Important gene therapy target cells such as resting human T cells are refractory to transduction with lentiviral vectors. Completion of reverse transcription, nuclear import, and subsequent integration of the lentiviral genome occur in these cells only if they have been activated. In T-cell–based gene therapy trials performed to date, cells have been activated via their cognate antigen receptor. To couple activation with gene transfer, we previously generated lentiviral vectors displaying an anti-CD3 scFv fragment that allowed up to 48% transduction of freshly isolated T cells. However, transduction of highly purified resting T cells with these anti-CD3–displaying lentiviral vectors was inefficient and shifted the T cells from the naive to the memory phenotype. Here, we describe interleukin-7 (IL-7)–displaying HIV-1–derived vectors. Like recombinant IL-7, these modified particles could promote the survival of primary T cells placed in culture without inducing a naive-to-memory phenotypic switch. Furthermore, a single exposure to the IL-7–displaying vectors resulted in efficient gene transfer in both resting memory adult T cells and naive cord blood T cells. With adult naive T cells, preactivation with recombinant IL-7 was necessary for efficient gene transfer. Altogether, these results suggest that IL-7–displaying vectors could constitute interesting tools for T-cell–targeted gene therapy.
Introduction
Transfer of genes into T cells is a crucial step in the development of therapeutic strategies for diseases such as cancers and acquired immunodeficiency syndrome.1 The population of mature adult T cells can be divided into 2 different subsets, namely memory and naive T cells. Naive T cells are especially important as gene therapy target cells since they maintain the capacity to respond to novel antigens. It is also of utmost importance that the responses of T cells to antigens are not dramatically altered by the gene transfer protocol. It is now generally accepted that resting T cells, which make up most of the circulating T-cell pool in vivo, cannot be transduced by lentiviral vectors despite improvements brought into their vector structure that allow optimized gene transfer in other cell types.2-4 That the parental virus, HIV-1, can enter into resting T lymphocytes but does not replicate5-9 has been attributed to multiple postentry blocks. These include, in particular, defects in initiation and completion of the reverse-transcription process,10 lack of adenosine triphosphate–dependent nuclear import, and lack of integration of the proviral genome.8,9,11 We and others have reported that inducing cell-cycle entry into G1b, via stimulation through the T-cell receptor (TCR), allows efficient transduction of adult naive T cells by HIV-1 or HIV-1–based vectors.12,13 However, the transduced naive T cells switch to the memory phenotype upon TCR stimulation. In this regard, it is interesting to note that exposure of adult T cells to cytokines such as interleukin-2 (IL-2), IL-15, and IL-7 renders them permissive to lentiviral infection.14 IL-7 is an especially appealing candidate because it appears to function as a master regulator of T-cell survival and homeostatic proliferation.15-17 Moreover, it has been reported that IL-7 administration after allogenic bone marrow transplantation improves immune reconstitution in a mouse model.18 Thus, IL-7 may have the potential to stimulate lymphocytes under conditions of bone marrow transplantation, high-dose chemotherapy, and acquired immunodeficiency syndrome.
IL-7 is a 25-kDa glycoprotein that interacts with the IL-7 receptor heterodimer, composed of a common γ-chain that is shared with the IL-2, IL-4, IL-9, IL-15, and IL-21 receptors and a unique α-chain (CD127).19,20 The IL-7 receptor is present on developing thymocytes, as well as on mature CD4+ and CD8+ naive and memory T cells.21 IL-7 induces the proliferation of thymocytes and of umbilical cord blood (CB) T lymphocytes, largely composed of recent thymic emigrants, in the absence of any other stimulus.22,23,29 In contrast to naive CB T cells, long-term resident naive T cells in adults do not proliferate in vitro in response to IL-7.22,23Importantly, IL-7 treatment does not induce T cells to switch from a naive to a memory phenotype24,25 and triggers only a slight up-regulation of activation markers such as CD25, CD98, CD71, CD11a, and CD40 ligand in vitro.26 Thus, this cytokine does not appear to induce the massive changes in phenotype observed in cells stimulated through the TCR. Furthermore, IL-7 can render resting T cells permissive to HIV and HIV-derived lentiviral vectors.14,23 29
Here, we coupled lentiviral transduction to IL-7 stimulation by displaying IL-7 on the surface of lentiviral vectors. Using these modified lentiviral vectors, we were able to induce survival of both naive and memory T cells in vitro. Moreover, these vectors allowed efficient transduction of up to 48% of naive CB CD4+ T cells, and of up to 41% of adult memory CD4+ T cells, in the absence of any other exogenous stimulus. Notably, these transduction levels were equivalent to those obtained in the presence of recombinant (r)IL-7. In contrast, significant transduction of adult naive CD4+ T cells was only achieved following preactivation with this cytokine.
Materials and methods
T-cell isolation
Umbilical cord blood (CB) and adult peripheral blood (APB) were collected in ACD-containing tubes. CD4+T cells were isolated from fresh blood using the Rosette system (StemCell Technologies, Meylan, France) according to the manufacturer's protocol. Resting CD4+ T cells were then purified by negative selection using anti–HLA-DR and anti-CD69 monoclonal antibodies (mAbs) in combination with anti-CD45RA or anti-CD45RO mAbs to recover memory and naive T cells, respectively. Cells were separated following addition of anti–mouse IgG–conjugated magnetic beads (Dynal, Compiégne, France) and depletion of antibody-bound cells. Purities were monitored by staining with anti-CD4, anti-CD3, anti-CD69, anti–HLA-DR, anti-CD45RO, and anti-CD45RA mAbs and ranged from 97% to 99% with less than 0.5% CD69 expression and less than 0.5% HLA-DR expression. The isolated T cells were resuspended in RPMI-1640 medium suplemented with 10% fetal calf serum (FCS). In experiments performed with total peripheral blood lymphocytes (PBLs), peripheral blood mononuclear cells (PBMCs) were separated from adult blood using a Ficoll-Hypaque gradient, and lymphocytes were enriched by overnight plastic adherence. All isolated T cells were cultured in RPMI-1640 medium supplemented with 10% FCS.
Envelope construction
The IL-7 cDNA (456 base pair [bp]) was obtained by polymerase chain reaction (PCR) using the primers: 5′IL-7–SfiI: GGCCCAGCCGGCCATGGCCGATTGTGATATTGAAGGTAAAGATG and 3′IL-7NotI: GCGGCCGCGTGTTCTTTAGTGCCCATCAAAATTTT, which encode for the SfiI and NotI sites at the 5′ and 3′ ends, respectively. The SfiI/NotI PCR fragment was fused in the 4070A (amphotropic) murine leukemia virus (MLV)env gene at position + 1 of the surface (SU) subunit using the SfiI/NotI backbone fragment of CMVOKT3SU.13 The resulting chimeric glycoprotein is termed IL7SU. The SUx mutation,27 that inhibits furin-mediated cleavage of the MLV glycoprotein in the virus-producer cells, was inserted in the IL7SU construct, thus resulting in a second chimera, named IL7SUx. Both the IL7SU and IL7SUx chimeras were expressed in the phCMV-G expression vector backbone.28 Both the OKT3SU and IL-2SU chimeric glycoproteins are described elsewhere.13 27
Production of retroviral vectors
Pseudotyped HIV-1–derived vectors were generated as previously described13 by transient transfection of 293T cells.
