Plasmodium falciparum may cause severe forms of malaria when excessive sequestration of infected and uninfected erythrocytes occurs in vital organs. The capacity of wild-type isolates of P falciparum–infected erythrocytes (parasitized red blood cells [pRBCs]) to bind glycosaminoglycans (GAGs) such as heparin has been identified as a marker for severe disease. Here we report that pRBCs of the parasite FCR3S1.2 and wild-type clinical isolates from Uganda adhere to heparan sulfate (HS) on endothelial cells. Binding to human umbilical vein endothelial cells (HUVECs) and to human lung endothelial cells (HLECs) was found to be inhibited by HS/heparin or enzymes that remove HS from cell surfaces.35S-labeled HS extracted from HUVECs bound directly to the pRBCs' membrane. Using recombinant proteins corresponding to the different domains of P falciparum erythrocyte membrane protein 1 (PfEMP1), we identified Duffy-binding–like domain–1α (DBL1α) as the ligand for HS. DBL1α bound in an HS-dependent way to endothelial cells and blocked the adherence of pRBCs in a dose-dependent manner. 35S-labeled HS bound to DBL1α-columns and eluted as a distinct peak at 0.4 mM NaCl.35S-labeled chondroitin sulfate (CS) of HUVECs did not bind to PfEMP1 or to the pRBCs' membrane. Adhesion of pRBCs of FCR3S1.2 to platelet endothelial cell adhesion molecule–1 (PECAM-1)/CD31, mediated by the cysteine-rich interdomain region 1α (CIDR1α), was found be operative with, but independent of, the binding to HS. HS and the previously identified HS-like GAG on uninfected erythrocytes may act as coreceptors in endothelial and erythrocyte binding of rosetting parasites, causing excessive sequestration of both pRBCs and RBCs.
Introduction
Mature trophozoites, the later intraerythrocytic form of Plasmodium falciparum, are bound in the deep microvasculature, primarily to endothelial cells (cytoadherence) and to erythrocytes (rosetting), whereas ring-stage trophozoites circulate in the peripheral blood. The sequestration of parasitized red blood cells (pRBCs) and RBCs is suggested to be mediated by P falciparum erythrocyte membrane protein 1 (PfEMP1), a parasite-derived polypeptide expressed at the surface of the infected pRBCs. The resulting binding of pRBCs in the inner organs1 helps the parasite to hide from the immune system and withdraw from splenic clearance. Yet binding of the pRBCs may be lethal to the parasite because excessive sequestration may cause severe malaria and the death of its human host.
PfEMP1 is a high–molecular weight (200 to 350 kDa) transmembrane polypeptide consisting of 4 to 7 extracellular domains encoded by the var gene family.2,3 PfEMP1 mediates the sequestration of pRBCs through interactions with receptors such as CD36, platelet endothelial cell adhesion molecule–1 (PECAM-1)/CD31, intercellular adhesion molecule–1 (ICAM-1), immunoglobulin G (IgG), and IgM.4-12 Lectinlike interactions13 of PfEMP1 have been described with glycans such as the blood group A antigen14,15; heparan sulfate (HS)–like glycosaminoglycans (GAGs) present on uninfected erythrocytes16,17; and chondroitin sulfate (CS), a galactosaminoglycan. Indeed, CS of the A type (CSA), containing 4-O-sulfated glucosamine units, has been found to act as a receptor for pRBC binding in the placenta18 and in the microvasculature.19
GAGs are long, linear carbohydrate chains attached to a core protein, forming a proteoglycan. HS is one such GAG, composed of alternating glucosamine and uronic acid residues in a repeating disaccharide unit (-4GlcAβ1-4GlcNAcα1-). During biosynthesis, this backbone becomes variably modified by N-deacetylation/N-sulfation and C5 epimerization of the glucuronic acid to iduronic acid, followed by O-sulfation at C2 of the uronic acid and at C3 and C6 of the glucosamine unit. Owing to biosynthetic constraints, HS chains are modified to different degrees, and the modified units, especially the sulfate groups, are unevenly distributed along the chain. Thus, HS in different species and tissues expresses distinct molecular characteristics.20-22 The related molecule heparin, which can be considered a highly sulfated form of HS, is more extensively modified and more uniformly sulfated over the whole chain. HS is found on almost all cell surfaces, including endothelial cells, whereas heparin is found only within granules in connective tissue mast cells.
