Interleukin-7 (IL-7) is important for thymopoiesis in mice and humans because IL-7 receptor α (IL-7Rα) mutations result in a severe combined immunodeficiency phenotype with severe thymic hypoplasia. Recent evidence has indicated that IL-7 also plays an important role as a regulator of T-cell homeostasis. Here we report the immunologic effects of recombinant human IL-7 (rhIL-7) therapy in normal and simian immunodeficiency virus (SIV)–infected nonhuman primates. Cynomolgus monkeys receiving 10 days of rhIL-7 showed substantial, reversible increases in T-cell numbers involving a dramatic expansion of both naive and nonnaive phenotype CD4+ and CD8+ subsets. Although IL-7 is known to have thymopoietic effects in mice, we observed marked declines in the frequency and absolute number of T-cell receptor excision circle-positive (TREC+) cells in the peripheral blood and dramatic increases in the percentage of cycling T cells in the peripheral blood as measured by Ki-67 expression (baseline less than 5% to approximately 50% after 6 days of therapy) and ex vivo bromodeoxyuridine (BrdU) incorporation. Similarly, moderately CD4- depleted SIV-infected macaques treated with rhIL-7 also had significant increases in peripheral blood CD4+ and CD8+ T cells following rhIL-7 therapy. Thus, rhIL-7 induces dramatic alterations in peripheral T-cell homeostasis in both T-cell–replete and T-cell–depleted nonhuman primates. These results further implicate IL-7 as a promising immunorestorative agent but illustrate that a major component of its immunorestorative capacity reflects effects on mature cells. These results also raise the possibility that IL-7 therapy could be used to temporarily modulate T-cell cycling in vivo in the context of immunotherapies such as vaccination.
Introduction
Interleukin-7 (IL-7) is required for T and B lymphopoiesis in mice.1,2 In humans, mutations in the IL-7 receptor α (IL-7Rα) chain, which is required for IL-7 signaling, result in a severe reduction in T-cell numbers with substantial numbers of B cells.3 Thus, IL-7 is critical for T-cell development in humans but may not be required for B-cell development. Studies in mice have demonstrated that pharmacologic administration of IL-7 increases thymopoiesis following bone marrow transplantation.4 5 Thus, based on the thymopoietic effects of IL-7 and the well-described importance of the thymus in regenerating T-cell repertoire following T-cell depletion occurring in the context of stem cell transplantation and HIV infection, there is currently substantial interest in the clinical development of IL-7 as an immunorestorative agent. However, it remains to be seen whether the potent effects of IL-7 observed in mice will extend to humans.
In addition to effects on the thymus, IL-7 also has potent effects on mature T cells by lowering the threshold for activation and increasing survival via up-regulation of bcl-2 family members (reviewed by Hofmeister et al6 and Fry and Mackall7). IL-7 is required for low-affinity antigen-induced T-cell proliferation, which occurs following T-cell depletion,8,9 and treatment of T-cell-replete mice with IL-7 leads to increased homeostatic expansion.10,11 Furthermore, there is an increased availability of circulating IL-7 in humans with CD4 lymphopenia,12-14 thus providing a plausible mechanism for the increased homeostatic expansion observed in T-cell–depleted hosts. Recently, it has also been demonstrated that IL-7 can partially substitute for IL-15 in the maintenance of memory T cells when overexpressed as a transgene in mice.15 Thus, in addition to thymopoietic effects, IL-7 plays a critical role in regulating peripheral T-cell homeostasis following T-cell depletion through effects on mature naive and memory T cells, occurring independent of thymopoietic effects.16
Thus far, study of the effects of IL-7 therapy has been limited to murine systems. In this report, we analyzed the immunologic changes that occur with IL-7 therapy in nonhuman primates. We demonstrate that IL-7 treatment alters peripheral T-cell homeostasis in nonhuman primates, resulting in substantial but reversible elevations in peripheral T-cell numbers through a dramatic increase in the number of peripheral T cells undergoing cell cycling. Importantly, immunologic effects of IL-7 were observed in simian immunodeficiency virus (SIV) infection despite predicted elevations of endogenous IL-7. These results provide further evidence for IL-7 as a central regulator of peripheral T-cell homeostasis and suggest that IL-7 therapy will potently immunomodulate both T-cell–depleted and T-cell–replete humans.