Transduction assays
To determine transduction efficiency and infectious titers of HIV-1–derived vectors, serial dilutions of vector preparations were added to the 293T cells. The infectious titers are expressed as 293T transducing units (TU/mL). To infect human CB or APB T cells, viral supernatants containing 1 × 107 to 2.5 × 107 TU were added to 1 × 106 lymphocytes resuspended in 0.5 mL of RPMI with 10% FCS in 24-well plates. Multiplicities of infection (MOIs) were determined on proliferating 293T cells and are indicated in all lymphocyte transduction experiments. Single transductions were maintained for 3 or 7 days in culture as indicated. Double transductions consisted of one transduction round of 3 days, followed by removal of viral supernatant, a single-wash step with RPMI/10% FCS. Fresh viral supernatant and rIL-7 (where indicated) were added for an additional 4 days and transduction efficiency was then determined by flow cytometry. Under conditions of preactivation, T cells were cultured for 3 or 6 (for adult naive T cells) days in the presence of 15 ng/mL rIL-7. For costimulation of T cells, anti-CD3 and anti-CD28 monoclonal antibodies were used at 1 μg/mL.
Cell-surface staining
Staining for CD25, CD69, CD71, and HLA-DR activation markers was performed using phycoerythrin (PE)–conjugated antibodies (1:25 dilution; BD Pharmingen, Le Pont-de-Claix, France) in order to simultaneously visualize cell-surface marker and enhanced green fluorescent protein (EGFP) expression by fluorescence-activated cell-sorter (FACS) analysis. Staining for T-cell subpopulations was performed with PE-coupled anti-CD3, anti-CD4, anti-CD8, anti-CD45RA, and anti-CD45R0 mAbs at a 1:25 dilution (BD Pharmingen). A PE-conjugated antibody against the α-subunit of the IL-7 receptor (CD127; Immunotech, Marseille, France) was used to detect expression of the high-affinity IL-7 receptor α-subunit. Expression was monitored on a FACScalibur (Becton Dickinson, Le Pont-de- Claix, France).
Intracellular cytokine staining
Following infection, cells were incubated for 5 hours in RPMI, 10% FCS in the presence of either Brefaldin A alone or a combination of PMA (4-α-phorbol 12-myristate 13-acetate), ionomycin, and Brefeldin A. Anti–IL-2–PE, anti–IL-4–PE, anti–IFNγ-PE, and isotype-matched controls were purchased from BD Pharmingen. For cytokine staining, cells were treated according to BD Pharmingen's instructions.
Cell-cycle analysis
Cell-cycle analysis was performed by staining DNA and RNA with 7-amino-actinomycin-d (7AAD; Sigma, St Quentin-Fallavier, France) and pyronin Y, respectively. A total of 5 × 105cells were labeled with 7AAD at a final concentration of 20 μM for 30 minutes at room temperature followed by 5 μM pyronin Y (Sigma) for 10 minutes on ice. Cells were immediately analyzed on a FACScalibur flow cytometer.
Results
OKT3-displaying lentiviral vectors fail to efficiently transduce highly purified resting T cells and do modify their naive phenotype
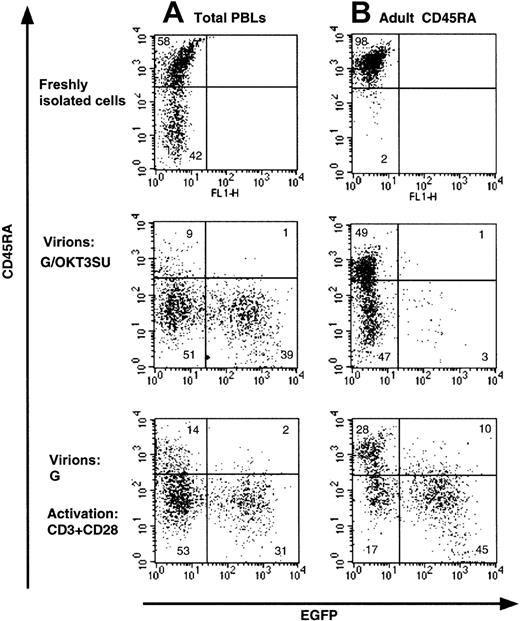
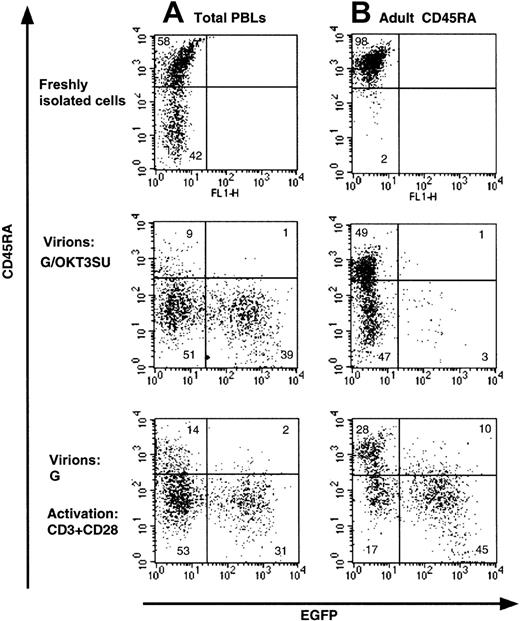
Infection of quiescent T cells by wild-type HIV-1 and HIV-1–derived vectors is blocked before completion of reverse transcription.9,12 Recently, we were able to overcome this block by exposing peripheral blood lymphocytes (PBLs) to lentiviral vectors displaying an anti-CD3 scFv fragment,13derived from the monoclonal antibody OKT3 (G/OKT3SU in Figure1). While high transduction levels were achieved using these vectors, we now demonstrate that the phenotype of the T cells was modified (Figure 1A). Specifically, we found that there was a near complete loss of the CD45RA isoform on naive T cells that was accompanied by an acquisition of the CD45RO memory marker (Figure1A and data not shown). Moreover, under conditions in which resting naive T-cell populations were purified (> 97% CD45RA+), transduction by the G/OKT3SU pseudotyped vectors was almost completely abrogated despite a similar change in phenotype as observed for nonpurified T cells (PBL in Figure 1B). The low transduction efficiency in the resting naive T-cell population, compared with the high transduction observed in T cells maintained in the context of total lymphocytes, was likely due to the depletion of antigen-presenting cells (APCs) in the former population. These APCs provide a costimulatory signal to CD3 engagement. This is emphasized by our findings that anti-CD3–displaying lentiviral vectors promoted cell-cycle entry in nonpurified PBL samples but not in purified resting T cells (not shown). Furthermore, resting T lymphocytes were efficiently transduced following costimulation with anti-CD3 and anti-CD28 mAbs, yet the T-cell population showed a similar loss of naive phenotype (Figure 1B). Thus, anti-CD3–displaying vectors allow T cells to be transduced without any prestimulation under conditions in which antigen-presenting cells are present. Nevertheless, these vectors may not be optimal for some T-cell–based gene therapy applications, as they require the presence of APCs for efficient transduction and result in a shift of T-cell phenotype.
