also induces bleeding in a significant number of patients.4
Traditionally, platelet concentrates are administered to patients with GT during bleeding episodes or prophylactically during surgery. However, platelet concentrates carry the risk of alloimmunization to human leukocyte antigens or to αIIbβ3, making further administration of donor platelets ineffective. Bleeding complications in patients receiving anti-αIIbβ3 drugs may be controlled simply by withdrawing infusion of the drug. Due to the relatively short half-life of these compounds,3 the induced platelet defect is rapidly reversed. When urgent bleeding problems occur, platelet transfusion has been shown to reverse the inhibitory effect of abciximab. However, in case of eptifibatide and tirofiban, which are dosed in a way the peak concentration is very high relative to the amount of αIIbβ3 molecules present in circulation, transfused platelets are also inhibited rapidly after infusion by free-circulating drug.3
A novel approach to treat patients with GT during bleeding episodes or surgery is the administration of recombinant factor VIIa (rFVIIa; Novo Nordisk, Bagsværd, Denmark).5,6 rFVIIa was originally developed for the treatment of inhibitor-complicated hemophilia A and B.7,8 Currently, novel indications for rFVIIa, including its use in patients with liver disease,9,10 thrombocytopenia,11 and platelet function defects,6,12 and in patients without coagulation disorders who are bleeding as a result of extensive surgery or major trauma,13,14 are explored in clinical trials. The use of rFVIIa in patients with GT appears to be safe and effective, although randomized controlled clinical trials have not been performed in this small patient group. The apparent success of rFVIIa in GT may possibly be translated to patients who suffer from uncontrollable bleeding as a consequence of administration of anti-αIIbβ3 drugs. A single case in which rFVIIa was used for bleeding management of a patient treated with tirofiban has recently been reported.15
The mechanism of action of rFVIIa in platelet-related bleeding disorders is still a matter of debate. To explain the efficacy of rFVIIa in hemophilia, both tissue factor–dependent and –independent enhancement of thrombin generation has been suggested to play a role.16-19 At first sight, the efficacy of rFVIIa in platelet-related bleeding disorders is curious because of the presence of a fully competent coagulation system in these patients. However, it has been suggested that enhancement of thrombin generation may enhance recruitment of defective platelets to the site of injury as well as enhance fibrin deposition, thereby compensating for the platelet defect.20 Thrombin has multiple actions on platelets, which are, at present, not fully understood. It has been proposed that the GPIb/V/IX complex is an important thrombin receptor on platelets.21,22 Thrombin binding to this complex initiates signaling events by enhancing activation of the classical thrombin receptor (protease activated receptor 1 [PAR-1]),23 and it has been proposed to facilitate the cleavage of GPV from the complex, resulting in a hyperresponsive platelet.24,25 Also, thrombin binding to GPIb appears essential for thrombin-mediated induction of platelet procoagulant activity.22 Furthermore, thrombin is able to activate the low-affinity thrombin receptor (PAR-4),26 but whether binding to the GPIb/V/IX complex also enhances PAR-4 cleavage is not known.
In this study, we have generated a model to study the effect of rFVIIa-mediated thrombin generation on platelet adhesion under flow conditions in a model system using αIIbβ3-inhibited platelets. Using a model system of isolated platelets and red cells, and purified clotting factors, we show an enhancement of platelet adhesion to subendothelial material. Important denominators in thrombin-mediated enhancement of platelet deposition in our system were subsequently investigated.
Materials and methods
Proteins, antibodies, and anti-αIIbβ3drugs
rFVIIa, a goat polyclonal inhibitory antibody against tissue factor, and a monoclonal antibody against FVIIa were generous gifts from Dr U. Hedner (Novo Nordisk, Måløv, Denmark). Factor X was purified from fresh-frozen plasma by immunoaffinity chromatography followed by Q-Sepharose chromatography as previously described27 or purchased from Kordia (Leiden, The Netherlands). Purified prothrombin was purified from fresh-frozen plasma according to Koedam et al.28 Collagen type III was from Sigma (St Louis, MO). Recombinant hirudin was a generous gift from R. Wallis (Ciba Geigy, Horsham, United Kingdom). Recombinant annexin V was a generous gift from Dr W. L. van Heerde (University Medical Centre St Radboud, Nijmegen, the Netherlands).
