The survival of viral mediated lymphomas depends upon constitutive nuclear factor kappa B (NF-κB) activity. AIDS-related human herpesvirus type 8–associated primary effusion lymphoma (PEL) responds poorly to chemotherapy and is almost invariably fatal. We have previously demonstrated that the antiviral combination of interferon alpha (IFN-α) and azidothymidine (AZT) induces apoptosis in PEL cell lines. We therefore used these agents as therapy for an AIDS patient with PEL. The patient had a dramatic response, with complete resolution of his malignant effusion in 5 days. In PEL cells, the death receptor ligand known as tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is markedly up-regulated by IFN-α; however, signals transduced by death receptors may also activate an antiapoptotic response mediated by NF-κB. In both the primary tumor cells from our patient and PEL cell lines, AZT selectively blocked nuclear entry of the NF-κB heterodimer p50 and p65, an effect not seen with other nonthymidine antiviral nucleosides. AZT monophosphate, the principal intracellular metabolite, inhibited phosphorylation and degradation of IκB by the IκB kinase complex. AZT- and IFN-α-mediated apoptosis was blocked by expression and nuclear localization of an IκB-resistant form of NF-κB (the p50 subunit linked to the transactivation domain of herpes simplex virus VP16). The proapoptotic effect of AZT and IFN-α in PEL occurs through the concomitant activation of TRAIL and blockade of NF-κB and represents a novel antiviral therapy for a virally mediated tumor.
Introduction
Immunodeficient patients are predisposed to the development of a variety of malignancies, including non-Hodgkin lymphomas (NHLs).1,2 Many of these lymphomas are associated with oncogenic herpesviruses.3 Primary effusion lymphoma (PEL), a recently described subtype of NHL, presents as a malignant effusion in patients with advanced AIDS, but may also occur in human immunodeficiency virus (HIV)–negative individuals.4,5 A hallmark of PEL is its association with human herpesvirus type 8 (HHV-8), although Epstein-Barr virus (EBV) sequences are usually detected in malignant cells.6,7 PELs rarely express T- or B-cell surface antigens but are almost always genotypically B-cell lymphomas.8 The pathogenesis of PEL is unclear; however, a number of genes encoded by HHV-8 with antiapoptotic or transforming potential have been identified.9-11 The prognosis in AIDS-related PEL is dismal, and most patients die despite treatment with conventional chemotherapy.
One molecular feature of PEL that is common to other viral mediated lymphoproliferative diseases (eg, human T-lymphotropic virus type I–associated adult T-cell leukemia [HTLV-I ATLL], EBV-associated lymphomas) is elevated DNA-binding activity of the transcriptional activator nuclear factor kappa B (NF-κB).12 Constitutive activity of the NF-κB pathway has also been shown recently to be an important characteristic of a subset of diffuse large cell lymphomas that have a particularly poor prognosis.13 The NF-κB family (RelA[p65], cRel, RelB, p50, and p52) form heterodimers that bind to κB sites and regulate transcription. In quiescent cells, NF-κB dimers are sequestered in the cytoplasm bound to members of the inhibitor of kappa B (IκB) family.14 Under the stimuli of malignant transformation, viral infection, or cytokines, IκB is phosphorylated at specific serine sites by the IκB kinase (IKK) complex and degraded via the ubiquitin proteosome pathway.15 NF-κB proteins then translocate to the nucleus, where they bind to specific target sequences, activating transcription of various genes.16NF-κB target genes encode a variety of immunomodulatory and antiapoptotic proteins.17,18 Recent work has demonstrated that the proapoptotic effects of death receptor (DR) ligands, including tumor necrosis factor (TNF), Fas ligand, and TNF-related apoptosis-inducing ligand (TRAIL), may be abrogated by concomitant activation of NF-κB or potentiated in the absence of RelA.19-21 Because constitutive NF-κB nuclear activity is an inherent antiapoptotic property of many malignancies, agents that inhibit this process are being evaluated in clinical trials. Investigators have demonstrated that blockade of nuclear localization of NF-κB in EBV and HHV-8 lymphoma lines results in the apoptotic death of these tumors.22,23 The combination of the antiviral thymidine analog azidothymidine (AZT) and interferon alpha (IFN-α) has been used to treat several viral associated cancers, including HTLV-I-associated ATLL and AIDS-related Kaposi sarcoma.24,25 While studying the in vitro activity of these 2 agents in other types of viral lymphomas, we recently observed an interesting property in established PEL lines. Marked apoptosis was induced by the combination of AZT and IFN-α, whereas apoptosis was negligible with either agent alone.26 Further investigation revealed that in PEL cells, IFN-α induced expression of TRAIL to a level far exceeding that seen in other types of B-cell lymphomas. AZT- and IFN-α-mediated apoptosis was blocked by expression of dominant-negative Fas-associated death domain (FADD), soluble TRAIL receptors (DR-4 and DR-5), and overexpression of a decoy receptor, which indicated that cytotoxicity occurred through a TRAIL/DR interaction.27
To elucidate this proapoptotic mechanism induced by AZT and IFN-α in PEL, we focused on the cooperative effect of AZT in facilitation of the IFN-α (TRAIL)–activated apoptosis. We demonstrate here that AZT monophosphate (AZTMP), which is generated at high levels in PEL cells, blocks NF-κB nuclear localization through inhibition of IKK-mediated phosphorylation of IκB and markedly potentiates IFN-α (TRAIL)–mediated apoptosis. These processes were demonstrated in cell lines and primary PEL cells derived at the time of diagnosis from a patient who subsequently experienced a complete remission after treatment with parenteral AZT and IFN-α. The proapoptotic activity of AZT and IFN-α in PEL occurs through a concomitant activation of TRAIL and blockade of NF-κB constitutive nuclear localization.