About 50% of rosettes formed by cultured strains or fresh isolates have been found to be sensitive to the disruption by HS and heparin.13,16,17 Furthermore, a heparinase III–sensitive receptor has been suggested to be involved in the formation of rosettes.16 The capacity to bind heparin to the pRBC surface has also been found to be relatively common among clinical samples and associated with the severity of disease.23Duffy-binding–like domain–1α (DBL1α) of FCR3S1.2, an N-terminally located domain of PfEMP1 present in almost all PfEMP1s known to date,24 has been demonstrated to be a heparin-binding protein.16,25 The finding that DBL1α also interacts with GAGs from other sources15 25 suggested to us that HS might be implicated in the cytoadherence of pRBCs to vascular endothelial cells. In this study, we show that human endothelial cell surface HS indeed functions as a receptor in the cytoadherence of pRBCs of FCR3S1.2 as well as of wild-type clinical isolates from Uganda. In addition, the binding to HS was found to be via the N-terminal DBL1α domain, which for the first time is demonstrated to be involved in cytoadherence.
Materials and methods
Parasites
The parasite FCR3S1.2,15 a highly rosetting clone obtained from FCR3S1 by micromanipulation, was used in some experiments. The rosetting rate was routinely greater than 75%. The parasites were cultivated in malaria culture medium (RPMI 1640–HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 25 mM sodium bicarbonate, 10 μg/mL gentamicin) containing 10% human serum (blood group AB Rh+) according to standard procedures.26 27
The P falciparum clinical isolates U10, U11-1, U11-2, U14, U15, U18, U22-1, U22-2, and U26 were obtained from venous blood sample taken from malaria patients in Kampala, Uganda. In all cases, informed consent was obtained from the patients and/or their parents. The erythrocytes were immediately separated from mononuclear cells on Polymorphprep (Axis-Shield, Oslo, Norway) and washed in RPMI 1640. Nonimmune AB Rh+ serum to a final hematocrit of 40% and freezing media (28% glycerol, 3% sorbitol, 0.65% NaCl) in a 1:1 ratio were added before freezing the cells in liquid N2. For the assays, the freshly frozen isolates were thawed and cultured in malaria culture medium in their own blood containing 15% human serum (blood group AB Rh+) until they had matured into trophozoites.26
Cells
Human umbilical vein endothelial cells (HUVECs) were isolated from fresh umbilical cords obtained from the Karolinska Hospital (Stockholm, Sweden) as previously described.28 Briefly, human umbilical veins were flushed with phosphate-buffered saline (PBS), then filled with PBS containing 0.2% trypsin and incubated for 30 minutes at room temperature. The HUVECs were removed from the vein by PBS wash, and the trypsin solution was added to HUVEC medium (MCDB-131 medium, 25 mM HEPES, 0.2% NaHCO3, 10 ng/mL epidermal growth factor [Sigma, St Louis, MO], 1 μg/mL hydrocortisone [GIBCO, Paisley, Scotland]), containing 10% fetal calf serum (FCS). Other HUVEC preparations used were purchased from Cytotech (Nivaa, Denmark). Cells were grown in HUVEC medium supplemented with 5% FCS. Human lung endothelial cells (HLECs) were a generous gift from Artur Scherf (Institut Pasteur, Paris, France) and Jürg Gysin (Faculté de Medecine, Université de la Mediterranée, Marseille, France). HLECs were grown in HLEC medium (Dulbecco modified Eagle [DME]/F12 medium, 1.2 g/L sodium bicarbonate, 10 mg/L gentamicin, 15 μg/mL endothelial cell growth supplement [BD Biosciences, Bedford, MA]) containing 10% fetal bovine serum.