Inhibitory antibodies against GPIb (AK-2, ascites fluid) and VWF (RAG-35, ascites fluid) were generous gifts from Dr M. Berndt (Baker Institute, Melbourne, Australia) and Dr J. A. van Mourik (Central Laboratory for Blood Transfusion [CLB], Amsterdam, The Netherlands), respectively. Fab fragments of a monoclonal antibody, which specifically inhibits thrombin binding to GPIb (LJIb-10), were a generous gift from Dr Z. M. Ruggeri (The Scripps Research Institute, La Jolla, CA). Fluorescein isothiocyanate (FITC)–labeled goat antimouse IgG was purchased from Calbiochem (La Jolla, CA).
The RGD-containing peptided-arginyl-glycyl-l-aspartyl-l-tryptophane (dRGDW) was generously provided by Dr J. Bouchaudon (Rhône Poulenc Rorer, Chemistry Department, Centre de Recherche de Vitry, Vitry sur Seine, France).
Abciximab was from Centocor (Malvern, PA). Eptifibatide was purchased from COR Therapeutics (South San Francisco, CA). Tirofiban was from Merck (White House Station, NJ).
Cell culture
Human umbilical vein endothelial cells were isolated and grown to confluence as described.29 Cells of the second passage were seeded on gelatin-coated Thermanox coverslips (Nunc, Naperville, IL). The cells were stimulated overnight with phorbol myristate acetate (PMA; Sigma; 20 ng/mL final concentration) for 16 hours. After stimulation, the endothelial cell matrix (ECM) was isolated by removing the cells with 0.1 M NH4OH for 15 minutes at room temperature, and subsequently the matrices were washed 3 times with phosphate-buffered saline (PBS; 10 mM phosphate buffer, 150 mM NaCl, pH 7.4).
Collagen-coated surfaces
Collagen type III was solubilized in 50 mM acetic acid and sprayed on Thermanox or glass coverslips (the latter were used for immunofluorescence studies) using a retouching airbrush (Badger model 100; Badger Brush, Franklin Park, IL) at a density of 30 μg/cm2. After the spraying procedure, coverslips were blocked for 1 hour at room temperature with 4% human albumin in PBS.
Blood collection
Blood was drawn from healthy volunteers who denied ingestion of aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) for the preceding 10 days into one-tenth volume 3.4% sodium citrate. For selected experiments, blood from 6 unrelated patients with type 1 GT was used.
Perfusion studies
Perfusions were carried out in a single-pass perfusion chamber as described previously.30 Perfusions were carried out with reconstituted blood, which was prepared as follows. Platelet-rich plasma (PRP) was prepared from whole blood by centrifugation (10 minutes at 200g at room temperature). The PRP was acidified by addition of one-tenth volume of ACD (2.5% trisodium citrate, 1.5% citric acid, and 2% d-glucose), and the platelets were spun down (500g, 15 minutes). The platelet pellet was resuspended in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)–Tyrode buffer (10 mM HEPES, 137 mM NaCl, 2.68 mM KCl, 0.42 mM NaH2PO4, 1.7 mM MgCl2, 5 mMd-glucose, pH 7.35). Prostacyclin (PGI2, 10 ng/mL) was added to prevent platelet activation during the subsequent washing step. Platelets were spun down and resuspended in a small volume of HEPES-Tyrode buffer. The platelets were diluted in human albumin solution (HAS; 4% human albumin, 4 mM KCl, 124 mM NaCl, 20 mM NaHCO3, 2 mM Na2SO4, 1.5 mM MgCl2, 5 mMd-glucose, pH 7.35). Red cells were washed twice with 0.9% NaCl containing 5 mM d-glucose (2000g, 5 minutes), and finally cells were packed (2000g, 15 minutes).