Patient, materials, and methods
The study was approved by the University of Miami Institutional Review Board/Medical Sciences Committee. Written informed consent was obtained from the patient before enrollment.
Apoptosis analyses
A total of 5.0 × 105 cells were grown in 10 mL Iscove modified Dulbecco medium (IMDM; GIBCO-BRL, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (FBS) in the presence of medium, 10 μg/mL AZT, 1000 U/mL IFN-α, or 10 μg/mL AZT plus 1000 U/mL IFN-α for 36 hours. After this, 2.0 × 105 cells were removed from the flask and stained with annexin V–fluorescein isothiocyanate/propidium iodide (PI), as described previously.27 Cells were then analyzed using fluorescence-activated cell sorting (FACScan flow cytometer; Becton Dickinson, San Jose, CA).
Preparation of nuclear extracts
Nuclear extracts were prepared as described by Dignam et al,28 with some modification. Cells (5 × 106) were washed twice with cold phosphate-buffered saline (PBS), and the cell pellet was suspended in 400 μL hypotonic buffer A (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 1 mM dithiothreitol [DTT], 0.5 mM phenylmethylsulfonyl fluoride, and protease inhibitor cocktail). After 15 minutes of incubation on ice, 25 μL of 10% Nonidet P-40 was added, and the sample was vigorously vortexed. The nuclei were collected by centrifugation at 3000g for 5 minutes at 4°C and washed once with buffer A, and the nuclear pellet was suspended in 75 μL buffer B (20 mM HEPES, pH 7.9, 400 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA [ethylenediaminetetraacetic acid], 1 mM DTT, 5% glycerol, and protease inhibitor cocktail) and incubated for 30 minutes at 4°C with brief mixing. The mixture was centrifuged at 10 000g for 5 minutes at 4°C. The supernatant was saved as the nuclear extract and stored at −80°C. Protein concentrations were measured using the Bradford assay (Bio-Rad, Richmond, CA).
Western blot
After the indicated treatment, 1.2 × 107 cells were suspended in lysis buffer (150 mM NaCl, 50 mM Tris-HCl; pH 7.5), 1 mM DTT, Triton X-100 (0.1%), and protease inhibitor cocktail (Sigma, St Louis, MO) and incubated on ice for 15 minutes. Protein content was determined with the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA). Forty micrograms of total protein was mixed with an equal volume of 2× sample buffer and fractionated on 12% sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE). Rainbow marker (Amersham Life Science, Arlington Heights, IL) was used as a low-molecular-weight standard. Proteins were transferred electrophoretically to nitrocellulose membranes and preblocked with 5% nonfat dry milk in Tris-buffered saline (TBS) for 60 minutes at room temperature. Incubation with the polyclonal anti-human IκBα antibody (PharMingen, San Diego, CA) and monoclonal anti-human IKKα/β antibody (Santa Cruz Biotechnology, Santa Cruz, CA) in 1% milk/TBS was performed for 60 minutes at room temperature. The blot was then washed 3 times with TBS for 5 minutes each. The membrane was incubated with horseradish peroxidase-conjugated secondary antibody (1:2000 dilution) for 60 minutes at room temperature, followed by washing with TBS 3 times for 10 minutes each. Immunoreactive proteins were detected after treatment with SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology, Rockford, IL) using autoradiography.
Electrophoretic mobility shift assay (EMSA)
NF-κB and activator protein-1 (AP-1) consensus oligonucleotide (Santa Cruz, CA) were end-labeled with [γ-32P]adenosine triphosphate (ATP) using T4 polynucleotide kinase. Unincorporated ATP was removed with G-50 spin columns (Amersham, Piscataway, NJ) and centrifugation. Before addition of oligonucleotide probe, 15 μg nuclear protein was incubated with binding buffer (2 μg poly(dI-dC), 12% glycerol, 20 mM HEPES, pH 7.0, 1 mM DTT, 1 mM EDTA, 50 mM NaCl) for 10 minutes at room temperature. A radiolabeled oligonucleotide (20 000-50 000 counts per minute [cpm]) was incubated with reaction mixture for 15 minutes at room temperature and subjected to 5% nondenaturing PAGE in 0.5× TBE buffer. The gels were dried and analyzed by autoradiography. Supershift was performed by adding antibodies to the incubation mixture of nuclear extract (p50, p52, p65, and c-rel; Santa Cruz Biotechnology) and incubating for 45 minutes on ice before the addition of radiolabeled probe.
Phosphorylation of AZT
A total of 5.0 × 106 cells of HHV-8+PEL (primary cells BCLM and the PEL cell line BCBL-1) and viral-negative B-cell lymphoma (BJAB) were grown in 1.0 mL IMDM (GIBCO-BRL) supplemented with 10% heat-inactivated FBS in the presence of medium alone or medium containing 5 μg/mL AZT for 21 hours. Growth medium was then replaced with 1.0 mL of medium containing 5 μg/mL 3H-AZT (at a ratio of 1:203H-AZT [Moravek Biochemicals, Brea, CA] to cold AZT), and cells were grown for 3 additional hours. Cells were harvested, washed 3 times with ice-cold PBS, extracted in 0.5 mL 65% ice-cold methanol, and kept at −20°C overnight. Samples were then centrifuged at 14 000g in a refrigerated Eppendorf microcentrifuge (Brinkman Instruments, Westbury, NY) for 20 minutes. Supernatants containing AZT metabolites were harvested, dried, and dissolved in 60 μL ddH2O. The extracts of AZT metabolites were analyzed by high-performance liquid chromatography (HPLC). An anion exchange column (Whatman Partisil 5 SAX; Whatman, Clifton, NJ) was eluted at a rate of 1 mL/min in the gradient mode by using solvent A (10 mM NH4H2PO4 buffer, pH 3.8, containing 7% methanol) and solvent B (750 mM NH4H2PO4 buffer, pH 3.8, containing 7% methanol). The gradient condition used was as follows: 0 to 8 minutes, 0% B; 8 to 16 minutes, 0% to 80% B; 16 to 30 minutes, 80% B; 30 to 35 minutes, 80% to 0% B; and 35 to 40 minutes, 0% B. The retention times observed were 3.78, 9.42, 18.80, and 22.56 minutes for AZT, AZTMP, AZT diphosphate (AZTDP), and AZT triphosphate (AZTTP), respectively.