Polysaccharides
GAGs were radiolabeled byN-3H–acetylating free amino groups to a specific activity of 125 000 cpm/μg, 70 000 cpm/μg, and 475 000 cpm/μg for bovine lung heparin, swine liver HS, and swine intestine HS, respectively, as described.29Selective chemical modification of bovine lung heparin was prepared as described previously.29,30 The proteoglycan pool from HUVECs was purified from Na235SO4(NEN)–metabolically labeled cells essentially as described.31 To isolate the GAGs HS and CS from the intact proteoglycan, a proteolytic digestion of the core proteins with pronase E (0.3 mg/mg protein) was performed. To purify HS from the GAG pool, CS was cleaved off with chondroitinase ABC followed by anion-exchange chromatography as described.31 CS was isolated by treating the GAG pool with nitrous acid at pH 1.5 to cleave HS, followed by separation on anion-exchange chromatography. Heparin, HS, and CSA from porcine intestine used in rosetting, cytoadherence, and immunofluorescence assays were obtained from Løvens Kemiske Fabrik (Ballerup, Denmark). Bovine lung heparin was a gift from Pharmacia (Kalamazoo, MI) and was purified as described.32 HS from swine liver and swine intestine was purified as previously described.20
Expression of recombinant PfEMP1 domains
Gene constructs encoding 3 PfEMP1 domains (DBL1α, cysteine-rich interdomain region 1α [CIDR1α], and DBL2δ) of FCR3S1.2 were expressed as glutathioneS-transferase (GST) fusion proteins and purified as described.16 Briefly, the pGEX-4T-1 system was used, and individual fragments were inserted downstream of a GST sequence. The proteins were expressed in Escherichia coli (BL21) and induced with 0.1 mM isopropyl-β-D-thiogalactoside, following a 4-hour incubation at 30°C. The fusion proteins were purified on glutathione-sepharose according to the manufacturer (GST Gene Fusion System; Pharmacia-Upjohn, Uppsala, Sweden). The fusion proteins were extensively dialyzed against PBS before use.
Cytoadherence assay
The cytoadherence assay was performed as described27 with some modifications. Briefly, HUVECs or HLECs were seeded out onto gelatin-treated Thermion coverslips (13 mm2) (Nunc, Labassco, Sweden). The cells were seeded at a density of 30 000 cells per well and incubated at 37°C for 24 to 48 hours before use to allow for attachment. Then, pRBCs freed of rosettes by mechanical disruption with a syringe were resuspended in binding medium (RPMI 1640; 25 mM HEPES, pH 6.8; 25 μg/mL gentamicin) with or without 10% human serum and added to the RPMI 1640–prewashed target cells. The cultures were allowed to incubate for 1 hour with intermediate resuspension of sedimented erythrocytes every 15 minutes. After incubation, unbound pRBCs were removed by washes in binding medium, and the remaining cells were fixed with 1% glutaraldehyde in PBS for 1 hour at room temperature. The cells were stained with 1% Giemsa for 1 hour at room temperature. To estimate the number of bound pRBCs per cell, 300 to 500 endothelial cells were counted by means of light microscopy (Optiphot-2; Nikon, Tokyo, Japan) at magnification × 1000.
Inhibition of adhesion was tested by addition of different GAGs (HS, heparin, and CSA at 0.01 to 10 mg/mL); antibodies (antihuman PECAM-1/CD31 monoclonal antibodies, and clone JC/70A [DAKO, Copenhagen, Denmark] at 0.001 to 10 μg/mL); or proteins (DBL1α-GST and GST at 50, 100, and 200 μg/mL) to cells before addition of pRBCs or together with pRBCs. Enzyme treatment of the cells was performed with heparinase III (25°C, pH 7.4), chondroitinase ABC (37°C, pH 7.5), neuraminidase (37°C, pH 6.0), or hyaluronidase (22°C, pH 6.0) at 0.002, 0.02, and 0.2 IU/mL for 2 hours before adding parasites (all purchased from Sigma).
Rosette disruption assay
The assay was performed essentially as described.27 33 Briefly, GAGs (0.001 to 1 mg/mL) were added to 25 μL aliquots of a rosetting FCR3S1.2 culture in a microtiter plate. The mixture was incubated for 30 minutes at 37°C. The rosetting rate was subsequently estimated after staining with acridine orange and compared with mock-treated controls. The level of rosetting was expressed as the number of rosette-forming late-stage pRBCs per total number of late-stage pRBCs × 100.