Platelets were mixed with red cells to obtain reconstituted blood containing 200 000 platelets/μL and a hematocrit of 40%. The reconstituted blood was preincubated with buffer or clotting factors for 5 minutes at 37°C and perfused for 5 minutes at a shear rate of 1600 s−1 After perfusion, slides were washed with HEPES buffer (10 mM HEPES,150 mM NaCl, pH 7.35) and fixed in 0.5% glutaraldehyde in PBS. Subsequently, slides were dehydrated in methanol and stained with May-Grünwald and Giemsa as described previously.31 Platelet adhesion was evaluated using computer-assisted analysis with OPTIMAS 6.0 software (Dutch Vision Systems [DVS], Breda, The Netherlands), and was expressed as the percentage of the surface covered with platelets.
Immunofluorescence microscopy
To investigate direct binding of rFVIIa to platelets adhered under flow conditions, washed platelets and red cells were perfused over a collagen-coated surface (5 minutes at a shear rate of 1600 s−1) in the presence and absence of rFVIIa (1.2 μg/mL) and calcium chloride (5 mM). After perfusion, the coverslips were washed with HEPES buffer containing 5 mM calcium chloride and fixed with 3% paraformaldehyde and 0.002% glutardialdehyde in PBS. Subsequently, coverslips were washed with PBS and blocked with 1% bovine serum albumin (BSA), 0.1% glycine in PBS for 10 minutes at room temperature. Coverslips were incubated with a monoclonal anti-FVII antibody (10 μg/mL in PBS) for 45 minutes at 37°C. After washing and subsequent blocking, coverslips were incubated with FITC-labeled goat antimouse IgG (1:20 diluted in PBS) for 45 minutes at 37°C. After washing, coverslips were mounted in Mowiol 40-80 containing 0.1% paraphenylenediamine. Bound rFVIIa was visualized using confocal laser scanning microscopy using Leica TCS 4D (Heidelberg, Germany) equipment.
Statistical analysis
Statistical analysis was performed using the GraphPad InStat (San Diego, CA) software package. Comparison of mean surface coverage values was undertaken by standard Student t test.P < .05 was considered statistically significant.
Results
Enhancement of deposition of αIIbβ3-deficient platelets to PMA-ECM on addition of coagulation factors VIIa, X, and II
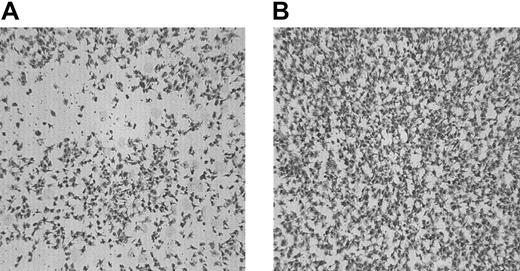
Washed platelets and red cells obtained from 6 unrelated patients with type 1 GT were perfused over PMA-stimulated endothelial cell matrix (PMA-ECM) at 1600 s−1 for 5 minutes. In Figure1A, the morphologic appearance of a typical experiment performed with GT platelets to PMA-ECM is presented. The surface coverage was 37.5% ± 5.0%. (mean ± SEM of 16 coverslips; with 4 patients experiments were performed in triplicate; with the other 2 patients, experiments were performed in duplicate). No platelet aggregates were observed. On addition of a thrombin-generating system, consisting of rFVIIa (1.2 μg/mL), factor X (10 μg/mL), and prothrombin (20 ng/mL), a significant increase in platelet adhesion was observed (67.0% ± 4.3%; mean ± SEM of 16 coverslips,P < .0001; Figure 1B shows a typical example of the morphology). Platelet aggregates were still not formed.
The effect of coagulation factors VIIa, X, and II on adhesion of platelets from patients with type 1 GT to the subendothelial matrix of PMA-stimulated endothelial cells under flow conditions.
Washed platelets and red cells (200 000 platelets/μL; hematocrit 40%) were perfused over PMA-ECM for 5 minutes at 1600 s−1in absence (A) or presence (B) of a thrombin-generating system (1.2 μg/mL rFVIIa, 10 μg factor X, 20 ng/mL prothrombin, 3 mM CaCl2). Shown is a typical example from one patient with GT. Original magnification × 400 for panels A-B.