Assay of IKK activity
Drug-treated cells were washed twice with PBS buffer, then lysed in kinase lysis buffer (KLB: 25 mM Tris [pH 8.0], 150 mM NaCl, 25 mM β-glycerophosphate, 250 μM Na-orthovanadate, 5 mM EDTA, 5 mM EGTA [ethyleneglycoltetraacetic acid], 1% Triton X-100, 10% glycerol, 25 mM NaF, and protease inhibitor cocktail [Sigma]) for 20 minutes at 4°C. The cell lysates were centrifuged at 10 000g for 10 minutes, and the resulting supernatants (total cell extracts) were collected.
To analyze IKK activity, 100 μg cytoplasmic extract was rotated with 2 μg anti-IKKα/β polyclonal antibody (SC-7607; Santa Cruz Biotechnology) in KLB for 1 hour at 4°C and then for an additional 2 hours with protein A–agarose (20 μL; Santa Cruz Biotechnology). The immunoprecipitates were washed twice with KLB and then once with kinase assay buffer (KAB: 20 mM HEPES, pH 7.6, 10 mM MgCl2, 0.1 mM orthovanadate, 10 mM β-glycerophosphate, 1 mM DTT, 50 mM NaCl, and protease inhibitor cocktail). The washed bead was incubated with 20 μL KAB containing 1 μg glutathione S–transferase (GST)–IκBα (1-317) (SC-4094; Santa Cruz Biotechnology), substrate 50 μM ATP, and 2.5 μCi (0.0925 MBq) [γ-32P]ATP (Amersham, Arlington Heights, IL) for 30 minutes at 30°C. The reactions were stopped by addition of 5× SDS-PAGE sample buffer. The proteins were resolved by 10% SDS-PAGE and transferred onto a polyvinylidene difluoride membrane. IKKα/β activity was evaluated from the formulation of γ32P-IκBα-GST detected by autoradiography. The same membrane was used for Western blot with polyclonal antibodies to IKKα/β to quantitate and normalize the kinase activities.
DNA transfection
Plasmid DNA containing NF-κB p50 subunit coupled to the herpes simplex virus (HSV) VP16 (a generous gift from Dr Collin Duckett, National Institutes of Health) was cotransfected with episomal expression vector pBMG-Neo at a ratio of 5:1. The construct was introduced into BC-3 cells by electroporation. Cells were then transferred to a 75-cm flask and grown in 50 mL IMDM supplemented with 10% heat-inactivated FBS and 500 μg/mL G-418 for selection of positive transfectants. Medium was changed every 4 days for a duration of 3 weeks. Single clones were selected by limiting dilution and confirmed by Western blot.
Results
AZT and IFN-α induce remission in a PEL patient and apoptosis in primary tumor cells
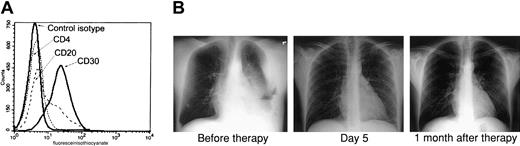
Because the combination of AZT and IFN-α exhibited a potent proapoptotic effect in PEL lines and both of these antiviral agents have been used extensively in AIDS patients, we developed a clinical protocol for this relatively rare disease. Recently an HIV-positive man, 35 years of age, presented to the emergency room complaining of shortness of breath. His absolute CD4+ T-cell count was 222/mm3 with an undetectable HIV load, and he had not taken any antiretroviral medications for several months before admission. Chest x-rays revealed a large left-sided pleural effusion and fluid accumulation in the mediastinum. Examination of this pleural fluid revealed an anaplastic lymphoproliferative process, and flow cytometry performed on tumor cells demonstrated the typical phenotype of PEL: CD4−, CD20−, and CD30+ (Figure1A). Multiple analyses of tumor cells by polymerase chain reaction (PCR) repeatedly demonstrated the presence of HHV-8 and absence of EBV (data not shown). The patient was advised of his condition, and written informed consent was obtained for our clinical protocol, which uses twice-daily parenteral AZT 1.5 g and IFN-α 5 million units. No other antineoplastic, corticosteroid, or antiretroviral agents were administered. The patient had a remarkable response with resolution of his effusion within 5 days, as demonstrated by chest x-rays (Figure 1B) and computed tomography scan (not shown). Ten days after starting parenteral therapy, he was discharged on oral AZT 600 mg daily and subcutaneous IFN-α 5 million units daily. A repeated chest film performed on the patient one month after diagnosis was clear. He continued taking AZT and IFN-α only for an additional 2 months and then stopped taking antiviral agents, although he was seen 6 months later at our AIDS clinic and was symptom free.
AZT and IFN-α induce remission in a PEL patient.
(A) Primary tumor cells (BCLM) from an HHV-8+ PEL patient demonstrate typical phenotype of PEL. Surface expression of CD4, CD20, and CD30 on 1 × 106 cells from pleural fluid was determined by FACS analysis. (B) AZT and IFN-α induce remission in the PEL patient. Left: Chest x-ray of the same patient 2 days after diagnostic thoracentesis and before therapy with twice-daily parenteral AZT 1.5 g and IFN-α 5 million units. Middle: Chest x-ray 5 days after initiation of therapy. Right: Chest x-ray 1 month after therapy.
AZT and IFN-α induce remission in a PEL patient.