Immunofluorescence
HUVECs or HLECs were detached from the culture flasks with a “rubber policeman” and resuspended in PBS to a cell density of 2 to 4 × 106. HUVECs were used with or without 0.3% bovine serum albumin (BSA). Fusion proteins, consisting of GST-DBL1α, GST-CIDR1α, or GST alone, were added at 50, 100, or 200 μg/mL to the cells and incubated for 1 hour. The cells were washed 3 times in PBS, and the binding of the fusion proteins was visualized by incubating the cells with mouse anti-GST monoclonal antibody (clone GST-2, IgG2b, diluted 1:100) (Sigma) following incubation with fluorescein isothiocyanate (FITC)–conjugated antimouse immunoglobulin antibody (diluted 1:20) (DAKO). All incubations were for 1 hour at room temperature and with slow rotation. Surface fluorescence was studied in incident ultraviolet (UV) light by means of a Nikon Optiphot-2 microscope. In some of the experiments, 1 mg/mL HS was added together with the ligand, or the cells were treated with 10 μg/mL anti–PECAM-1/CD31 antibodies or with 0.2 IU/mL heparinase III.
Binding of GAGs to pRBCs
Trophozoite-infected erythrocytes (2 × 108) were incubated with 0.01 nM [3H]HS or 3000 cpm [35S]HS and [35S]CS in PBS for 1 hour at room temperature with intermediate resuspension every 15 minutes. After 3 washes in PBS, the cells were lysed in 1 mM Tris (tris(hydroxymethyl)aminomethane), pH 7.4, and 0.1 mM EDTA (ethylenediaminetetraacetic acid). Samples were centrifuged, and membranes were separated from the supernatant. Radioactivity bound to the membranes was analyzed by liquid scintillation counting.
Binding of GAGs to recombinant PfEMP1 domains
DBL1α-GST, CIDR1α-GST, DBL2δ-GST, or GST alone was coupled to a 1 mL NHS-Hi-trap-column (Pharmacia-Upjohn) as suggested by the manufacturer. Radiolabeled [35S]HS and [35S]CS, purified from HUVECs, or [3H]heparin purified from bovine lung were added to the column equilibrated in Tris-buffered saline (TBS) (50 mM Tris, pH 7.4; 150 mM NaCl) and incubated for 20 minutes. Unbound material was washed out with 5 mL TBS. Elution of bound GAGs was performed with a stepwise gradient of 0.2 to 2 M NaCl in TBS. Fractions were analyzed for radioactivity by liquid scintillation counting.
Results
pRBCs bind to HS available on the endothelial cell surface
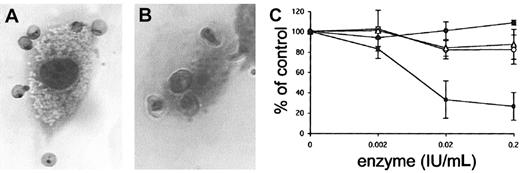
To evaluate the function of GAGs as receptors for pRBCs on human endothelial cells, FCR3S1.2 pRBC adhesion to HUVECs and HLECs was studied as described. An average of 487 ± 44 FCR3S1.2 pRBCs bound per 100 HUVECs and 342 ± 62 FCR3S.2 pRBCs bound per 100 HLECs, as estimated from 3 individual experiments (Figure 1A-B). When the HUVECs were pretreated with heparinase III to remove HS from the cell surface, adhesion decreased by more than 70% (73.5%) (Figure 1C), and an average of 129 ± 37 pRBCs bound per 100 HUVECs. Adhesion was not affected when HUVECs were pretreated with neuraminidase, chondroitinase ABC, or hyaluronidase (Figure 1C), indicating that neither sialic acid, CS, nor hyaluronan participated in the binding of infected erythrocytes to the cells. The binding capacity to HLECs decreased by more the 40% upon heparinase III treatment, and an average of 189 ± 56 pRBCs bound per 100 HLECs (44%; data not shown).
Heparinase sensitivity in adhesion of infected erythrocytes to human endothelial cells.