The effect of coagulation factors VIIa, X, and II on adhesion of platelets from patients with type 1 GT to the subendothelial matrix of PMA-stimulated endothelial cells under flow conditions.
Washed platelets and red cells (200 000 platelets/μL; hematocrit 40%) were perfused over PMA-ECM for 5 minutes at 1600 s−1in absence (A) or presence (B) of a thrombin-generating system (1.2 μg/mL rFVIIa, 10 μg factor X, 20 ng/mL prothrombin, 3 mM CaCl2). Shown is a typical example from one patient with GT. Original magnification × 400 for panels A-B.
Enhancement of platelet deposition to PMA-ECM in a model for GT on addition of coagulation factors VIIa, X, and II
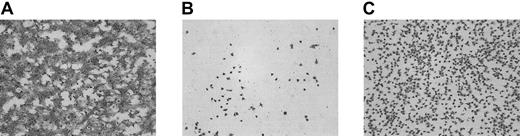
To study the mechanism by which addition of coagulation factors VIIa, X, and II lead to a significant increase in adhesion of αIIbβ3-deficient platelets to PMA-ECM, a model system for GT was created. Perfusion experiments were performed with platelets from healthy volunteers pretreated with a peptide containing the RGD sequence to block ligand binding to αIIbβ3. In the absence of the RGD-containing peptide, perfusion of washed platelets and red cells over PMA-ECM at 1600 s−1 resulted in a surface coverage of 69.2% ± 3.0% (mean ± SEM of 6 coverslips), and extensive aggregate formation was observed (Figure2A). Addition of the dRGDW peptide (200 μM, final concentration in platelet suspension) resulted in an extensive reduction in platelet adhesion (7.4% ± 2.1%; mean ± SEM of 18 coverslips), and aggregate formation was completely absent (Figure 2B).On addition of purified coagulation factors VIIa, X, and II to αIIbβ3-inhibited platelets, a significant enhancement of platelet adhesion was observed (48.2% ± 2.9%; mean ± SEM of 18 coverslips;P < .0001; Figure 2C). When only rFVIIa was added to the perfusate, no increase in platelet adhesion was observed (data not shown). The morphologic appearance of GT platelets adhered to PMA-ECM was similar to that observed in the dRGDW model, although the surface coverage observed with GT platelets was slightly higher than that of dRGDW-inhibited normal platelets.
Development of a model system for adhesion of thrombasthenic platelets to subendothelial structures under flow conditions and the effect of the thrombin-generating system.
Washed normal platelets and red cells (200 000 platelets/μL; hematocrit 40%) were perfused over PMA-ECM for 5 minutes at 1600 s−1 in the absence (A) or presence (B-C) of an RGD-containing peptide (rRGDW; 200 μM). Panel C shows the effect of the thrombin-generating system (1.2 μg/mL rFVIIa, 10 μg factor X, 20 ng/mL prothrombin, 3 mM CaCl2) on adhesion of RGD-inhibited platelets. Original magnification × 400 for panels A-C.
Development of a model system for adhesion of thrombasthenic platelets to subendothelial structures under flow conditions and the effect of the thrombin-generating system.
Washed normal platelets and red cells (200 000 platelets/μL; hematocrit 40%) were perfused over PMA-ECM for 5 minutes at 1600 s−1 in the absence (A) or presence (B-C) of an RGD-containing peptide (rRGDW; 200 μM). Panel C shows the effect of the thrombin-generating system (1.2 μg/mL rFVIIa, 10 μg factor X, 20 ng/mL prothrombin, 3 mM CaCl2) on adhesion of RGD-inhibited platelets. Original magnification × 400 for panels A-C.
Enhancement of deposition of platelets treated with αIIbβ3-blocking drugs to PMA-ECM on addition of coagulation factors VIIa, X, and II
When normal platelets were pretreated with the registered anti-αIIbβ3 drugs abciximab (10 μg/mL), eptifibatide (10 μg/mL), and tirofiban (1 μg/mL), a similar morphologic pattern of adhesion was seen compared to the studies using dRGDW to block αIIbβ3. As shown in Table1, a low adhesion was observed when platelets treated with an anti-αIIbβ3 drug were perfused over PMA-ECM. On addition of the thrombin-generating system, platelet deposition was significantly enhanced.