(A) Primary tumor cells (BCLM) from an HHV-8+ PEL patient demonstrate typical phenotype of PEL. Surface expression of CD4, CD20, and CD30 on 1 × 106 cells from pleural fluid was determined by FACS analysis. (B) AZT and IFN-α induce remission in the PEL patient. Left: Chest x-ray of the same patient 2 days after diagnostic thoracentesis and before therapy with twice-daily parenteral AZT 1.5 g and IFN-α 5 million units. Middle: Chest x-ray 5 days after initiation of therapy. Right: Chest x-ray 1 month after therapy.
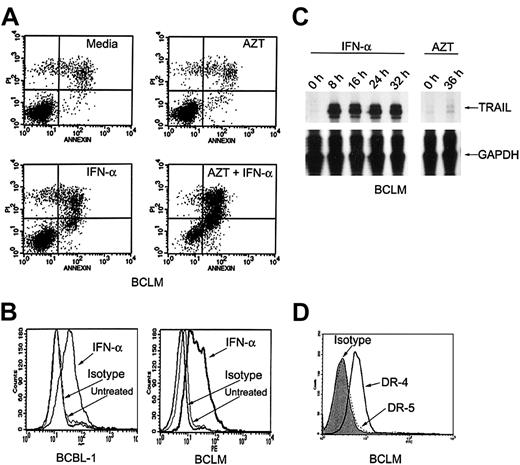
Primary PEL cells express TRAIL in response to IFN-α
We studied fresh residual primary tumor cells that had been obtained from the initial procedure on our patient. As we had previously noted in HHV-8+ PEL lines, AZT (10 μg/mL) and IFN-α (1000 U/mL) together induced apoptosis in 64% of the primary PEL cells (called BCLM) within 36 hours (Figure2A). To determine whether IFN-α activated the expression of TRAIL in primary PEL cells, we studied primary BCLM cells and the PEL line BCBL-1. In BCLM and BCBL-1 cells, IFN-α (1000 U/mL) induced marked expression of TRAIL mRNA (measured by RNAse protection assays) as well as surface protein (measured by flow cytometry). TRAIL mRNA induction occurred rapidly within 8 hours after treatment with IFN-α, and surface expression was maximal by 24 to 36 hours, whereas AZT had no effect on its expression (Figure 2B-C). Flow cytometric analysis of the patient's cells demonstrated a high level of expression of death receptor 4 (DR-4), but little DR-5 expression (Figure 2D). Expression of DR-4 was not affected by pretreatment with AZT (data not shown). Therefore, the in vitro effect of AZT and IFN-α in primary PEL cells and cell lines correlated closely with the clinical response to these agents.
IFN-α induces expression of TRAIL in primary PEL cells.
(A) AZT and IFN-α synergize to induce apoptosis in primary PEL cells (BCLM). Cells were treated for 36 hours with medium, AZT 10 μg/mL, IFN-α 1000 U/mL, or AZT 10 μg/mL plus IFN-α 1000 U/mL. Cells were analyzed for apoptosis by PI/annexin-V staining and FACS analysis. Top and bottom right quadrants of each box are the apoptotic populations. (B) IFN-α induces TRAIL surface expression in primary PEL cells. BCBL-1 and BCLM cells were treated for 24 hours with medium or IFN-α 1000 U/mL and assayed for TRAIL expression by FACS analysis. (C) TRAIL mRNA is induced by IFN-α but not by AZT in primary PEL cells. BCLM cells were treated with medium; IFN-α for 8, 16, 24, and 32 hours; or AZT 10 μg/mL for 36 hours and examined for TRAIL mRNA expression by RNAse protection assay. Reduced glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was used as an internal control. (D) Primary PEL cells express TRAIL receptors. BCLM surface expression of DR-5 (dotted line) and DR-4 (solid line) was determined by FACS analysis. Shaded area is mouse isotype control (IgG1).
IFN-α induces expression of TRAIL in primary PEL cells.
(A) AZT and IFN-α synergize to induce apoptosis in primary PEL cells (BCLM). Cells were treated for 36 hours with medium, AZT 10 μg/mL, IFN-α 1000 U/mL, or AZT 10 μg/mL plus IFN-α 1000 U/mL. Cells were analyzed for apoptosis by PI/annexin-V staining and FACS analysis. Top and bottom right quadrants of each box are the apoptotic populations. (B) IFN-α induces TRAIL surface expression in primary PEL cells. BCBL-1 and BCLM cells were treated for 24 hours with medium or IFN-α 1000 U/mL and assayed for TRAIL expression by FACS analysis. (C) TRAIL mRNA is induced by IFN-α but not by AZT in primary PEL cells. BCLM cells were treated with medium; IFN-α for 8, 16, 24, and 32 hours; or AZT 10 μg/mL for 36 hours and examined for TRAIL mRNA expression by RNAse protection assay. Reduced glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was used as an internal control. (D) Primary PEL cells express TRAIL receptors. BCLM surface expression of DR-5 (dotted line) and DR-4 (solid line) was determined by FACS analysis. Shaded area is mouse isotype control (IgG1).