Adhesion of infected erythrocytes to human endothelial cells is heparinase sensitive. (A-B) Adhesion of FCR3S1.2-infected erythrocytes to HUVECs (panel A) and HLECs (panel B) stained with Giemsa (“Materials and methods”). Original magnification × 1000. (C) HUVECs were treated with the enzymes neuraminidase (▵), chondroitinase ABC (○), hyaluronidase (●), or heparinase III (▪) for 2 hours before incubation with FCR3S1.2 for 1 hour at 37°C. All samples are compared with controls in which pRBCs alone were incubated with untreated HUVECs. All data are expressed as the mean of 3 independent experiments ± SD.
Heparinase sensitivity in adhesion of infected erythrocytes to human endothelial cells.
Adhesion of infected erythrocytes to human endothelial cells is heparinase sensitive. (A-B) Adhesion of FCR3S1.2-infected erythrocytes to HUVECs (panel A) and HLECs (panel B) stained with Giemsa (“Materials and methods”). Original magnification × 1000. (C) HUVECs were treated with the enzymes neuraminidase (▵), chondroitinase ABC (○), hyaluronidase (●), or heparinase III (▪) for 2 hours before incubation with FCR3S1.2 for 1 hour at 37°C. All samples are compared with controls in which pRBCs alone were incubated with untreated HUVECs. All data are expressed as the mean of 3 independent experiments ± SD.
To confirm that the use of an HS receptor is true not only for the cloned parasite FCR3S1.2, wild-type clinical isolates from Uganda were analyzed. Of 9 isolates, 8 grow into mature trophozoites, with parasitemia ranging between 2.2% and 7.2%. These were tested for binding to HUVECs. Four of these 8 isolates, U10, U11-2, U14, and U18, showed adhesion over 100 pRBCs per 100 cells (140, 207, 101, and 232 pRBCs per 100 cells, respectively) (Table1). These were considered to be binders to HUVECs and are discussed further. Isolates generating lower cytoadherence than 100 pRBCs per 100 cells were considered to be nonbinders to HUVECs in this study (U11-1, U15, U22-1, and U22-2 with 51, 11, 6, and 38 pRBCs per 100 cells, respectively). After heparinase III treatment of the target cells, U10, U11-2, U14, and U18 decreased in binding by 44, 29, 94, and 10%, respectively (79, 147, 6, and 209 pRBCs per 100 cells, respectively) (Table 1).
HS blocks the adhesion of pRBCs to endothelial cells
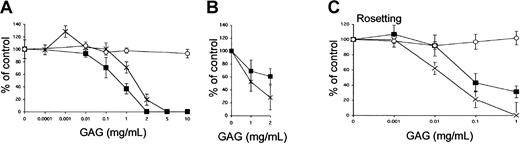
In a complementary assay, we tested whether soluble GAGs could compete for the binding of pRBCs to endothelial cells. To estimate the competing effect of the different GAGs, HS, heparin, and CSA (0.1 to 10 mg/mL) were added together with the infected erythrocytes (FCR3S1.2) to HUVECs and HLECs in the cytoadherence assay. HS and heparin inhibited adhesion to HUVECs in a dose-dependent manner, whereas CSA had no effect even at the highest concentrations tested (Figure 2A). Cytoadherence of pRBCs to HLECs was also affected by the addition of the GAGs HS and heparin in a dose-dependent manner (Figure 2B). On HUVECs, HS was found to be more effective than heparin in the cytoadhesion assay with a 50% inhibitory concentration (IC50) of 0.3 mg/mL as compared with 1.2 mg/mL for heparin. The opposite was found on HLECs, on which heparin, with an IC50 of 1 mg/mL, was a more effective inhibitor than HS. The IC50 for HS could not be determined at the concentrations tested (Figure 1B). In parallel, the same GAG preparations were examined for inhibition of rosette formation (Figure 2C). For rosette disruption, heparin was a better inhibitor than HS. Heparin inhibited rosetting with an IC50of 0.02 mg/mL, whereas HS did not completely inhibit rosetting even at the highest concentration tested.
GAG-dependent cytoadherence and rosetting of infected erythrocytes.
(A-B) Inhibition of cytoadherence to HUVECs (panel A) and HLECs (panel B) was studied by incubating pRBCs on endothelial cells as described in “Materials and methods.” The GAGs HS (▪), heparin (×), or CSA (○) were added at the indicated concentrations together with the erythrocytes. (C) In the rosetting assay, rosettes were mechanically disrupted and allowed to re-form in the absence or presence of HS (▪), heparin (×), or CSA (○) for 1 hour at 37°C. All data are expressed as the mean of 3 independent experiments ± SD.