The generation of thrombin independently of tissue factor results in enhancement of platelet deposition in the GT model
When hirudin (5 U/mL) was added to a dRGWD-inhibited platelet suspension together with the thrombin-generating system, the increase in platelet deposition to PMA-ECM induced by the thrombin-generating system was completely abolished (Table2). Similarly, addition of annexin V (50 μg/mL) to dRGDW-inhibited platelets completely abrogated the enhancement in adhesion induced by the thrombin-generating system. However, when all tissue factor activity in the system was neutralized by an inhibitory polyclonal antibody (500 μg/mL in PBS; both the PMA-ECM as well as the platelet suspension were treated for 45 minutes at room temperature), addition of the thrombin-generating system still enhanced platelet deposition to the same extent as in the absence of tissue factor blockade (Table 2). The inhibitory capacity of the antibody was demonstrated using a standard prothrombin time assay and by a factor Xa generation assay on PMA-ECM. Preincubation of the tissue factor source with the antibody prolonged the prothrombin time from 12 to over 200 seconds, and preincubation of PMA-ECM with the antibody completely abolished rFVIIa-induced Xa generation in a static assay.
Thrombin-mediated enhancement of platelet deposition in the GT model is dependent on the GPIb-VWF interaction and on thrombin binding to GPIb
When the platelets were pretreated with an inhibitory antibody against either GPIb or VWF (45 minutes at room temperature; both antibodies were used in a dilution of 1:250), the enhancement in adhesion induced by the thrombin-generating system was completely inhibited (Table 3).
If platelets are pretreated with Fab fragments of an antibody that specifically blocks the binding of thrombin to GPIb (LJIb-10; 50 μg/mL), the enhancement of platelet adhesion induced by the thrombin-generating system was completely abolished (Table 3). The antibody did not affect platelet adhesion to purified VWF (not shown).
Tissue factor–independent thrombin generation enhances platelet deposition to collagen type III on αIIbβ3blockade
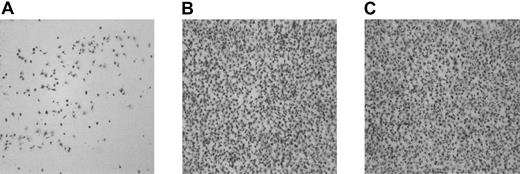
To confirm that rFVIIa is able to generate thrombin independently of tissue factor in our model, experiments were performed using a tissue factor–poor adhesive surface. Platelet adhesion to collagen (type III) in presence of dRGDW was low (6.4% ± 2.0%, mean ± SEM of 12 coverslips), presumably due to the absence of VWF in the perfusate, which is necessary for reduction of the velocity of the platelets. Aggregate formation was completely absent (Figure3A). On addition of the thrombin-generating system to αIIbβ3-inhibited platelets, the adhesion to collagen was significantly increased (57.0% ± 1.4%, mean ± SEM of 12 coverslips; P < .001; Figure 3B). Pretreatment of both the surface and the platelet suspension with anti–tissue factor IgG (500 μg/mL) did not abolish the enhanced platelet deposition induced by the thrombin-generating system (54.7% ± 1.3%, mean ± SEM of 12 coverslips; Figure3C), confirming that the thrombin generation also occurred independently of tissue factor.
Adhesion of αIIbβ3-inhibited platelets to collagen type III under flow conditions; increased adhesion by the thrombin-generating system is independent of tissue factor.
Washed normal platelets and red cells (200 000 platelets/μL; hematocrit 40%) were perfused over collagen type III for 5 minutes at 1600 s−1 in the presence of an RGD-containing peptide (rRGDW; 200 μM) in the absence (A) or presence (B-C) of the thrombin-generating system (1.2 μg/mL rFVIIa, 10 μg factor X, 20 ng/mL prothrombin, 3 mM CaCl2). In panel C both the surface and the platelet suspension were preincubated with inhibitory tissue factor IgG. Original magnification × 400 for panels A-C.