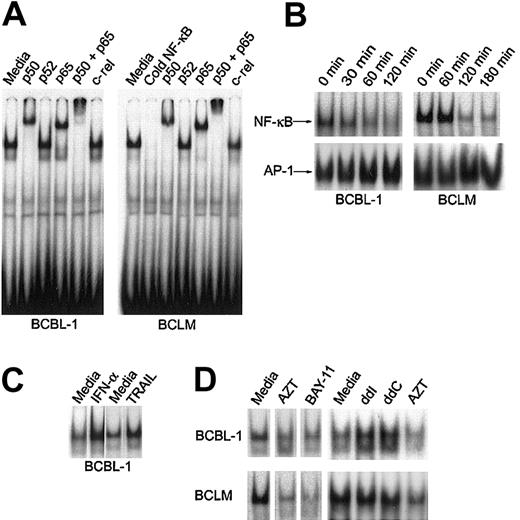
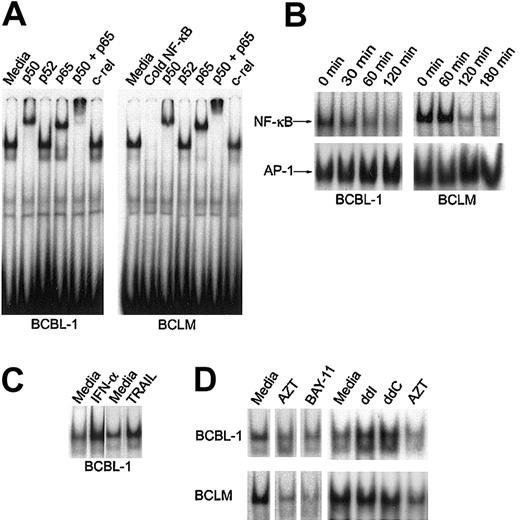
AZT inhibits constitutive NF-κB nuclear activity in PEL
The signal transduced by interaction between several death receptors and their constitutive ligands (including TRAIL) has been shown in some cell types to also activate NF-κB nuclear activity.20 AZT potentiated the proapoptotic effect of IFN-α (TRAIL), and we had previously demonstrated that it also potentiated the proapoptotic effect of Fas ligand in PEL lines.26 We therefore investigated the effect of AZT on constitutive NF-κB activity in primary PEL cells and the BCBL-1 line. Consistent with previous work by other investigators, we found that the predominant form of NF-κB in nuclear extracts of primary BCLM and BCBL-1 cells was the p50/p65 heterodimer23 (Figure3A). To study the effect of AZT on NF-κB localization in PEL, we cultured primary and BCBL-1 cells in 10 μg/mL AZT and measured NF-κB nuclear activity by EMSA. Within 1 to 2 hours, AZT clearly inhibited NF-κB nuclear signal in the PEL cells (Figure 3B). We measured the nuclear AP-1 signal as a control, and this demonstrated no decrease in intensity. Treatment of BCBL-1 with IFN-α (1000 U/mL for 16 hours) or TRAIL (50 ng/mL for 1 hour) actually up-regulated nuclear NF-κB to even higher levels than baseline, which indicated that in this tumor, the proapoptotic effect of TRAIL might be blunted by the concomitant antiapoptotic effect of NF-κB activity (Figure 3C). We compared the effect of AZT with that of BAY-11, an inhibitor of NF-κB that has been shown to induce apoptosis in PEL,23 and found that both agents effectively blocked NF-κB nuclear signal (Figure 3D). In contrast to AZT, the commonly used antiretroviral nucleosides didanosine (ddI) and zalcitabine (ddC) actually enhanced NF-κB nuclear activity (Figure 3D). This was in agreement with our previous finding that these agents in combination with IFN-α did not induce apoptosis in PEL cells.27 This finding demonstrated that the effect of AZT in blocking NF-κB nuclear activity in PEL is a specific feature of this antiviral agent.
AZT inhibits constitutive NF-κB nuclear activity in PEL.
(A) NF-κB complexes in primary PEL (BCLM) and BCBL-1 are composed of p50/p65 heterodimer. Purified nuclear extracts (15 μg) from PEL cells were preincubated with the indicated antibodies and subjected to EMSA using the 32P-labeled NF-κB oligonucleotide probe. The results are representative of 3 independent experiments. (B) AZT blocks NF-κB nuclear colocalization in PEL. Nuclear extracts were prepared from cells treated with AZT (10 μg/mL) for the indicated times, and binding activity was assayed by EMSA using either NF-κB (top panels) or AP-1 (bottom panels) specific consensus oligonucleotides as probes. (C) Induction of NF-κB by IFN-α and soluble TRAIL in BCBL-1. BCBL-1 cells were treated with either IFN-α (1000 U/mL) for 18 hours or soluble TRAIL (50 ng/mL) for 1 hour. Nuclear extracts were then assayed by EMSA for NF-κB. (D) Effect of nucleoside analogs and BAY-11 on NF-κB translocation. BCBL-1 and BCLM cells were treated with AZT (10 μg/mL), BAY-11 (10 μM), ddI (10 μg/mL), or ddC (10 μg/mL) for 2 hours and assayed by EMSA using NF-κB consensus oligonucleotides as probes.
AZT inhibits constitutive NF-κB nuclear activity in PEL.
(A) NF-κB complexes in primary PEL (BCLM) and BCBL-1 are composed of p50/p65 heterodimer. Purified nuclear extracts (15 μg) from PEL cells were preincubated with the indicated antibodies and subjected to EMSA using the 32P-labeled NF-κB oligonucleotide probe. The results are representative of 3 independent experiments. (B) AZT blocks NF-κB nuclear colocalization in PEL. Nuclear extracts were prepared from cells treated with AZT (10 μg/mL) for the indicated times, and binding activity was assayed by EMSA using either NF-κB (top panels) or AP-1 (bottom panels) specific consensus oligonucleotides as probes. (C) Induction of NF-κB by IFN-α and soluble TRAIL in BCBL-1. BCBL-1 cells were treated with either IFN-α (1000 U/mL) for 18 hours or soluble TRAIL (50 ng/mL) for 1 hour. Nuclear extracts were then assayed by EMSA for NF-κB. (D) Effect of nucleoside analogs and BAY-11 on NF-κB translocation. BCBL-1 and BCLM cells were treated with AZT (10 μg/mL), BAY-11 (10 μM), ddI (10 μg/mL), or ddC (10 μg/mL) for 2 hours and assayed by EMSA using NF-κB consensus oligonucleotides as probes.