GAG-dependent cytoadherence and rosetting of infected erythrocytes.
(A-B) Inhibition of cytoadherence to HUVECs (panel A) and HLECs (panel B) was studied by incubating pRBCs on endothelial cells as described in “Materials and methods.” The GAGs HS (▪), heparin (×), or CSA (○) were added at the indicated concentrations together with the erythrocytes. (C) In the rosetting assay, rosettes were mechanically disrupted and allowed to re-form in the absence or presence of HS (▪), heparin (×), or CSA (○) for 1 hour at 37°C. All data are expressed as the mean of 3 independent experiments ± SD.
The effect of GAGs on cytoadherence of wild-type clinical isolates from Uganda was analyzed on the isolates according to the same criteria (U-10, U11-2, U14, and U18). Isolates U10 and U14 decreased by 44% and 54% after adding 1 mg/mL HS and by 54% and 94% upon addition of 1 mg/mL heparin, respectively (Table 1). Heparin (1 mg/mL) affected cytoadhesion of sample U11-2 and binding decreased by 36%, whereas double HS concentration was needed to exert a modest effect. No loss of adhesion from the use of any of the GAGs was seen in sample U18.
Two different domains of PfEMP1 mediate binding to HS and PECAM-1/CD31
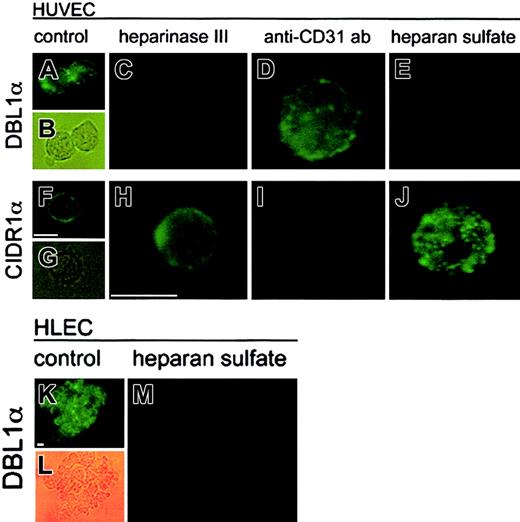
Because PECAM-1/CD31 is a known receptor involved in cytoadherence of pRBCs and is expressed by HUVECs, we wanted to examine the relative involvement of PECAM-1/CD31 and HS as receptors for pRBCs of FCR3S1.2. The interaction between recombinant domains of PfEMP1 and the receptors when they are located on the human endothelial cell surface was studied. The 2 most N-terminal domains of PfEMP1var1, DBL1α and CIDR1α, were tested for binding to HUVECs by means of an immunofluorescence assay as described. Recombinant GST-fusion proteins of DBL1α or CIDR1α were incubated with HUVEC, and the adhesion was detected by means of a fluorescent antibody to GST. Strong fluorescence was observed when DBL1α and CIDR1α bound to normal HUVECs, whereas GST alone did not bind to the cells (Figure 3A-B, F-G, and data not shown). When the HUVECs were pretreated with heparinase III to remove cell surface HS, no binding was detected with DBL1α (Figure3C), whereas CIDR1α continued to bind well to the HS-free cells (Figure 3H). In contrast, incubation of the cells with an anti–PECAM-1/CD31 antibody blocking PECAM-1/CD31 on the cell surface abolished binding of CIDR1α (Figure 3I) but not binding of DBL1α (Figure 3D). DBL1α also bound to HLECs in an HS-sensitive manner (Figure 3K-M). With these results, we show for the first time that DBL1α acts as a ligand to an endothelial receptor and add HS to the receptor map of DBL1α for cytoadherence, but also confirm earlier data with PECAM-1/CD31 as a CIDR receptor.15 The role of HS as a receptor for DBL1α, but not for CIDR1α, could be further confirmed by competition of adhesion with soluble HS (Figure 3E,J, respectively). These findings together suggest that the 2 domains are binding to distinct receptor molecules at the cell surface. On HUVECs, the DBL1α domain binds to an HS receptor, and CIDR1α binds to PECAM-1/CD31. No difference was seen in the absence or presence of BSA.