Materials and methods
Animals
All animals were housed and handled in accordance with standards of the American Association for the Accreditation of Laboratory Animal Care, the Guide for the Care and Use of Laboratory Animals,38 and the US Department of Agriculture through the Animal Welfare Act (public law 91-579). Healthy juvenile cynomolgus monkeys (Macaca fascicularis) were injected subcutaneously with recombinant human IL-7 (rhIL-7) once daily for 10 days at a dose of 50, 200, or 500 μg/kg/d. Blood samples were obtained from anesthetized monkeys prior to treatment, on day 6, day 11, day 21, and day 27 or 28. Inguinal, axillary, and popliteal lymph nodes were examined at baseline, day 2, day 6, day 11, and day 21. The 8 juvenile colony-bred rhesus macaques(Macaca mulatta) used here were obtained from Covance Research Products (Alice, TX) and were infected with SIVmac251(561).17 These animals were treated with daily antiretroviral therapy (ART) consisting of 20 mg/kg/d of (R)-9-2-phosphonylmethoypropyladenine (PMPA), subcutaneously, and 5 mg/kg/d of didanosine, intravenously (Videx; Bristol-Meyers Squibb, Princeton, NJ) during primary infection. Plasma virus levels were measured by nucleic acid sequence–based amplification (NASBA) as previously described.18 IL-7 was administered for 9 consecutive days at a dose of 100 μg/kg/d subcutaneously. All SIV-infected rhesus macaques studied in this report were vaccinated with NYVAC-SIV-gag-pol-env (gpe)19and NYVAC-SIV-Retanef (RTN)20 together on day 3 of IL-7 therapy. Details of the effects of IL-7 on vaccine immune response are the focus of a separate report.
RhIL-7 production
RhIL-7 was manufactured by the Biopharmaceutical Development Program, SAIC Frederick, using an Escherichia coliexpression system (kindly supplied by Dr J. Murphy, Boston Children's Hospital). RhIL-7 was expressed as an insoluble inclusion body. Following cell disruption, the inclusion bodies were washed extensively and solubilized in 6 M guanidine hydrochloride. Denatured monomeric IL-7 was purified and refolded to an active form. The refolded rhIL-7 was extensively purified by a series of chromatographic purification steps. The final formulation was more than 97% pure by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and reverse-phase high-performance liquid chromatography (HPLC) and contained less than 0.05 EU/mg of residual endotoxin. Biologic activity was confirmed using the IL-7-dependent cell line 2E8. All of the rhIL-7 used in this study was from the same bulk production lot. Prior to injection, IL-7 was stored in aliquots at −20°C. Once thawed, an aliquot was used within 48 hours.
Flow cytometry
Within 24 hours of collection, EDTA (ethylenediaminetetraacetic acid)–anticoagulated peripheral blood samples were analyzed by flow cytometry. Antibody staining and whole blood lysis was performed using standard techniques. For surface immunoglobulin M (IgM) staining, antibody was added after red cell lysis and washing. Flow cytometric analysis was performed on a dual laser FACSCalibur (Becton Dickinson, San Jose, CA) using CellQuest software. Viable lymphocyte populations were gated based on forward scatter and side scatter characteristics and back-gating with CD45 and CD14. The antibodies used were CD3ε fluorescein isothiocyanate (FITC) and peridinin chlorophyll protein (PerCP) (clone SP34), CD45RA FITC (clone 5H9), CD16 FITC (clone 3GB), CD14 FITC (clone M5E2), HLA-DR R-phycoerythrin (R-PE) and Cy-Chrome (clone G46-6), CD25 R-PE (clone M-A251), CD69 R-PE (clone FN50), CD95 R-PE (clone DX2), CD27 R-PE (clone M-T271), CD11a R-PE and allophycocyanin (APC) (clone HI111), CD28 Cy-Chrome and APC (clone CD28.2), CD56 R-PE (clone MY31), CD20 R-PE and APC (clone 2H7), CD4 PerCP (clone L200), CD45 PerCP (clone TU116), CD10 Cy-Chrome (clone HI10a), CD8 APC (clone RPA-T8), and isotype controls (all from BD PharMingen, San Diego, CA). In addition, goat antimonkey IgM FITC (Kirkegard & Perry, Gaithersburg, MD), CD8β R-PE and APC (clone 2ST8.5H7), and CD127 (IL-7Rα) R-PE (clone R34.34) (Immunotech, Marseille, France) were used.