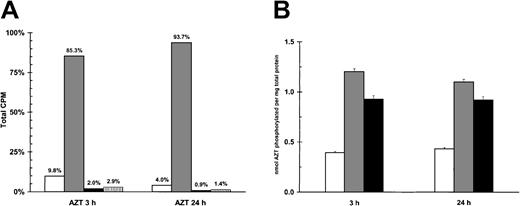
Elevated levels of AZTMP in PEL cells
AZT, a prodrug, is initially phosphorylated to its monophosphate form, a process dependent upon thymidine kinase (TK).29Both viral and cellular TK can catalyze this step.30 The subsequent rate-limiting step by thymidylate kinase results in the production of relatively low levels (compared with the predominant form of AZTMP) of AZTDP and AZTTP.31 Because AZT blocked NF-κB, we investigated its intracellular metabolism in PEL cells. Primary BCLM cells, BCBL-1 cells, and a control viral-negative B-cell lymphoma (BJAB) line were treated with 5 μg/mL of a combination of unlabeled AZT (95%) and tritiated AZT (3H-AZT; 5%) and then assayed by HPLC for AZT phosphorylation. In all cells, the metabolite AZTMP was repeatedly found to be the predominant species of AZT. Figure 4A demonstrates that more than 90% of the phosphorylated species of AZT in BCLM cells was AZTMP. When we compared relative levels of total phosphorylated AZT between the PEL cells and BJAB, 200% to 300% greater levels of phosphorylated forms of AZT were detected in the HHV-8+ lines (Figure 4B). This demonstrated that the predominant species of AZT in PEL cells was AZTMP, and this metabolite was detected at substantially higher levels in PEL cells than in viral-negative BJAB cells.
AZT is phosphorylated in PEL cells.
(A) Primary PEL cells (BCLM) phosphorylate AZT to AZTMP. A total of 5 × 106 BCLM cells were treated with 3H-AZT and cold AZT (5 μg/mL) for 3 and 24 hours. Methanol extracts were then subjected to HPLC fractionation to separate the different forms of AZT. White bar (■) indicates AZT; gray bar (░), AZTMP; black bar (▪), AZTDP; and vertical lined bar (▥), AZTTP. (B) Quantification of total phosphorylation of AZT in HHV-8+ PEL cells versus viral negative B-cell lymphoma (BJAB). A total of 5 × 106 HHV-8+ PEL (BCLM, BCBL-1) and BJAB cells were treated with 3H-AZT and cold AZT (5 μg/mL) for 3 and 24 hours. Methanol extracts were then subjected to HPLC, and all forms of phosphorylated AZT were quantitated by β-scintillation counting. White bar (■) indicates BJAB; gray bar (░), BCLM; and black bar (▪), BCBL-1.
AZT is phosphorylated in PEL cells.
(A) Primary PEL cells (BCLM) phosphorylate AZT to AZTMP. A total of 5 × 106 BCLM cells were treated with 3H-AZT and cold AZT (5 μg/mL) for 3 and 24 hours. Methanol extracts were then subjected to HPLC fractionation to separate the different forms of AZT. White bar (■) indicates AZT; gray bar (░), AZTMP; black bar (▪), AZTDP; and vertical lined bar (▥), AZTTP. (B) Quantification of total phosphorylation of AZT in HHV-8+ PEL cells versus viral negative B-cell lymphoma (BJAB). A total of 5 × 106 HHV-8+ PEL (BCLM, BCBL-1) and BJAB cells were treated with 3H-AZT and cold AZT (5 μg/mL) for 3 and 24 hours. Methanol extracts were then subjected to HPLC, and all forms of phosphorylated AZT were quantitated by β-scintillation counting. White bar (■) indicates BJAB; gray bar (░), BCLM; and black bar (▪), BCBL-1.
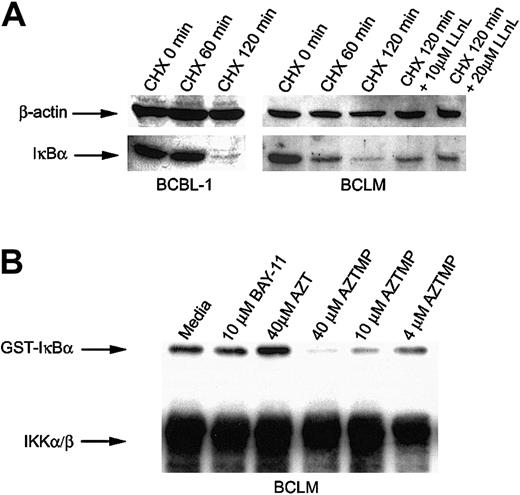
AZTMP blocks IKKβ-mediated phosphorylation of IκBα
The 2 principal regulators of NF-κB are IκBα and its kinase, the IKK complex (IKKα/β/δ). IKKβ has a substantially higher specific activity toward IκBα than IKKα. Therefore, most of its kinase activity is attributable to IKKβ.32,33IKK-induced phosphorylation of IκBα triggers its degradation in cells, followed by the translocation of NF-κB to the nucleus. Constitutive IKK activity in activated B-cell-type NHLs has been demonstrated as a mechanism that results in NF-κB nuclear translocation.13 Because we had established that AZT blocked NF-κB translocation, we decided to study its effect on the components of this pathway. To determine whether primary PEL cells constitutively express IKK, we cultured BCLM and BCBL-1 cells in the protein synthesis inhibitor cycloheximide (CHX) and measured IκBα by Western blot. The level of IκBα declined after 2 hours, which indicated progressive phosphorylation and degradation of IκBα, mediated by the intrinsically high level of IKK in PEL cells. A proteosome inhibitor, N-acetyl-L-leucinyl-L-leucinyl-L-norleucinal (LLnL; Sigma), partially blocked degradation of IκBα in CHX-treated PEL cells (Figure 5A). Because AZTMP was the predominant form of AZT produced in PEL cells, we studied its effect on the activity of IKK by an in vitro kinase assay. IKK was immunoprecipitated from BCLM cells, and equivalent aliquots were treated with PBS, BAY-11, AZT, or AZTMP and then assayed for the ability to phosphorylate recombinant IκBα. Interestingly, only AZTMP (in a dose-dependent fashion), and not AZT or BAY-11, inhibited IKK activity (Figure 5B). This mechanism may differ from that of BAY-11, another NF-κB inhibitor, which blocks TNF-α-inducible rather than constitutive phosphorylation of IκBα.34 To confirm that this was not a property of a kinase variant found only in PEL cells, we performed similar experiments on IKK immunoprecipitated from BJAB cells and demonstrated the same effect (data not shown). These experiments demonstrated that AZTMP inhibited NF-κB by blocking phosphorylation and degradation of IκBα.