Adhesion of αIIbβ3-inhibited platelets to collagen type III under flow conditions; increased adhesion by the thrombin-generating system is independent of tissue factor.
Washed normal platelets and red cells (200 000 platelets/μL; hematocrit 40%) were perfused over collagen type III for 5 minutes at 1600 s−1 in the presence of an RGD-containing peptide (rRGDW; 200 μM) in the absence (A) or presence (B-C) of the thrombin-generating system (1.2 μg/mL rFVIIa, 10 μg factor X, 20 ng/mL prothrombin, 3 mM CaCl2). In panel C both the surface and the platelet suspension were preincubated with inhibitory tissue factor IgG. Original magnification × 400 for panels A-C.
rFVIIa binds to collagen-adhered platelets under flow conditions
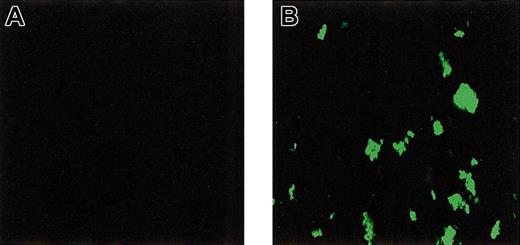
To investigate direct binding of rFVIIa to platelets adhered and activated under flow conditions, perfusion experiments were performed in which washed platelets and red cells were perfused over a collagen-coated surface in the presence and absence of rFVIIa and calcium chloride. After perfusion, platelets were fixed and bound rFVIIa was visualized using immunofluorescence. As shown in Figure4, intense staining for rFVIIa was observed. No staining was observed when rFVIIa or the primary antibody (not shown) was omitted. Also, no fluorescence was observed when annexin V or EDTA (ethylenediaminetetraacetic acid) was added to the perfusate (data not shown). Addition of an inhibitory antibody against tissue factor did not affect the signal (not shown).
Binding of rFVIIa to collagen-adhered platelets under flow conditions.
Washed platelets and red cells were perfused over a collagen-coated glass coverslip in the absence (A) or presence (B) of rFVIIa (1.2 μg/mL). After perfusion, coverslips were fixed and bound rFVIIa was visualized by confocal laser scanning fluorescence microscopy using a monoclonal antibody against factor VII followed by a FITC-labeled secondary antibody. Original magnification × 400 for panels A-B.
Binding of rFVIIa to collagen-adhered platelets under flow conditions.
Washed platelets and red cells were perfused over a collagen-coated glass coverslip in the absence (A) or presence (B) of rFVIIa (1.2 μg/mL). After perfusion, coverslips were fixed and bound rFVIIa was visualized by confocal laser scanning fluorescence microscopy using a monoclonal antibody against factor VII followed by a FITC-labeled secondary antibody. Original magnification × 400 for panels A-B.
Tissue factor–independent thrombin generation is associated with the release GPV from the platelet surface
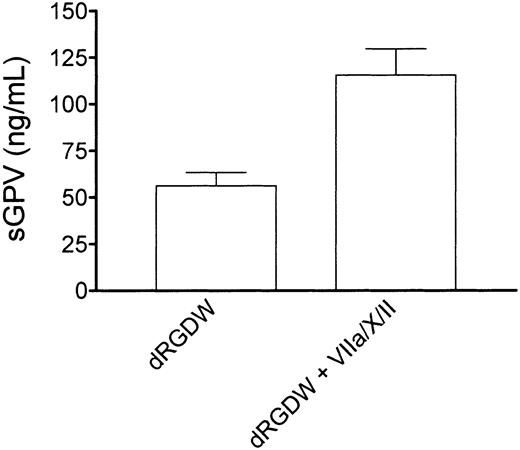
Thrombin binding to GPIb has been proposed to facilitate the release of a soluble fragment of GPV from the platelet surface. To investigate whether thrombin generation in our system also results in the release of GPV, we performed perfusion experiments with washed platelets and red cells over a collagen-coated surface in the presence of RGD and in the presence or absence of purified factors VIIa, X, and II. The perfusate was collected in 50 mM EDTA (1:10, vol/vol), cells were removed by centrifugation, and soluble GPV was measured in the supernatant by enzyme-linked immunosorbent assay (ELISA; asserachrom-soluble GPV, Diagnostica Stago, Asnieres, France). On addition of the thrombin-generating system, a significant increase in soluble GPV was found in the supernatant as shown in Figure5 (control, 56 ± 7 ng/mL, mean ± SEM of 4 independent experiments in duplicate, in presence of rFVIIa/X/II 116 ± 14 ng/mL; P = .002).