Loss of naive phenotype after transduction of T cells with HIV-1–derived vectors displaying a CD3/TCR-stimulating ligand.
(A) Freshly isolated total human peripheral blood lymphocytes (PBLs) or (B) highly purified naive adult CD4+ T cells (> 98% CD45RA+) were transduced with anti-CD3 scFv-displaying lentiviral vectors pseudotyped with VSV-G (G/OKT3SU) in the absence of other exogenous factors using MOIs of 10 to 15. Control PBLs and naive T cells were transduced with VSV-G–pseudotyped lentiviral vectors (G) in the presence of soluble anti-CD3 and anti-CD28 monoclonal antibodies (1 μg/mL). The percentage of EGFP+ and naive T cells (CD45RA+) was determined 6 days after transduction by FACS analysis. The results are representative of 5 independent experiments.
Loss of naive phenotype after transduction of T cells with HIV-1–derived vectors displaying a CD3/TCR-stimulating ligand.
(A) Freshly isolated total human peripheral blood lymphocytes (PBLs) or (B) highly purified naive adult CD4+ T cells (> 98% CD45RA+) were transduced with anti-CD3 scFv-displaying lentiviral vectors pseudotyped with VSV-G (G/OKT3SU) in the absence of other exogenous factors using MOIs of 10 to 15. Control PBLs and naive T cells were transduced with VSV-G–pseudotyped lentiviral vectors (G) in the presence of soluble anti-CD3 and anti-CD28 monoclonal antibodies (1 μg/mL). The percentage of EGFP+ and naive T cells (CD45RA+) was determined 6 days after transduction by FACS analysis. The results are representative of 5 independent experiments.
Lentiviral vectors displaying human IL-7 mimic the functional properties of recombinant IL-7
The data presented above suggest that under certain conditions it is desirable to transduce resting T cells in the absence of TCR engagement. In this regard, IL-7 is a promising candidate because (1) it maintains the maturational state of T cells25; (2) it promotes long-term T-cell survival in vitro22; and (3) it can induce a state of HIV permissiveness.14,23,29 We therefore generated lentiviral vectors displaying IL-7 on their surface to assess whether these viral particles could be used to transduce T cells in the absence of any other stimulus. The human IL-7 gene was fused to the amino-terminus of the SU subunit of the MLV envelope glycoprotein, resulting in the expression of an IL7SU chimeric glycoprotein. The position of insertion of the IL-7 gene was chosen to allow optimal display of the cytokine on HIV-1–derived vectors.27 To avoid partial dissociation of IL-7 from the viral surface, an IL7SUx chimera was constructed in which the cleavage site between the SU and transmembrane (TM) envelope subunits was inactivated. Since lentiviral vectors displaying the IL7SU or IL7SUx chimeric glycoproteins demonstrated only reduced infectivity, in all experiments described below, they were generated with both the chimeric glycoproteins and vesicular stomatitis virus G (VSV-G).
To determine whether human IL-7 displayed in the context of IL7SU or IL7SUx chimeric glycoprotein–pseudotyped lentiviral vectors could interact with the IL-7 receptor, we first monitored the down-regulation of the high-affinity IL-7 receptor α subunit (CD127). These experiments were performed on naive as well as memory CD4+T cells, as their characteristics are known to be distinct.22,25 Additionally, because IL-7 has distinct biologic effects on naive T cells isolated from APB and umbilical cord blood (CB),22 experiments were also performed on naive CD4+ CB T cells. CD4+subsets were isolated by negative selection and activated T cells were eliminated with anti-CD69 and anti–HLA-DR antibodies. Most, if not all of the cells of the 3 T-cell subsets, expressed CD127 (supplemental figure on the Blood website; see the Supplemental Figure link at the top of the online article). When these quiescent T-cell populations were incubated for 12 hours with lentiviral vectors coexpressing IL7SU or IL7SUx with VSV-G (G/IL7SU or G/IL7SUx), a strong reduction in surface expression of the IL-7 high-affinity receptor was observed on naive as well as memory adult T cells (supplemental figure). Slightly higher levels of IL-7Rα were detected on naive CB T cells, as previously described,29 but down-regulation of this receptor was as pronounced on these cells as on adult T cells, following a 12-hour incubation with either G/IL7SU or G/IL7SUx pseudotyped lentiviral vectors (supplemental figure). Notably, the level of down-regulation of the IL-7Rα in the 3 different T-cell subsets was equivalent to that observed in the presence of rIL-7 (G+rIL7). This response was specific, as it was observed neither with lentiviral vectors pseudotyped with VSV-G alone nor with HIV-1–derived vectors codisplaying a chimeric MLV envelope N-terminally fused to interleukin-2 (IL2SU) and VSV-G (supplemental figure).