AZTMP inhibits IKKβ-mediated phosphorylation of IκBα.
(A) PEL cells constitutively express IKK. BCBL-1 and BCLM cells were treated with CHX (50 μg/mL) for 1 and 2 hours, and Western blot analysis of whole-cell extracts was performed using antibodies specific for IκBα, with β-actin used as a control. BCLM cells were also treated with CHX for 2 hours in the presence of the proteosome inhibitor LLnL (10 and 20 μM) and assayed for IκBα by Western blot analysis. (B) Phosphorylated AZT (AZTMP) specifically inhibits IKK activity. Top panel: 100 μg cytosolic extract from BCLM cells was immunoprecipitated with anti-IKKα/β polyclonal antibody. The purified enzyme was incubated in vitro with medium, BAY-11 (10 μM), AZT (40 μM), or AZTMP (40, 10, and 4 μM). IKK activity was measured using recombinant GST-IκBα (1-317) as a substrate in the presence of 32P-ATP. Bottom panel: Western blot analysis using the same IKK antibody.
AZTMP inhibits IKKβ-mediated phosphorylation of IκBα.
(A) PEL cells constitutively express IKK. BCBL-1 and BCLM cells were treated with CHX (50 μg/mL) for 1 and 2 hours, and Western blot analysis of whole-cell extracts was performed using antibodies specific for IκBα, with β-actin used as a control. BCLM cells were also treated with CHX for 2 hours in the presence of the proteosome inhibitor LLnL (10 and 20 μM) and assayed for IκBα by Western blot analysis. (B) Phosphorylated AZT (AZTMP) specifically inhibits IKK activity. Top panel: 100 μg cytosolic extract from BCLM cells was immunoprecipitated with anti-IKKα/β polyclonal antibody. The purified enzyme was incubated in vitro with medium, BAY-11 (10 μM), AZT (40 μM), or AZTMP (40, 10, and 4 μM). IKK activity was measured using recombinant GST-IκBα (1-317) as a substrate in the presence of 32P-ATP. Bottom panel: Western blot analysis using the same IKK antibody.
AZT- and IFN-α–mediated apoptosis in PEL is NF-κB dependent
To determine whether forced expression of NF-κB could prevent apoptosis induced by AZT and IFN-α, we used the NF-κB p50 subunit coupled to the HSV VP16 transactivation domain. p50 is found in the nucleus in quiescent lymphocytes, which suggests that it lacks a transactivation domain. When bound to DNA as a homodimer, it functions as an inhibitor of NF-κB activity; however, with the addition of the HSV transactivator (VP16), p50 can function as a transcription factor despite the absence of p65 and independent of IκB control.35 BC-3 cells were transfected with this construct, and Western blots performed on nuclear protein extracts from these cells demonstrated high levels of p50 compared with wild-type BC-3 cells (Figure 6A).
AZT- and IFN-α-mediated apoptosis in PEL is NF-κB dependent.
(A) BC-3 p50/VP16 cells express high levels of nuclear NF-κB compared with wild-type BC-3 cells. Purified nuclear extracts (15 μg) from wild-type BC-3 cells and BC-3/VP16 transfectants were assayed by Western blot for expression of NF-κB. (B) Effect of AZT on NF-κB nuclear signal in p50/VP16 transfectants. Untransfected BC-3 cells (WT) and 2 clones of BC-3 p50/VP16 transfectants were treated with AZT (10 μg/mL) for 30 minutes. Nuclear extracts (15 μg) were assayed for NF-κB by EMSA. (C) Transfection of p50/VP16 renders BC-3 cells resistant to AZT and IFN-α. BC-3 and BC-3 p50/VP16 transfectants were treated with medium or AZT (10 μg/mL) plus IFN-α (1000 U/mL) for 36 hours. Viability was assessed by trypan blue exclusion.
AZT- and IFN-α-mediated apoptosis in PEL is NF-κB dependent.
(A) BC-3 p50/VP16 cells express high levels of nuclear NF-κB compared with wild-type BC-3 cells. Purified nuclear extracts (15 μg) from wild-type BC-3 cells and BC-3/VP16 transfectants were assayed by Western blot for expression of NF-κB. (B) Effect of AZT on NF-κB nuclear signal in p50/VP16 transfectants. Untransfected BC-3 cells (WT) and 2 clones of BC-3 p50/VP16 transfectants were treated with AZT (10 μg/mL) for 30 minutes. Nuclear extracts (15 μg) were assayed for NF-κB by EMSA. (C) Transfection of p50/VP16 renders BC-3 cells resistant to AZT and IFN-α. BC-3 and BC-3 p50/VP16 transfectants were treated with medium or AZT (10 μg/mL) plus IFN-α (1000 U/mL) for 36 hours. Viability was assessed by trypan blue exclusion.
BC-3 wild-type cells were compared with BC-3/p50 transfectants for sensitivity to AZT blockade of NF-κB. Transfectants were unaffected by AZT, whereas in wild-type cells, AZT blocked nuclear localization of NF-κB (Figure 6B).