T-cell receptor excision circle (TREC) analysis
Frozen peripheral blood mononuclear cells (PBMCs) were rapidly thawed and washed in RPMI supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Gaithersburg, MD). Cells were counted, washed, and labeled with either mouse antihuman CD4 or CD8 antibodies conjugated to magnetic beads (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. Cells were positively selected using an MS column. Three separate aliquots of the positively selected fraction were carefully counted and averaged. Cells were pelleted and stored at −80°C until analysis. Quantification of T-cell receptor excision circles (TRECs) in sorted CD4+ and CD8+ T cells was performed by quantitative polymerase chain reaction (PCR) with an ABI7700 system (Perkin-Elmer, Norwalk, CT) using the conditions exactly as previously described.21 Primer and probe sequences were as follows: forward-CACATCCCTTTCAACCATGCT, reverse-GCCAGCTGCAGGGTTTAGG, and probe-ACGCCTCTGGTTTTTGTAAAGGTGCTCACT. A standard curve was plotted and TREC values for samples calculated by the ABI7700 software. Samples were analyzed in duplicate.
Ki67 and BrdU
Frozen PBMCs were thawed and washed in RPMI 1640 with FBS; 1 × 106 cells were washed and surface-labeled with antibodies as indicated. Cells were fixed, permeabilized, and labeled with anti-Ki67 FITC or isotype antibodies for 30 minutes on ice. For bromodeoxyuridine (BrdU) analysis, washed PBMCs were added to a 12-well plate at a concentration of 2 × 106/mL and pulsed with BrdU (BD Pharmingen) at a concentration of 10 μM. At 18 hours and 42 hours, cells were harvested and labeled with anti-BrdU FITC (BD Pharmingen) according to the manufacturer's instructions.
Western blotting
Analysis of protein levels in thawed PBMCs was performed using standard Western blotting techniques. The monoclonal antibodies used were as follows: anti-p27kip1 IgG1 (F-8), anti-p16Ink4a IgG2a (F-12) (Santa Cruz, Santa Cruz, CA), antihuman α tubulin (0.1 μg/mL) (Ab-1) (Oncogene Research Products, Cambridge, MA), and sheep antimouse horseradish peroxidase (HRP)–conjugated IgG (Amersham Pharmacia Biotech, Piscataway, NJ)
In vitro IL-7Rα expression
Frozen PBMCs from untreated cynomolgus monkeys were thawed, washed, and counted. Half of the cells were fixed with 5% paraformaldehyde for 5 minutes at 37°C. Fixed and unfixed cells were then incubated at either 4°C or 37°C, 5% CO2, with rhIL-7 at a concentration of 10 ng/mL in RPMI supplemented with 10% heat-inactivated fetal bovine serum, penicillin, streptomycin, and glutamine (all from Gibco Life Technologies, Gaithersburg, MD) or in media alone. At 14 hours, cells were analyzed by flow cytometry as already described.