Tissue factor–independent thrombin generation is associated with release of a soluble fragment of GPV from the platelet surface.
Washed normal platelets and red cells (200 000 platelets/μL; hematocrit 40%) were perfused over collagen type III for 5 minutes at 1600 s−1 in presence of an RGD-containing peptide (dRGDW; 200 μM) in the absence or presence of the thrombin-generating system (1.2 μg/mL rFVIIa, 10 μg factor X, 20 ng/mL prothrombin, 3 mM CaCl2). The perfusate was collected in EDTA (1:10, vol/vol), cells were removed by centrifugation, and soluble GPV was measured in the supernatant. Shown is the mean of 4 independent experiments performed in duplicate. Error bars indicate SEM.
Tissue factor–independent thrombin generation is associated with release of a soluble fragment of GPV from the platelet surface.
Washed normal platelets and red cells (200 000 platelets/μL; hematocrit 40%) were perfused over collagen type III for 5 minutes at 1600 s−1 in presence of an RGD-containing peptide (dRGDW; 200 μM) in the absence or presence of the thrombin-generating system (1.2 μg/mL rFVIIa, 10 μg factor X, 20 ng/mL prothrombin, 3 mM CaCl2). The perfusate was collected in EDTA (1:10, vol/vol), cells were removed by centrifugation, and soluble GPV was measured in the supernatant. Shown is the mean of 4 independent experiments performed in duplicate. Error bars indicate SEM.
Discussion
This study shows that rFVIIa-mediated thrombin generation profoundly enhances deposition of platelets with a congenital or drug-induced defect in the integrin αIIbβ3to the extracellular matrix of cultured human umbilical vein endothelial cells and to purified collagen type III. Because the enhancement in platelet deposition by the thrombin-generating system (coagulation factors VIIa, X and II) seems independent of tissue factor, but dependent on the presence of procoagulant surface, we propose a model in which thrombin is generated via activation of factor X by rFVIIa bound to adhered platelets. The generated factor Xa subsequently assembles into a prothrombinase complex, of which the factor Va in our assay setup is presumably secreted by already adhered platelets. Finally, the thrombin generated in situ binds to GPIb on platelets, which are either in suspension or rolling on the surface, resulting in platelet activation. This thrombin-mediated activation of platelets somehow results in enhanced platelet deposition. The exact mechanism that is responsible for the thrombin-induced enhancement of platelet deposition is at present not clear, but might involve PAR-1–mediated activation or cleavage of GPV from the GPIb/V/IX complex.24
A tissue factor–independent enhancement of thrombin generation by rFVIIa involving binding to activated platelets or monocytes has been proposed to explain efficacy of rFVIIa in hemophilia as well as other hemostatic disorders in which rFVIIa has been shown beneficial.19,20,32 It has also been proposed that rFVIIa exerts its hemostatic effect in a tissue factor–dependent manner, possibly involving competition or rFVIIa with zymogen plasma factor VII for tissue factor.16 18 Whether a tissue factor–dependent or –independent mechanism applies in vivo is currently debated in the literature.
In this study we now show that rFVIIa is able to generate thrombin on platelets adhered to a collagen surface under flow conditions without requirement for tissue factor, by binding directly to the platelet surface, supporting the tissue factor–independent mechanism of rFVIIa as suggested by Monroe et al.19 A mechanistic explanation for the efficacy of rFVIIa in patients with GT is still lacking. Our study suggests that a local enhancement of thrombin generation, either in a tissue factor–dependent or –independent manner, is able to increase adhesion of αIIbβ3-deficient platelets. Although enhancement of adhesion still does not result in a stable platelet plug, due to the absence of αIIbβ3-mediated platelet-platelet interaction, it does provide an increase of procoagulant surface at the site of injury. The increased procoagulant surface facilitates a further enhancement of thrombin generation and subsequent fibrin formation. This enhancement in fibrin deposition might compensate for the lack of aggregate formation.