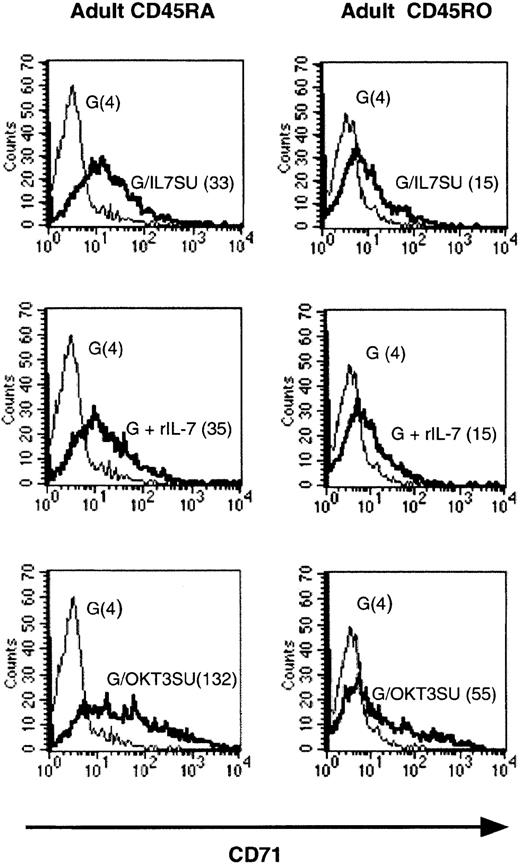
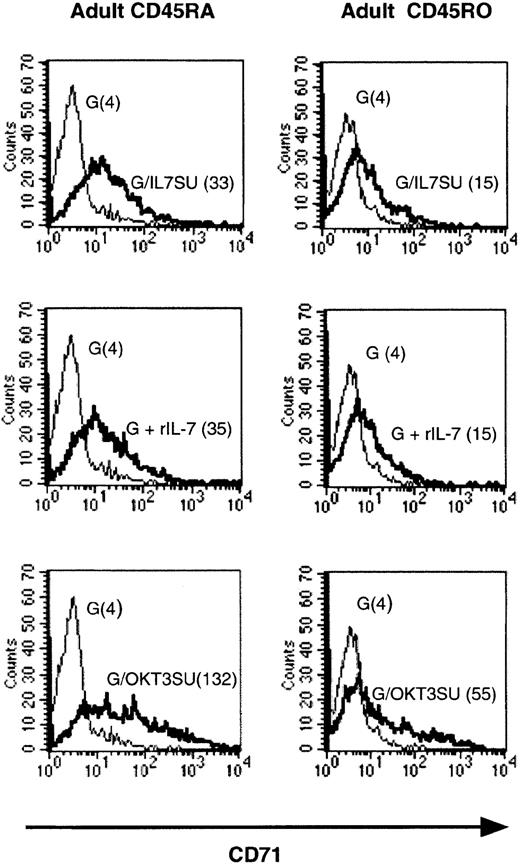
A low level of up-regulation of T-cell activation markers such as CD25 and CD71 has been reported after in vitro incubation of resting T cells with rIL-7.26 We therefore evaluated the levels of up-regulation of either activation marker by IL-7–displaying HIV-1–derived vectors. Importantly, the increase in CD71 expression in T cells cultured in the presence of either rIL-7 or IL-7–displaying vectors was similarly low (Figure 2). In contrast, VSV-G–pseudotyped vectors did not induce CD71 expression. It should be noted though that the up-regulation of CD71 induced by rIL-7 or IL-7–displaying vectors was significantly lower than that observed following TCR engagement with anti-CD3–displaying lentiviral vectors, as demonstrated by the higher mean fluorescence intensity (MFI) of CD71 expression in resting T cells incubated with the latter vectors (Figure 2). Likewise, the IL-7–displaying virions only induced a similar modest increase of CD25 (IL-2Rα) expression (data not shown).
HIV-1–derived vectors displaying human IL-7 share biologic properties with recombinant IL-7.
Purified adult memory and naive CD4+ T cells were incubated with IL-7–displaying lentiviral vectors (G/IL7SU, G/IL7SUx) for 6 days and expression of the CD71 activation marker was monitored. Expression was compared with that observed following incubation with VSV-G–pseudotyped vectors alone (G). As controls, T cells were incubated with VSV-G–pseudotyped vectors in the presence of rIL-7 (15 ng/mL), G+rIL-7, or with anti-CD3 scFv-displaying lentiviral vectors (G/OKT3SU). For each histogram, the mean fluorescence intensity of CD71 expression (MFI) is indicated in brackets. The data presented are representative of 3 independent experiments.
HIV-1–derived vectors displaying human IL-7 share biologic properties with recombinant IL-7.
Purified adult memory and naive CD4+ T cells were incubated with IL-7–displaying lentiviral vectors (G/IL7SU, G/IL7SUx) for 6 days and expression of the CD71 activation marker was monitored. Expression was compared with that observed following incubation with VSV-G–pseudotyped vectors alone (G). As controls, T cells were incubated with VSV-G–pseudotyped vectors in the presence of rIL-7 (15 ng/mL), G+rIL-7, or with anti-CD3 scFv-displaying lentiviral vectors (G/OKT3SU). For each histogram, the mean fluorescence intensity of CD71 expression (MFI) is indicated in brackets. The data presented are representative of 3 independent experiments.
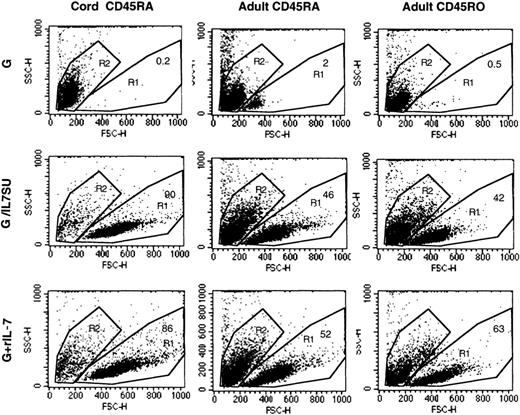
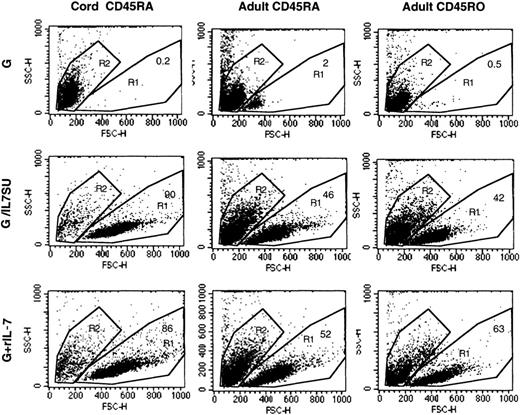
IL-7 has been identified as a master regulator of T-cell survival, particularly for naive T cells.25 IL-7–displaying virions were as effective as rIL-7 at preventing the death of naive and memory adult T cells. While virtually all resting T cells placed in culture were dead after 12 days in the presence of VSV-G–pseudotyped lentiviral vectors alone, the death rate of adult T cells was reduced to 40% or 60% in the presence of IL-7–displaying lentiviral vectors or rIL-7 (Figure 3). The cell death observed in these latter cultures was probably due to the fact that the culture medium was not refreshed and neither viral supernatant nor rIL-7 was supplemented during the 12 days of culture. In the case of naive CB T cells, protection from death was even more pronounced since at day 12, all CB T cells were dead when incubated with VSV-G–pseudotyped lentiviral vectors alone while incubation with either IL-7–displaying vectors or rIL-7 resulted in 85% to 90% viability (Figure 3). Protection of CB T cells from apoptosis was confirmed by staining of cells with annexin V–FITC/PI (data not shown). In conclusion, the biologic effects induced by IL-7–displaying lentiviral vectors largely recapitulated those induced by recombinant human IL-7.
IL-7–displaying HIV-1–derived vectors promote T-cell survival.
The forward/side scatter profiles of adult memory (CD45RO), adult naive (CD45RA), and cord blood–naive (CD45RA) CD4+ T cells that were incubated for 12 days with VSV-G–pseudotyped vectors (G), IL-7–displaying lentiviral vectors (G/IL7SU), or VSV-G–pseudotyped lentiviral vectors in the presence of rIL-7 (15 ng/mL; G+rIL-7) are shown. The percentage of viable cells, in the R1 gate, is indicated in each dot blot. Viability of all freshly isolated cells was higher than 98% (not shown).