Results
Recombinant human IL-7 therapy induces substantial but reversible increases in circulating CD4+ and CD8+ counts, lymphadenopathy, and a preservation of the CD4/CD8 ratio
IL-7 therapy dramatically increases T-cell numbers in normal10,22 and T-cell–depleted mice.11 To determine the immunologic effects of IL-7 therapy in nonhuman primates, we treated healthy juvenile cynomolgus monkeys with recombinant human IL-7 administered subcutaneously daily for 10 days using doses (50, 200, and 500 μg/kg/d) extrapolated from biologically active doses in mice. This short-term IL-7 therapy substantially increased the number of total lymphocytes and both CD4+ and CD8+ T cells in the peripheral blood. At 50 μg/kg/d and 200/μg/kg/d there were mixed responses, with 1 of 2 treated animals responding, whereas all 3 treated animals showed a substantial response at 500 μg/kg/d (Figure 1A-C). All responding animals had T-cell increases ranging from approximately 2-fold to 5-fold, with a median absolute increase in CD4 count of 5315 cells per microliter and CD8 count of 2849 cells per microliter (bothP values less than .05, Wilcoxon signed-rank test), with preservation of the CD4/CD8 ratio (Figure 1D). Although changes in lymphocyte trafficking can alter peripheral blood lymphocyte numbers without altering total body lymphocyte counts,23-25 this was not the case with IL-7 therapy because clinically evident lymphadenopathy was observed in IL-7–treated primates on days 2, 6, and 11, coincident with increases in peripheral blood lymphocytes (PBLs). Both the effects on PBL numbers and lymphadenopathy were reversible, returning to near baseline by day 21. There were no changes in absolute B-cell or natural killer (NK) cell counts (data not shown). Thus, similar to murine models, a short course of IL-7 therapy substantially and reversibly increased total body T-cell numbers in nonhuman primates. However, unlike murine models, no increases in B-cell counts were observed, and the effects on CD4+ and CD8+ T cells were equivalent.10
IL-7 therapy increases CD4+ and CD8+ T cells in the peripheral blood.
(A) Reversible increases in CD3+/CD4+lymphocytes in peripheral blood are expressed as percent change from baseline. Each line represents individual animals treated with 50 μg/kg/d (red), 200 μg/kg/d (blue), and 500 μg/kg/d (green). Dotted lines represent untreated animals. (B) Similar changes observed in peripheral blood CD3+/CD8+lymphocytes. (C) Absolute number of CD3+/CD4+ and CD3+/CD8+lymphocytes on day 11 (1 day after stopping IL-7 therapy). Each data point represents an individual animal. (D) No change in CD4/CD8 ratio.
IL-7 therapy increases CD4+ and CD8+ T cells in the peripheral blood.
(A) Reversible increases in CD3+/CD4+lymphocytes in peripheral blood are expressed as percent change from baseline. Each line represents individual animals treated with 50 μg/kg/d (red), 200 μg/kg/d (blue), and 500 μg/kg/d (green). Dotted lines represent untreated animals. (B) Similar changes observed in peripheral blood CD3+/CD8+lymphocytes. (C) Absolute number of CD3+/CD4+ and CD3+/CD8+lymphocytes on day 11 (1 day after stopping IL-7 therapy). Each data point represents an individual animal. (D) No change in CD4/CD8 ratio.
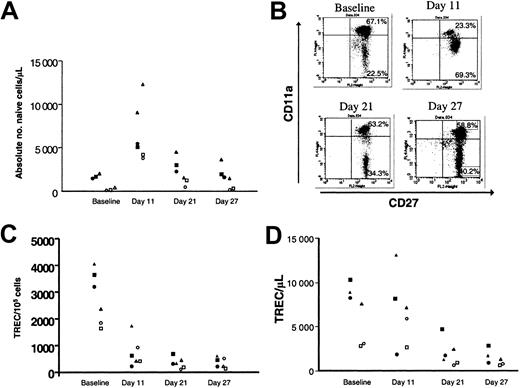
Treatment with rhIL-7 increases both naive and nonnaive T cells and induces declines in peripheral blood TREC levels
IL-7 therapy potently up-regulates thymopoiesis in murine models4,5,11 and in vitro in human fetal thymic organ culture systems.26 Thus, we anticipated that increases in the numbers of recent thymic emigrants would result in an increase in the absolute number of circulating T cells bearing a naive phenotype.