Interaction of 2 individual domains of PfEMP1 expressed as recombinant GST-fusion proteins to human endothelial cells.
Binding of DBL1α (panels A-E,K-M) and CIDR1α (panels F-J) to nontreated HUVECs (panels A-B,F-G) or HLECs (panels K-L); heparinase III–treated HUVECs (0.2 IU/mL for 2 hours) (panels C,H); PECAM-1/CD31–blocked HUVECs (10 μg/mL anti–PECAM-1/CD31 antibody) (panels D,I); and soluble HS–treated (1 mg/mL) HUVECs (panels E,J) and HLECs (panel M). Binding was visualized by incubating the cells with a mouse anti-GST antibody following incubation with an FITC-conjugated antimouse antibody and analyzed by light microscopy (× 1000). Scale bar equals 15 μm.
Interaction of 2 individual domains of PfEMP1 expressed as recombinant GST-fusion proteins to human endothelial cells.
Binding of DBL1α (panels A-E,K-M) and CIDR1α (panels F-J) to nontreated HUVECs (panels A-B,F-G) or HLECs (panels K-L); heparinase III–treated HUVECs (0.2 IU/mL for 2 hours) (panels C,H); PECAM-1/CD31–blocked HUVECs (10 μg/mL anti–PECAM-1/CD31 antibody) (panels D,I); and soluble HS–treated (1 mg/mL) HUVECs (panels E,J) and HLECs (panel M). Binding was visualized by incubating the cells with a mouse anti-GST antibody following incubation with an FITC-conjugated antimouse antibody and analyzed by light microscopy (× 1000). Scale bar equals 15 μm.
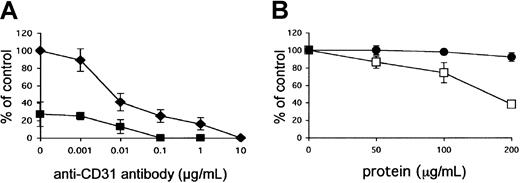
The role of the 2 receptor molecules was subsequently tested in the cell adhesion assay. When the endothelial cells were incubated with anti–PECAM-1/CD31 antibodies (0.001 to 10 μg/mL), 100% inhibition of cytoadherence was reached at a concentration of 10 μg/mL antibody (Figure 4A). Total inhibition could be achieved at a 100 × lower concentration of antibody when the cells were pretreated with heparinase III (0.2 IU/mL) (Figure 4A). Preincubation of HUVECs with recombinant GST-DBL1α fusion protein before the addition of pRBCs partially inhibited cytoadherence in a dose-dependent manner, whereas the GST control did not affect cytoadherence (Figure 4B), confirming a complementary role of the 2 receptors in endothelial binding.
Role of PECAM-1/CD31 and HS as endothelial receptors for pRBCs of FCR3S1.2.
(A) Adhesion of pRBCs was tested on nontreated HUVECs (♦) or after pretreatment (▪) with heparinase III (0.2 IU/mL for 2 hours) in the presence of anti–PECAM-1/CD31 antibody at the indicated concentrations. All samples were compared with controls in which pRBCs alone were incubated with nontreated HUVECs. (B) DBL1α-GST (●) or GST alone (■) was incubated with HUVECs for 1 hour before pRBCs were added to HUVECs and allowed to bind for 1 hour. All data are expressed as the mean of 3 independent experiments ± SD.
Role of PECAM-1/CD31 and HS as endothelial receptors for pRBCs of FCR3S1.2.
(A) Adhesion of pRBCs was tested on nontreated HUVECs (♦) or after pretreatment (▪) with heparinase III (0.2 IU/mL for 2 hours) in the presence of anti–PECAM-1/CD31 antibody at the indicated concentrations. All samples were compared with controls in which pRBCs alone were incubated with nontreated HUVECs. (B) DBL1α-GST (●) or GST alone (■) was incubated with HUVECs for 1 hour before pRBCs were added to HUVECs and allowed to bind for 1 hour. All data are expressed as the mean of 3 independent experiments ± SD.