These transfectants were also quite resistant to AZT and IFN-α. By 36 hours, AZT and IFN-α killed approximately 70% of wild-type BC-3 cells, whereas those transfected with p50/VP16 were 70% viable (Figure6C).
Discussion
We have demonstrated a mechanism whereby an antiviral thymidine analog potentiates IFN-α–mediated apoptosis in PEL, a highly aggressive viral lymphoma. Therapy based on these findings yielded a complete remission in the absence of other chemotherapy in an AIDS patient suffering from PEL. The mechanism of AZT- and IFN-α-mediated apoptosis in PEL was demonstrated in tumor cells obtained directly from the patient at the time of diagnosis and confirmed in other PEL lines. The outcome of HIV-positive PEL patients treated solely with conventional chemotherapy has been extremely poor, and a treatment that uses antiviral agents, especially those that combine antiretroviral activity, activation of TRAIL, and blockade of NF-κB, should be superior to nonspecific cytotoxic chemotherapy. We have previously demonstrated that IFN-α up-regulates TRAIL in PEL cell lines, and other investigators have demonstrated that type I interferons activate the expression of TRAIL in T cells, natural killer cells, and some malignant cell types.36-38 Anticancer therapy with TRAIL has been proposed; however, there is some concern about its lack of specificity and potential for causing end-organ damage.39The specific IFN-α–mediated induction of TRAIL in a malignancy provides a much more targeted approach to therapy.
Although the underlying mechanisms for the unique sensitivity of HHV-8+ PEL to AZT and IFN-α are not totally defined, our data demonstrated several important findings. PEL expresses very high levels of TRAIL in response to IFN-α. Therefore, PEL may be uniquely sensitive to death receptor ligand-mediated apoptosis compared with other types of viral and nonviral B-cell lymphomas. Both TRAIL and IFN-α activate NF-κB in PEL, which potentially inhibits their antineoplastic effect. On the basis of these findings, we reasoned that blockade of NF-κB should therefore potentiate the proapoptotic effect of IFN-α. A similar scenario has been described in multiple myeloma.40 The composition of NF-κB heterodimer has also been shown to be critical in TRAIL-induced apoptosis. Apoptosis is potentiated by cRel (not found in PEL cells) and absence of RelA (which was accomplished by AZT treatment).21 AZTMP accumulated to high levels in PEL, which led us to study the effect of this metabolite of AZT on the NF-κB signaling pathway. Our data indicate that AZTMP inhibits NF-κB nuclear translocation by blocking the phosphorylation of IκBα by IKK. The response observed in our patient also demonstrates in vivo the antitumor potential of inhibition of IKK in NF-κB-dependent tumors. The effect of AZT on antiapoptotic factors activated by NF-κB is unknown, although inhibition of elements that interfere with FADD-dependent apoptosis would facilitate IFN-α (TRAIL)-mediated apoptosis. One possibility demonstrated recently is that AZT inhibits the expression of vFLIP, a viral antiapoptotic protein that both inhibits FADD-dependent apoptosis and stimulates IκB kinase.41
Why AZT was so effectively phosphorylated in PEL is unclear. PEL cells did express a low level of viral TK, as measured by reverse transcriptase PCR (data not shown). This enzyme, as well as cellular TK, catalyzes monophosphorylation of AZT. However, expression of a lytic gene product would be expected to be quite low in a transformed tumor cell line. Recent data have indicated that a variety of noxious stimuli, including radiation and chemotherapy, induce the expression of lytic proteins in EBV-infected tumors.42 The disruption of viral latency may be linked to activation of cellular stress response elements, such as Jun N-terminal kinase and C-Jun/AP-1.43It is possible that AZT, originally designed as a chemotherapeutic agent, might also activate these pathways. Although the combination of AZT and IFN-α exhibited impressive antitumor activity in our patient, these tumors are infrequently diagnosed, and confirmation of the role of this combination will require a larger study involving multiple institutions. It should also be noted that the tumor lines as well as the patient material used in our studies were not coinfected with EBV. Most PELs carry EBV.11 The presence of another oncogenic herpesvirus may alter the expression of cellular and viral apoptotic factors and sensitivity to this therapy. Preliminary data indicate that the response to AZT and IFN-α therapy seen in our patient can be recapitulated in a PEL/severe combined immunodeficiency mouse model (G.F., unpublished observation, August 2002). This animal model should prove valuable to study dose response to antivirals and combinations of other therapeutic agents. Previous work has demonstrated that PEL is sensitive to BAY-11-mediated blockade of NF-κB. Our results indicate that AZT also blocks NF-κB, although perhaps with a degree of specificity toward herpesvirus lymphomas. There is also a wealth of clinical experience with both AZT and IFN-α. AZT is activated by phosphorylation and therefore might function as an inhibitor of NF-κB only in tumors that express high levels of TK. It is interesting to note that AZT and IFN-α have been used successfully to treat patients with ATLL,44another aggressive malignancy known for constitutive NF-κB activity. We have found that AZT and IFN-α also activate TRAIL expression in leukemic ATLL cells in vivo, coinciding with regression of tumor (W.J.H., unpublished data, July 2002). In addition, we have found that parenteral AZT is active in some cases of EBV lymphoproliferative disease. Therefore, AZT and IFN-α may be useful in viral lymphoproliferative diseases that share the same features: ability to induce death receptor signaling coupled with a dependence on NF-κB activity. Conventional chemotherapy for PEL produces poor results, and treatment protocols that incorporate AZT and IFN-α should be designed to study the effectiveness of these agents in this group of patients.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-08-2525.
Supported by grants UO1 CA 700580 (AIDS Malignancies Consortium) and RO1 CA 82274 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
William J. Harrington Jr, University of Miami School of Medicine, Sylvester Comprehensive Cancer Center, Rm 3400 (D8-4), 1475 NW 12th Ave, Miami, FL 33136; e-mail:wharring@med.miami.edu.