Using multiparameter flow cytometric analysis of peripheral blood, we monitored changes in T-cell subsets after IL-7 treatment. We defined naive CD8+ T cells as CD8+/CD45RAhi/CD27+/CD11alow/modand naive CD4+ T cells as CD4+/CD45RAhi/CD27+. While IL-7 therapy increased the absolute number of naive CD4+ and CD8+ T cells (Figure 2A), IL-7 therapy also induced a transient change in CD11a expression on CD8+ cells with the emergence of a dominant population of CD11amod cells (Figure 2B) suggesting a partial conversion of the naive subset to an activated/memory phenotype as has been described in peripherally expanding T cells in settings of T-cell depletion.27,28 Thus, although phenotypic categorization led to the impression that naive cell number was increased following short-term IL-7 therapy, changes in CD11a expression suggested that IL-7 may have been inducing cycling of the naive compartment, as has been described following IL-7 treatment of human cord blood naive T cells.29-31
Treatment with IL-7 results in increases in naive T cells but declines in TREC levels.
(A) Increases in the absolute number of phenotypically naive CD4+ and CD8+ lymphocytes with IL-7 treatment at 500 μg/kg/d. Open shapes represent CD8+ naive defined as CD45RAhi/CD27+/CD11adull/mod, and solid shapes represent CD4+ naive defined as CD45RAhi/CD27. Data from individual animals are represented by a consistent shape. (B) Transient change in CD11a expression on CD8+/CD45RA+ peripheral blood lymphocytes. (C) Rapid and persistent decline in TRECs per 100 000 CD4+ and CD8+ lymphocytes in the peripheral blood following IL-7 treatment. Open shapes represent CD8+, and solid shapes represent CD4+. (D) Decline in absolute number of TREC+ T cells per microliter with IL-7 therapy.
Treatment with IL-7 results in increases in naive T cells but declines in TREC levels.
(A) Increases in the absolute number of phenotypically naive CD4+ and CD8+ lymphocytes with IL-7 treatment at 500 μg/kg/d. Open shapes represent CD8+ naive defined as CD45RAhi/CD27+/CD11adull/mod, and solid shapes represent CD4+ naive defined as CD45RAhi/CD27. Data from individual animals are represented by a consistent shape. (B) Transient change in CD11a expression on CD8+/CD45RA+ peripheral blood lymphocytes. (C) Rapid and persistent decline in TRECs per 100 000 CD4+ and CD8+ lymphocytes in the peripheral blood following IL-7 treatment. Open shapes represent CD8+, and solid shapes represent CD4+. (D) Decline in absolute number of TREC+ T cells per microliter with IL-7 therapy.
To determine whether the increases in naive T cells reflected enhanced thymic output, we measured T-cell receptor excision circles (TRECs) in separated CD4+ and CD8+ T cells in IL-7–treated primates. Interestingly, IL-7 therapy induced a dramatic decline in the frequency (Figure 2C) and absolute number (Figure 2D) of circulating TREC+ T cells. Because TRECs are diluted by cell division, a marked increase in T-cell turnover would be expected to lead to dilution of the frequency of TREC+ T cells in the periphery. Thus, the decline in TRECs in both CD4+ and CD8+ T cells suggested that IL-7 therapy was inducing cycling of multiple T-cell subsets.