IL-7–displaying HIV-1–derived vectors promote T-cell survival.
The forward/side scatter profiles of adult memory (CD45RO), adult naive (CD45RA), and cord blood–naive (CD45RA) CD4+ T cells that were incubated for 12 days with VSV-G–pseudotyped vectors (G), IL-7–displaying lentiviral vectors (G/IL7SU), or VSV-G–pseudotyped lentiviral vectors in the presence of rIL-7 (15 ng/mL; G+rIL-7) are shown. The percentage of viable cells, in the R1 gate, is indicated in each dot blot. Viability of all freshly isolated cells was higher than 98% (not shown).
IL-7–displaying lentiviral vectors efficiently transduce memory adult CD4+ T cells and naive cord blood CD4+ T cells in the absence of any other stimulation
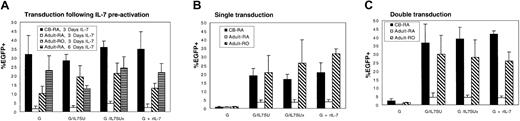
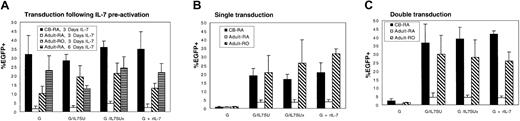
Conditions for IL-7–mediated lentiviral transduction were first optimized using rIL-7. As T lymphocytes present a heterologous population of cells, transductions were performed using purified either naive or memory T cells. Additionally, it has been reported that CD8+ T cells proliferate more vigorously than CD4+ T cells in response to IL-7.25 Since this could influence the outcome of the transduction assays, experiments were performed in purified CD4+ T cells. To assess precisely the efficiency of gene transfer in resting naive and memory CD4+ T-cell populations, each lymphocyte subset was purified by negative antibody-mediated selection. Although most CD4+ CB T cells are of the naive phenotype, this population was further purified by removing the approximately 10% of CD45RO+-expressing cells. The quiescent T-cell subsets were preactivated for 3 days with rIL-7, which resulted in efficient transduction of naive CB T cells with VSV-G–pseudotyped lentiviral vectors (up to 43%; mean, 32.0% ± 10.5%; n = 4; Figure4A). In the case in which rIL-7 was also added during transduction, either as a soluble cytokine (G+rIL-7) or via its display on the viral surface itself (G/IL7SUx or G/IL7SU), transduction levels were not significantly augmented (mean G/IL7SUx, 36.0% ± 3.6%; G/IL7SU, 28.5% ± 3.5%; G+rIL-7, 35.0% ± 9.6%, n = 4; Figure 4A). Memory adult CD4+ T cells were transduced at levels ranging from 10% to 28% under these conditions (mean VSV-G, 10.3% ± 3.9%; G/IL7SUx, 21.5% ± 5.6%; G/IL7SU, 19.5% ± 6.3%; G+rIL-7, 13.2% ± 2.7%, n = 3; Figure 4A). Identically treated naive adult CD4+T cells were transduced at significantly lower levels, not exceeding 5% (mean G, 1.7% ± 1.4%, G/IL7SUx, 4.6% ± 1.4%; G/IL7SU, 2.6% ± 0.7%; G+rIL-7, 2.5% ± 1.9%, n = 3; Figure 4A).
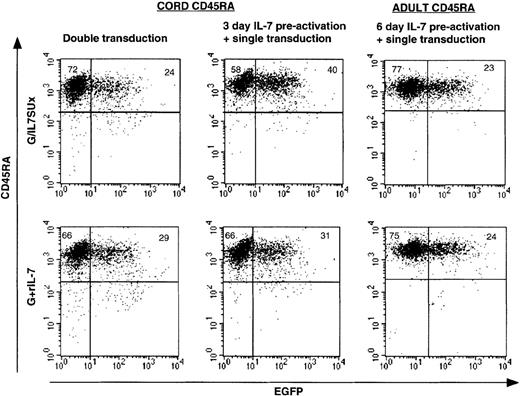
Differential transduction of naive CB and naive adult CD4+ T cells by IL-7–displaying lentiviral vectors.
Resting CD4+ naive cord blood, as well as quiescent naive and memory adult T cells were transduced with lentiviral vectors (A) following a preactivation of these CD4+ T-cell subsets for 3 or 6 days with rIL-7, (B) immediately upon isolation, or (C) upon isolation followed by a second transduction performed 3 days later. Cells were transduced with VSV-G–pseudotyped lentiviral vectors (G), IL-7–displaying VSV-G–pseudotyped lentiviral vectors (G/IL-7SU or G/IL7SUx), and VSV-G–pseudotyped lentiviral vectors in the presence of rIL-7 (G+rIL-7), using MOIs of 20 to 25. Cultures were continued for 3 days (A,C) or 7 days (B) after transduction, and EGFP expression was then monitored by FACS. Means ± SDs for at least 3 independent experiments are shown.
Differential transduction of naive CB and naive adult CD4+ T cells by IL-7–displaying lentiviral vectors.
Resting CD4+ naive cord blood, as well as quiescent naive and memory adult T cells were transduced with lentiviral vectors (A) following a preactivation of these CD4+ T-cell subsets for 3 or 6 days with rIL-7, (B) immediately upon isolation, or (C) upon isolation followed by a second transduction performed 3 days later. Cells were transduced with VSV-G–pseudotyped lentiviral vectors (G), IL-7–displaying VSV-G–pseudotyped lentiviral vectors (G/IL-7SU or G/IL7SUx), and VSV-G–pseudotyped lentiviral vectors in the presence of rIL-7 (G+rIL-7), using MOIs of 20 to 25. Cultures were continued for 3 days (A,C) or 7 days (B) after transduction, and EGFP expression was then monitored by FACS. Means ± SDs for at least 3 independent experiments are shown.