The assay setup used in this study features some simplifications that require explanation. First, in this study we compare adhesion of αIIbβ3-deficient platelets in the absence or presence of rFVIIa-mediated thrombin generation. To translate this experiment into the in vivo situation, one must realize that if a patient with αIIbβ3 deficiency receives rFVIIa, an increase in thrombin generation at the site of injury will occur (in contrast to the on/off situation in our experimental setup) and that the increase in adhesion is probably more subtle compared to the herein shown in vitro experiments. Second, our thrombin-generating system is simplified because it does not contain inhibitors of thrombin generation (tissue factor pathway inhibitor [TFPI], antithrombin, and the protein C system), and factors VIII, IX, and XI are lacking. Due to this simplification, dose-response experiments with rFVIIa will not provide physiologically relevant information. Finally, because no fibrinogen was added to the reconstituted blood, the interplay between platelet deposition and fibrin formation was not studied. Studying fibrin formation to a tissue factor–rich surface is accompanied by a number of technical difficulties. In case of perfusion of recalcified blood, thrombin generation is already initiated before perfusion by activation of the contact activation system. Moreover, fibrin deposition does not halt when the blood has left the tissue factor–rich surface, resulting in obstruction of the perfusion chamber with macroscopic clots. Using low-molecular-weight heparin-anticoagulated blood, which is commonly used to study fibrin deposition under flow conditions,33endogenous factor VII is partially activated by factor XIIa generated by the contact system, which is not blocked by low-molecular-weight heparin (T.L., unpublished observations, January 2001), resulting in an underestimation of the effect of exogenous added rFVIIa. However, Galan et al34 recently reported improvement of both platelet deposition and fibrin formation under flow conditions using low-molecular-weight heparin–anticoagulated blood from patients with GT, which corresponds to the hypothesized mechanism of action put forward in our current study.
In conclusion, in this study we show that a tissue factor–independent generation of thrombin via rFVIIa profoundly increases adhesion of αIIbβ3-deficient platelets under flow conditions in vitro. We hypothesize that administration of rFVIIa to patients with GT results in an increased platelet adhesion at the site of injury, followed by an enhanced deposition of fibrin. The increased fibrin formation might compensate for the lack of aggregation and therefore explain the therapeutic efficacy of rFVIIa in GT. We speculate that rFVIIa may also be an effective therapeutic agent for patients who are bleeding as a consequence of treatment with anti-αIIbβ3 drugs, with a mechanism of action comparable to that proposed here for patients with GT.
The authors would like to thank Dr U. Hedner for the generous gifts of rFVIIa and the antibodies against TF and VIIa. We would like to thank Dr R. Wallis for the generous gift of recombinant hirudin, and Dr W. L. van Heerde for annexin V. Dr M Berndt, Dr J. A. van Mourik, and Dr Z. M. Ruggeri are gratefully acknowledged for their gifts of antibodies against GPIb, VWF, and the thrombin binding site on GPIb, respectively. We thank Dr J. Bouchaudon for the gift of dRGDW. Finally we are grateful to Dr M. Peters (Academic Medical Centre Amsterdam, The Netherlands), and Dr M .C. Kappers-Klunne (University Hospital Dijkzigt, Rotterdam, The Netherlands) for their kind assistance with the patient studies.
Prepublished online as Blood First Edition Paper, October 31, 2002; DOI 10.1182/blood-2002-09-2761.
Supported in part by an unrestricted educational grant from Novo Nordisk (T.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ton Lisman, Thrombosis and Haemostasis Laboratory, Department of Haematology G.03.647, University Medical Centre, PO Box 85500, 3508 GA Utrecht, The Netherlands; e-mail:j.a.lisman@lab.azu.nl.