Dramatic increase in the percentage of cycling peripheral T cells with IL-7 therapy
To further investigate whether IL-7 therapy induced peripheral T-cell activation and/or turnover, we evaluated T-cell activation markers and T-cell cycling parameters before and after IL-7 treatment. We observed no evidence for up-regulation of classical activation markers such as CD69, HLA-DR, or CD25; however, Fas was up-regulated on both CD4+ and CD8+ T cells (data not shown). To assess whether IL-7 might induce T-cell cycling in the absence of classical activation, we evaluated Ki67 expression following IL-7 therapy. Ki67 is a nuclear antigen that is expressed in cells undergoing proliferation. At baseline the percentage of both CD4+ and CD8+ T cells expressing Ki67 was less than 2% (Figure 3A). By day 6 of IL-7 treatment, 30% to 60% of the circulating CD4+ and CD8+ T cells were Ki67+, a feature that persisted to day 11. By day 21 (11 days following cessation of therapy) the percentage of cells expressing Ki67 had declined to near baseline, providing evidence that the increase in proliferation induced by IL-7 is reversible. To confirm the observation of IL-7–induced cycling and to determine whether IL-7–induced effects can persist, at least transiently, in the absence of the cytokine, we cultured PBMCs from IL-7–treated animals in media without any specific T-cell stimulus and pulsed with BrdU for 18 hours. Because peripheral blood mononuclear cells do not produce IL-7, it would be expected that IL-7 was essentially absent from these cultures. In cultures containing PBMCs collected prior to IL-7 treatment, less than 0.1% of CD4+and CD8+ T cells incorporated BrdU (Figure 3B). By day 6 of IL-7 therapy, the fraction of CD4+ and CD8+ T cells that incorporated BrdU following overnight culture increased to 1% to 3% and was persistently elevated at day 11, returning to near baseline by day 21. To determine whether increases in cell cycling were observed in both naive and memory cells, Ki67 expression was analyzed in CD95loCD28+ “naive” cells and CD95hiCD28− memory cells.32 For both CD4+ T cells and CD8+ T cells, substantial increases in Ki67 occurred with rhIL-7 therapy in both naive and memory subsets as defined by these parameters (data not shown). Thus, treatment with IL-7 leads to a profound but transient increase in the number of cycling T cells, an effect that persists, at least temporarily, following removal from exposure to IL-7.
Treatment with IL-7 induces profound peripheral T-cell cycling.
(A) Transient and marked increase in the percentage of Ki67+/CD4+ and Ki67+/CD8β T cells with IL-7 treatment. The numbers in the upper quadrant are the percentage of CD4+ and CD8β+ cells that stain with Ki67. (B) Increased BrdU uptake by CD4+ and CD8β+ T cells during 18 hour ex vivo culture initiated following in vivo exposure to IL-7. (C) Reversible decrease in p27kip1 and p16Ink4a proteins in peripheral blood mononuclear cells following IL-7 treatment. The ratio of p27kip1 and p16Ink4a to tubulin densitometry readings are shown below each lane.
Treatment with IL-7 induces profound peripheral T-cell cycling.
(A) Transient and marked increase in the percentage of Ki67+/CD4+ and Ki67+/CD8β T cells with IL-7 treatment. The numbers in the upper quadrant are the percentage of CD4+ and CD8β+ cells that stain with Ki67. (B) Increased BrdU uptake by CD4+ and CD8β+ T cells during 18 hour ex vivo culture initiated following in vivo exposure to IL-7. (C) Reversible decrease in p27kip1 and p16Ink4a proteins in peripheral blood mononuclear cells following IL-7 treatment. The ratio of p27kip1 and p16Ink4a to tubulin densitometry readings are shown below each lane.
Barata et al recently reported that IL-7-induced cycling of T-cell acute lymphoblastic leukemia (ALL) cells was associated with a down-regulation of p27kip1 and that forced expression of p27 kip1 in this system prevented IL-7–induced cell cycle progression.33 To assess the status of p27kip1 in PBMCs from normal primates following IL-7 therapy, we performed Western blotting of lysates from PBMCs following IL-7 therapy. Treatment with IL-7 led to a decrease in p27kip1 protein levels by day 6 and day 11 as well as a decrease in p16Ink4a (Figure 3C), findings that further confirm cycling of PBMCs following IL-7 treatment. Together these results provide compelling evidence that IL-7–induced cycling of mature T cells is a primary effect of IL-7 therapy. Although these studies could not directly assess whether IL-7–induced cycling required concomitant signaling through the T-cell receptor (TCR), evidence from murine models has shown that homeostatic-induced proliferation, which is mediated by IL-7, requires signaling through the TCR and that low-affinity ligands, which normally are not capable of inducing T-cell cycling, result in TCR-induced proliferation in the presence of IL-7.34 35 Thus, it appears most likely that most cycling cells following IL-7 therapy are those that encounter low-affinity antigen, which, when coupled with IL-7 signaling, results in T-cell proliferation without full-scale activation.