Based on the encouraging transduction efficiencies obtained for naive CB CD4+ T cells and memory adult CD4+ T cells, we next assessed whether strictly coupling IL-7 stimulation to transduction by use of IL-7–displaying lentiviral vectors would be sufficient to promote efficient transduction of these T-cell subsets. Importantly, naive CB T cells as well as memory adult T cells could be efficiently transduced following a single exposure to either IL-7–displaying lentiviral vectors, in the absence of any other stimulus. Following a single transduction with IL-7–displaying lentiviral vectors at MOIs of 20 to 25, transduction levels ranging from 17% up to 40% were observed in naive CB T cells (mean G/IL7SUx, 17.1% ± 2.8%; G/IL7SU, 19.5% ± 4.2%; G+rIL-7, 21.0% ± 5.6%, n = 3; Figure 4B) and adult memory cells (mean G/IL7SUx, 26.5% ± 13.0%; G/IL7SU, 21.0% ± 9.8%; G+rIL-7, 32.2% ± 2.8%, n = 3; Figure 4B). As expected from the data obtained following a prestimulation with rIL-7, a single transduction of naive adult T cells with IL-7–displaying vectors did not result in important transduction levels (< 5%; Figure 4B). No significant differences in transduction efficiencies were observed with the 2 different chimeras: IL7SU, in which IL-7 may dissociate from the virions, and IL7SUx, in which the cytokine is “locked” onto the viral particle. This is not remarkable since both envelopes were expressed at equivalent levels on the viral surface, as detected by Western blot analysis (data not shown), and both types of vector particles equivalently down-regulated the IL-7R and protected T cells from death (supplemental figure and data not shown). Moreover, the efficient transduction obtained with IL7SUx strongly suggests that stimulation at the time of viral entry permitted gene transfer into naive CB and memory adult T cells. Expression of EGFP after a single transduction of the memory adult T-cell subset remained stable, at least over a 12-day period (data not shown), suggesting that it represented a true gene transfer. Furthermore, in naive CB T cells, the percentage of EGFP+ cells increased by 5% to 10% between 7 and 12 days after transduction (mean G/IL7SUx, 27.0% ± 9.8%; G/IL7SU, 29.0% ± 8.5%; G+rIL-7, 31% ± 7.5%, n = 3). This may reflect the fact that the most transduction-permissive cells in a population of IL-7–treated CB T lymphocytes are those cycling more actively.
We next assessed whether stimulation via IL-7–displaying lentiviral vectors followed by a second exposure to these vectors could augment transduction, especially in the context of naive adult CD4+resting T cells. Thus, T-cell subsets were incubated for 3 days with G/IL7SU or G/IL7SUx pseudotyped lentiviral vectors followed by a second transduction of 4 days. These conditions resulted in a higher transduction efficiency of naive CB T cells ranging from 30% to up to 48% (mean G/IL7SUx, 39.0% ± 7.1%; G/IL7SU, 36.6% ± 11.0%, n = 3; Figure 4C). These transduction levels were similar to those observed after a double transduction with VSV-G–pseudotyped lentiviral vectors in the presence of rIL7 (G+rIL-7, 42.0% ± 2.0% for naive CB T cells; Figure 4C). Again, after a double transduction of naive CB cells with IL-7–displaying lentivectors, an increase in transduction rates of 20% was observed at day 12 after infection compared with transduction rates determined at day 7 after infection (mean G/IL7SUx, 62% ± 7.8%; G/IL7SU, 65% ± 4.9%; G+rIL-7, 63.6% ± 6.6%, n = 3) (data not shown). Nevertheless, despite this double transduction protocol, adult naive T cells remained relatively refractory to transduction by IL-7–displaying vectors as well as in the presence of rIL-7 (mean G/IL7SUx, 4.5% ± 1.4%; G/IL7SU, 4.6% ± 2.6%; G+rIL-7, 4.3% ± 0.9%, n = 3; Figure 4C). Importantly, when the duration of IL-7 prestimulation was increased from 3 to 6 days, up to 32% transduction of adult naive T cells could be obtained (Figure 4A), indicating that the different T-cell subsets respond differently to IL-7 treatment and establishing that a precise timing of IL-7 treatment is required for optimal gene transfer. Finally, we determined that within the CB T-cell population, CD4+ and CD8+ T cells were transduced to equivalent levels after a single or a double exposure to IL-7–displaying vectors without a change in the ratio of CD4+/CD8+ T cells in the CB sample (data not shown).
CB T cells transduced by IL-7–displaying lentiviral vectors retain their naive phenotype
We demonstrated that incubation of adult naive T cells with the anti-CD3–displaying lentiviral vectors was accompanied by loss of the naive phenotype (Figure 1). We therefore sought to verify that IL-7–displaying lentiviral vectors did not modify the phenotype of transduced naive adult and cord blood T cells. A purified population of resting CD4+ naive CB T cells was transduced by a double transduction protocol with either IL-7–displaying vectors (G/IL7SUx) or with VSV-G–pseudotyped lentiviral vectors in the presence of rIL-7 (Figure 5). Resting adult naive CD4+ T cells were preactivated for 6 days with rIL-7 and transduced for 4 days with IL-7–displaying vectors (G/IL7SUx) or with VSV-G–pseudotyped lentiviral vectors in the presence of rIL-7. The phenotype of the transduced cells was then evaluated. Importantly, the transduced EGFP+ population in both T-cell subsets maintained the expression of the CD45RA naive marker, regardless of whether IL-7 was presented on the viral surface or added as a soluble cytokine (Figure 5).
Transduction of naive CB CD4+ T cells with IL-7–displaying lentiviral vectors maintain their naive phenotype.
CD4+ naive CB T cells were transduced with IL-7–displaying lentiviral vectors (G/IL7SUx) or with VSV-G–pseudotyped lentiviral vectors in the presence of rIL-7 (G+rIL-7). Cells were either transduced for 3 days followed by a second transduction for 4 days (double transduction) or preactivated with rIL-7 (15 ng/mL) for 3 days and then transduced for 4 days (preactivation + single transduction). CD4+ adult naive T cells were preactivated with rIL-7 for 6 days and transduced for 4 days. Expression of CD45RA and EGFP was simultaneously monitored by FACS analysis 4 days after transduction. The percentages of CD45RA+/ EGFP− and CD45RA+/EGFP+ cells are indicated.
Transduction of naive CB CD4+ T cells with IL-7–displaying lentiviral vectors maintain their naive phenotype.
CD4+ naive CB T cells were transduced with IL-7–displaying lentiviral vectors (G/IL7SUx) or with VSV-G–pseudotyped lentiviral vectors in the presence of rIL-7 (G+rIL-7). Cells were either transduced for 3 days followed by a second transduction for 4 days (double transduction) or preactivated with rIL-7 (15 ng/mL) for 3 days and then transduced for 4 days (preactivation + single transduction). CD4+ adult naive T cells were preactivated with rIL-7 for 6 days and transduced for 4 days. Expression of CD45RA and EGFP was simultaneously monitored by FACS analysis 4 days after transduction. The percentages of CD45RA+/ EGFP− and CD45RA+/EGFP+ cells are indicated.
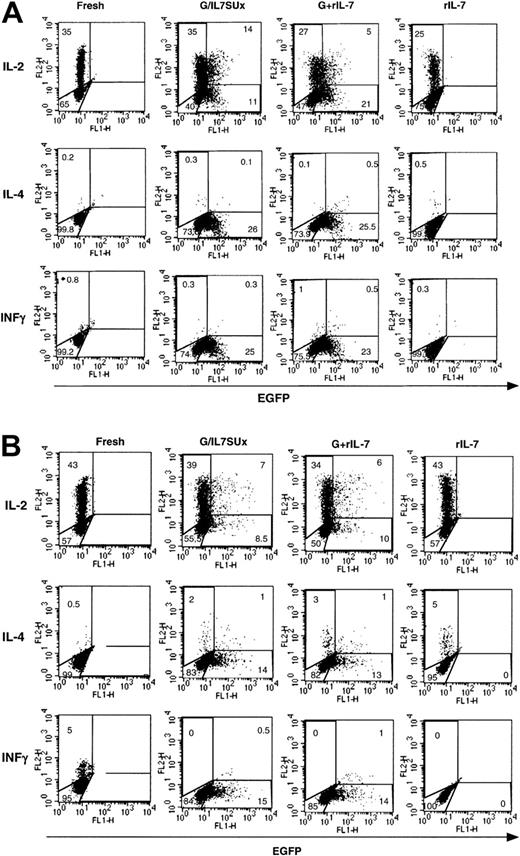
We then assessed whether IL-7–mediated lentiviral transduction did not induce differentiation of these transduced naive T-cell subsets. It is now generally agreed that naive CD4+ T cells essentially produce IL-2 and that only when they are primed through their TCR do they produce multiple cytokines.30 Therefore we assessed secretion of IL-2 by IL-7–mediated transduced naive cells and secretion of IL-4 and INFγ as markers for Th2 or Th1 differentiation, respectively. In agreement with their naive phenotype, IL-2 was expressed by 35% to 50% of naive CB CD4+ T cells following a 5-hour PMA and ionomycin stimulation, regardless of whether the cells were prestimulated with rIL-7 or infected with IL-7–displaying virions (Figure 6A). Moreover, only a minimal percentage (< 1%) of cells expressed IL-4 or INFγ under either of these conditions (Figure 6A). Of note, in the absence of PMA and ionomycin, no accumulation of cytokines was detected, whereas after an extended PMA/ionomycin stimulation, a small proportion of INFγ-expressing cells (10%-20%) and a negligible proportion IL-4–expressing cells could be detected (data not shown). Similarly, in adult naive T cells, a high percentage of fresh and IL-7–treated cells were found to express IL-2 following PMA/ionomycin stimulation (40%-60%). Expression of IL-4 and INFγ remained minimal following IL-7 stimulation (Figure 6B). Thus, collectively, these data suggested that IL-7–mediated transduction of naive T cells does not appear to promote their significant differentiation to either Th1 or Th2 phenotypes. Moreover, expression of IL-2 was not associated with either an enhanced or diminished susceptibility to lentiviral transduction in naive CB or adult T cells (Figure 6).
Cytokine profile of IL-7 mediated lentiviral-transduced naive T cells.
Freshly isolated CD4+ naive CB T cells (A) and 6-day IL-7–prestimulated CD4+ adult naive T cells (B) were incubated for 6 days and 4 days, respectively, with IL-7–displaying VSV-G–pseudotyped vectors (G/IL7SUx) or VSV-G–pseudotyped vectors in the presence of IL-7. Control incubations were performed of CB T cells and adult T cells for 6 and 10 days, respectively, in the presence of rIL-7. At 5 hours prior to assessment of IL-2, IL-4, and INFγ accumulation, these T-cells subsets were activated with PMA and ionomycin. The percentage of cells in each region is indicated. The results are representative of 3 independent experiments.
Cytokine profile of IL-7 mediated lentiviral-transduced naive T cells.
Freshly isolated CD4+ naive CB T cells (A) and 6-day IL-7–prestimulated CD4+ adult naive T cells (B) were incubated for 6 days and 4 days, respectively, with IL-7–displaying VSV-G–pseudotyped vectors (G/IL7SUx) or VSV-G–pseudotyped vectors in the presence of IL-7. Control incubations were performed of CB T cells and adult T cells for 6 and 10 days, respectively, in the presence of rIL-7. At 5 hours prior to assessment of IL-2, IL-4, and INFγ accumulation, these T-cells subsets were activated with PMA and ionomycin. The percentage of cells in each region is indicated. The results are representative of 3 independent experiments.
Differential transduction of CD4+ T-cell subsets correlates with the cell-cycle progression induced by IL-7–displaying lentiviral vectors
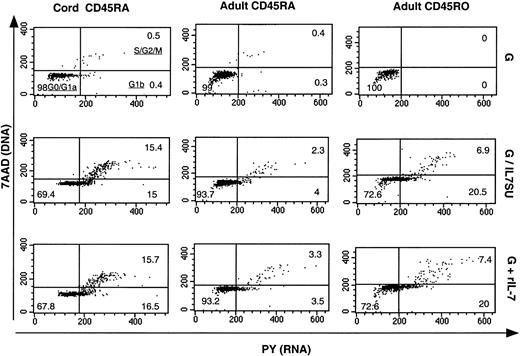
It has previously been reported that IL-7 differentially mediates cell-cycle progression in CB and adult CD4+ T cells.22 As infection of circulating peripheral blood T cells with HIV-1 requires progression into the G1b phase of the cell cycle12 and as we observed a relative refractoriness of naive adult T cells to transduction with IL-7–displaying lentiviral vectors, it was important to determine whether these vectors induced cell-cycle entry to equivalent levels in the 3 different CD4+ T-cell subsets: naive CB, and naive and memory adult T cells. Using a method that permits simultaneous visualization of DNA (7-amino-actinomycin staining) and RNA (pyronin Y staining), we were able to distinguish the percentage of T cells that were in G0/G1a, G1b, S, and G2/M phases of the cell cycle (Figure7). All T-cell subsets incubated with VSV-G–pseudotyped lentiviral vectors for 6 days remained in G0/G1a phase of the cell cycle (> 99%). In the presence of either G/IL7SUx pseudotyped lentiviral vectors or rIL-7 for 6 days, more than 27% of naive CB T cells as well as adult memory T cells entered into G1b and further progressed into S/G2/M (Figure 7). In contrast, the percentage of naive adult T cells entering into cycle under these conditions was markedly lower (< 7%; Figure 7). These data most likely explain why naive CB and memory adult T cells were susceptible to transduction with IL-7–displaying HIV-1–derived vectors, whereas naive adult T cells were more refractory. In agreement with previous reports,12,23 29 these results therefore indicated that the susceptibility of T-cell subsets to IL-7–displaying lentiviral vectors appears to correlate with their progression into cell cycle.
Comparison of cell-cycle entry of naive CB T cells and naive and memory adult T cells following exposure to IL-7–displaying lentiviral vectors.
CD4+ naive CB (CD45RA) and naive and memory (CD45RO) adult T cells were incubated for 6 days with VSV-G–pseudotyped lentiviral vectors (G), IL7SU-displaying VSV-G–pseudotyped lentiviral vectors (G/IL7SU) or VSV-G–pseudotyped lentiviral vectors in the presence of rIL-7 (G+rIL-7). Cell-cycle progression was monitored by simultaneously visualizing the RNA (PY) and DNA (7AAD) content of these T-cell subsets. The percentages of cells in the G0/G1a, G1b, S/G2/M phase of the cell cycle are indicated in the dot blots.
Comparison of cell-cycle entry of naive CB T cells and naive and memory adult T cells following exposure to IL-7–displaying lentiviral vectors.