Immunologic effects of IL-7 therapy are preserved in SIV-infected macaques
Increased levels of IL-7 are observed in humans with T-cell depletion due to HIV infection or due to treatment with chemotherapy for cancer.12-14 The results presented thus far in normal T-cell–replete primates provide evidence that IL-7 therapy induces profound changes in peripheral T-cell homeostasis, but it was important to determine whether similar immunologic effects of IL-7 therapy would occur in situations where endogenous levels of IL-7 are expected to be elevated. Thus, we treated rhesus macaques with moderate CD4 depletion due to infection with simian immunodeficiency virus with rhIL-7 (100 μg/kg/d for 9 days). Plasma levels of more than 1000 pg/mL were achieved (mean at day 3, 1159 ± 60 pg/mL), markedly increased from undetectable levels present at baseline in these animals and substantially higher than endogenous levels observed in humans with T-cell depletion (Figure 4A). Interestingly, these levels are still 1 log less than standard concentrations used in vitro (10 ng/mL). As with normal cynomolgus monkeys, responding SIV-infected primates showed significant increases in the absolute numbers of CD4+ (Figure 4B) and CD8+ T cells (Figure 4C) (mean CD4 increase, 449 cells/mm3; mean CD8 increase, 501 cells/mm3, both P values less than .05, Wilcoxon signed-rank test). Furthermore, in these SIV-infected animals that received continuing antiretroviral therapy during IL-7 treatment, no change in viral load was observed (data not shown).
IL-7 treatment of SIV-infected macaques (100 μg/kg/d) leads to increased levels of circulating IL-7, increased CD4+ and CD8+ peripheral blood T-cell numbers, and down-regulation of IL-7Rα.
(A) Plasma levels of IL-7 detected using enzyme-linked immunosorbent assay (ELISA). Lines represent individual animals. (B) Absolute numbers of CD4+ T cells following vaccination with (solid lines) and without (dotted lines) IL-7 treatment. (C) Absolute numbers of CD3+/CD8+ T cells following vaccination with (solid lines) and without (dotted lines) IL-7 treatment. The increase by day 10 was significant for both CD4+ and CD8+ T cells (P < .05, Wilcoxon signed-rank test). (D-E) IL-7 therapy decreases IL-7Rα expression on circulating CD4+ and CD8+ T cells.
IL-7 treatment of SIV-infected macaques (100 μg/kg/d) leads to increased levels of circulating IL-7, increased CD4+ and CD8+ peripheral blood T-cell numbers, and down-regulation of IL-7Rα.
(A) Plasma levels of IL-7 detected using enzyme-linked immunosorbent assay (ELISA). Lines represent individual animals. (B) Absolute numbers of CD4+ T cells following vaccination with (solid lines) and without (dotted lines) IL-7 treatment. (C) Absolute numbers of CD3+/CD8+ T cells following vaccination with (solid lines) and without (dotted lines) IL-7 treatment. The increase by day 10 was significant for both CD4+ and CD8+ T cells (P < .05, Wilcoxon signed-rank test). (D-E) IL-7 therapy decreases IL-7Rα expression on circulating CD4+ and CD8+ T cells.
Marked but transient declines in IL-7 receptor expression on circulating T cells following rhIL-7 treatment
Further evidence of biologic effects of IL-7 treatment in moderately T-cell–depleted SIV-infected primates is the marked decline in IL-7Rα expression observed on both CD3+CD8+ and CD3+CD8−T cells coincident with IL-7 therapy (Figure 4D-E).
To determine whether these observations reflected true decreases in receptor level or simply blocking of antibody binding by saturating levels of IL-7, PBMCs form normal cynomolgus monkeys were incubated with IL-7 for 24 hours. Marked decreases in IL-7Rα were seen (Figure 5A-B) on cells incubated at 37°C in the presence of IL-7, whereas incubation of fixed cells at 37°C with IL-7 or unfixed cells at 4°C with IL-7 did not lead to diminished expression of IL-7Rα. There was no change in IL-7Rα expression on cells incubated at 37°C in the absence of rhIL-7 (data not shown). These results indicate that that the decline in IL-7Rα expression following IL-7 therapy results from receptor-mediated events requiring a metabolically active cell. Interestingly, in these studies, IL-7 levels showed progressive declines from day 3 onward despite continued IL-7 therapy through day 10. It remains unclear to what extent this results from receptor-mediated endocytic clearance and/or the development of antihuman IL-7–neutralizing antibodies, because these were found at later time points in the IL-7–treated SIV-infected animals, thus precluding analysis of more long-term therapy or effects of repeated cycles.
IL-7Rα down-regulation by IL-7 requires metabolic activity.
Expression of IL-7Rα on CD3+CD4+ (A) and CD3+CD8+ (B) T cells following incubation of cynomolgus PBMCs with IL-7 (10 ng/mL) for 24 hours. (Thin solid line, paraformaldehyde-fixed cells; gray line, unfixed cells incubated at 4°C; thick black line, unfixed cells incubated at 37°C; dotted line, isotype control).
IL-7Rα down-regulation by IL-7 requires metabolic activity.
Expression of IL-7Rα on CD3+CD4+ (A) and CD3+CD8+ (B) T cells following incubation of cynomolgus PBMCs with IL-7 (10 ng/mL) for 24 hours. (Thin solid line, paraformaldehyde-fixed cells; gray line, unfixed cells incubated at 4°C; thick black line, unfixed cells incubated at 37°C; dotted line, isotype control).
Discussion
In summary, pharmacologic dosing of IL-7 induces obvious measurable biologic responses that include dramatic rises in peripheral T-cell numbers in T-cell–replete and T-cell–depleted hosts. Further, these modest doses, which were well tolerated, result in circulating levels greatly exceeding those observed in T-cell–depleted humans.12-14 Together, these results provide evidence that physiologic elevations in circulating IL-7 that occur following T-cell depletion will not preclude responses to pharmacologic dosing of this agent and indicate that IL-7 therapy will likely augment peripheral T-cell numbers in humans following T-cell depletion. Importantly, when administered as a short course, the peripheral effects of IL-7 predominate due to a dramatic increase in peripheral T-cell cycling, which is physiologically similar to the increased homeostatic expansion known to occur in T-cell–depleted hosts. In both normal cynomolgus and SIV-infected rhesus macaques (M.M., T.F., C.L.M., G.F., manuscript in preparation), increases in cycling were observed in both naive and nonnaive T cells, indicating that the effects of pharmacologic dosing will not be confined to the naive subset. With regard to IL-7's capacity for immune reconstitution, the peripheral effects preclude a clear enumeration of thymopoiesis in this model system. Indeed, the dilution of TREC levels in cycling cells has been cited as a possible limitation of this assay for measuring thymopoiesis, especially in states of robust peripheral cell cycling.36 This suggests that alternative approaches for measuring thymic functions such as evaluation of repertoire diversification may be necessary to accurately assess thymopoietic effects in this setting. Importantly, it should also be noted that enhanced homeostatic expansion can result in substantial improvements in host immunocompetence-independent thymopoietic effects.37 Therefore, these findings provide further evidence to suggest that IL-7 is a promising immunorestorative agent. They also raise the intriguing possibility that IL-7 may be a useful adjunct in the context of immunotherapies such as tumor vaccines wherein enhanced T-cell cycling may increase the magnitude and/or the breadth of the response to therapeutic or protective vaccination. Indeed, preliminary studies suggest that IL-7 administration increases the number of effector and memory T-cell populations to immunodominant and subdominant epitopes when administered concomitantly with a T-cell–based vaccine (T.F., C.L.M., manuscript in preparation).
We thank Dr Fariba Navid for her indispensable help with the Western blotting. We also acknowledge John Merrill, Patricia J. Tosca, and the other staff at Battelle Memorial Institute.
Prepublished online as Blood First Edition Paper, October 31, 2002; DOI 10.1182/blood-2002-07-2297.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
T. Fry, Bldg 10, Rm 13N240, MSC 1928, 10 Center Dr, Bethesda, MD 20892-1928; e-mail:tf60y@nih.gov.