CD4+ naive CB (CD45RA) and naive and memory (CD45RO) adult T cells were incubated for 6 days with VSV-G–pseudotyped lentiviral vectors (G), IL7SU-displaying VSV-G–pseudotyped lentiviral vectors (G/IL7SU) or VSV-G–pseudotyped lentiviral vectors in the presence of rIL-7 (G+rIL-7). Cell-cycle progression was monitored by simultaneously visualizing the RNA (PY) and DNA (7AAD) content of these T-cell subsets. The percentages of cells in the G0/G1a, G1b, S/G2/M phase of the cell cycle are indicated in the dot blots.
Discussion
This is the first demonstration that a cytokine-displaying vector can efficiently transduce quiescent T cells in the absence of any other stimulation, and without changing their phenotype. We were able to achieve high transduction efficiencies in both naive CB T cells and adult memory T cells via a lentiviral vector that presented IL-7 on its surface, without preactivation of the resting T cells. However, efficient transduction of adult naive T cells required prolonged treatment with IL-7. Importantly, the phenotype and functional characteristics of the transduced T cells were conserved. HIV-derived vectors displaying the IL-7 cytokine also promoted the survival of naive as well as memory resting T cells. From previous work performed by others and ourselves, it appears that the transduction of naive CB and adult T cells following IL-7 stimulation is very sensitive to various parameters including the timing of transduction, the multiplicity of infection, the presence of fibronectin, and the timepoints at which transduction is monitored14,23,29 (also L. Swainson, unpublished results, May 2002). Although the relative importance of these various parameters remains to be determined, transuction of IL-7–stimulated naive CB T cells can be achieved under conditions of high MOI and an extended culture period following transduction as shown here and in Ducrey-Rundquist et al.23
CB T cells differ from their adult counterpart in that the former represent mainly recent thymocyte emigrants. Compared with naive adult T cells, they are immature with respect to alloantigen recognition, lymphokine production, and T-cell activation profile.31,32In agreement with recent results obtained with rIL-7,23,29we found that IL-7–displaying vectors had distinct effects on cell-cycle entry of naive and memory T-cell subsets. CB T cells exposed to IL-7–displaying lentiviral vectors moved into the G2/M phase of the cycle, whereas adult T cells, and more particularly adult naive T cells, displayed a much slower rate of progression in the cell cycle and hardly progressed further than into G1b (Figure 7). The basis for this differential responsiveness remains to be elucidated but probably accounts for the high transduction efficiencies observed in the CB and adult memory T-cell subsets compared with the relatively refractory naive adult T-cell population. Indeed, no preactivation of naive CB and adult memory T cells with rIL-7 was required for transduction by IL-7–displaying virions, suggesting that these vectors may be capable of specifically transducing some IL-7R–expressing T-cell populations in vivo. In contrast, preactivation of naive adult T cells with recombinant IL-7 was necessary for efficient transduction with the IL-7–displaying vectors. Correspondingly, Unutmaz et al14and Ducrey-Rundquist et al23 previously demonstrated that several days of rIL-7 treatment are necessary for inducing maximal permissiveness to infection with HIV-1 or HIV-derived vectors in naive adult T lymphocytes. This also correlates the recent report of Steffens et al33 that IL-7 treatment can prime adult naive T cells for productive infection by T-cell–adapted and primary HIV isolates.
Introducing a gene into naive CB T cells that remain phenotypically naive is an important step forward for T-cell–based gene therapies. Unrelated CB samples are being used with increasing frequency as an alternative source of allogenic stem cells to treat both malignant and nonmalignant disorders.34-36 This source of stem cells is attractive because there are indications that there is a reduced level of severe graft-versus-host disease in patients who received CB transplants compared with patients reconstituted with allogenic bone marrow. This is ascribed to the functional and phenotypic immaturity of naive cord blood T cells.37-39 Thus, if an increased T-cell response (eg, a graft-versus-leukemia response) is desirable, introduction of specific signaling molecules or cytokines into naive CB T cells by lentiviral gene transfer may result in a beneficial outcome for the patient. Furthermore, transduction of naive CB T cells with IL-7–displaying vectors may have a secondary beneficial effect: IL-7 stimulation sensitizes cells and may therefore augment immune responsiveness against weak antigens.40 It is known that IL-7–primed T cells respond more rapidly to antigenic stimulation and react to antigens at lower concentrations than unprimed cells.40-42 As we found that naive CB T cells could be transduced by a single exposure to IL-7–displaying lentiviral vectors, such vectors could be used to immediately transduce the T-cell population within the CB sample and the cells could then be reinfused into the patient without any ex vivo culture. While this application is clearly not ready for clinical use, it is tempting to speculate that it will allow a rapid and selective transduction of T lymphocytes.
Altogether these results demonstrate that specific subsets of resting T cells can be efficiently transduced by a lentiviral vector that simultaneously delivers an activating signal and a transgene. One major advantage of the system described here is that the activating signal, IL-7, does not modulate the phenotype of the T cells. Moreover, in comparison with a TCR-mediated signal, IL-7 induces a much more restricted number of cell divisions, which may have important consequences for further antigen-mediated responses in vivo as well as for the maintenance of diversity in the reinfused lymphocyte population. In this regard, anti-CD3–displaying lentiviral vectors13 led to a loss of the naive T-cell phenotype and a strong activation of T cells. The efficiency of a vector in which IL-7 is “locked” onto the virion particles has important potential for in vivo applications, allowing these virions to be used for selective gene delivery to T lymphocytes. Finally, in agreement with results previously obtained with rIL-7,22,23 29 our data show that naive adult T cells and naive CB T cells respond distinctly to IL-7 displayed in the context of virions. An understanding of the characteristics of recent thymic emigrants that permit IL-7–induced cell-cycle entry and distinguish these lymphocytes from adult T cells awaits further investigation. Elucidation of these biologic parameters will enable us to develop new strategies permitting efficient and rapid gene delivery to all subpopulations of lymphocytes.
We thank Louise Swainson for sharing results and helpful discussions. We thank Delphine Olivier for expert technical assistance.
Prepublished online as Blood First Edition Paper, November 21, 2002; DOI 10.1182/blood-2002-07-2224.
Supported by the Agence Nationale pour la Recherche contre le SIDA (ANRS), the European Community (QLK3-1999-00859), Association Franco-Israélienne pour la Recherche Scientifique et Technologique (AFIRST), Association Française contre les Myopathies (AFM), Association pour la Recherche contre le Cancer (ARC), Centre National de la Recherche Scientifique (CNRS), and Institut National de la Santé et de la Recherche Médicale (INSERM). E.V. is supported by the Marie Curie Fellowship of the European Community.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
François-Loı̈c Cosset, LVRTG, INSERM U412, ENS de Lyon, 46 Allée d'Italie, 69007 Lyon, France; e-mail: flcosset@ens-lyon.fr.