35S-labeled human endothelial HS binds to infected erythrocytes and to DBL1α
To study the binding properties of the receptor purified from host target cells, human endothelial GAGs were purified. HUVECs were metabolically labeled with 35S, and [35S]HS and [35S]CS were isolated and purified. These human endothelial HS and CS preparations were tested for binding to the surface of pRBCs of FCR3S1.2, as were chemically radiolabeled swine liver [3H]HS and intestinal [3H]HS. GAGs were incubated with pRBCs and allowed to bind to the erythrocytes. Whereas the different types of HS bound to the infected erythrocytes, endothelial CS did not bind (data not shown). None of the GAG preparations bound to uninfected RBCs above background level (data not shown).
In the next step, the purified [35S]HS and [35S]CS fractions obtained from HUVECs were examined for direct binding to either of the 3 different recombinant PfEMP1 domains (DBL1α, CIDR1α, or DBL2δ) coupled to NHS-Hi-trap sepharose. HUVEC HS and heparin bound avidly to DBL1α (Figure 5A-B), but not to CIDR1α or to DBL2δ (Figure 5D-E,G-H). The major fraction of bound endothelial HS eluted from the DBL1α column at 0.4 M NaCl, the same concentration of salt required to elute heparin from DBL1α. Endothelial CS did not bind to any of the domains (Figure 5C,F,I), nor did any of the GAGs interact with GST (data not shown).
Binding of endothelial HS to DBL1α, CIDR1α, or DBL-2δ.
Human endothelial [35S]HS, bovine lung [3H]heparin, and human endothelial [35S]CS were incubated on columns with immobilized DBL1α-GST (panels A-C), CIDR1α-GST (panels D-F), and DBL2δ-GST (panels G-I) for 20 minutes followed by washing with TBS and elution with a stepwise gradient of increasing salt concentration in the same buffer. Then, 1-mL fractions were collected and analyzed for radioactivity. Control experiments carried out with GST NHS-sepharose did not show any binding (not shown).
Binding of endothelial HS to DBL1α, CIDR1α, or DBL-2δ.
Human endothelial [35S]HS, bovine lung [3H]heparin, and human endothelial [35S]CS were incubated on columns with immobilized DBL1α-GST (panels A-C), CIDR1α-GST (panels D-F), and DBL2δ-GST (panels G-I) for 20 minutes followed by washing with TBS and elution with a stepwise gradient of increasing salt concentration in the same buffer. Then, 1-mL fractions were collected and analyzed for radioactivity. Control experiments carried out with GST NHS-sepharose did not show any binding (not shown).
Discussion
Adhesion of P falciparum–infected erythrocytes to uninfected erythrocytes and to endothelial cells in deep microvasculature is thought to play a major role in the fatal outcome of severe malaria.1 Multiple receptors, which include both proteins and carbohydrates, are known to be involved in sequestration.1 Disruption of these interactions with adhesion antagonists may therefore be one of the strategies to treat severe malaria. Here, we demonstrate the involvement of human endothelial HS as receptor for infected erythrocytes via its interaction with the N-terminal domain DBL1α of the P falciparum adhesion molecule PfEMP1.
The present study was conducted to gather more insight into the role of GAGs as potential endothelial receptors for cytoadherence because HS was suggested as a cytoadhesion receptor for pRBCs on the basis of results obtained with an epithelial cell line (Chinese hamster ovary [CHO] cells).15 We show for the first time that DBL1α is implicated in cytoadherence and uses HS as receptor, which was confirmed at both the cellular and the molecular levels. We demonstrate that both pRBCs from the in vitro cloned parasite FCR3S1.2 and wild-type clinical isolates from Uganda, as well as the recombinant DBL1α, bound in an HS-dependent fashion to human endothelial cells where HS is situated on its core protein at the cell surface. Binding of pRBCs and DBL1α was abolished either by stripping the endothelial cell surface from HS or by addition of competing HS. Likewise, human endothelial HS bound to the membrane of pRBCs expressing DBL1α as part of PfEMP1. Furthermore, DBL1α alone was an inhibitor of the binding between pRBCs and HUVECs. On a molecular level, purified human endothelial HS displays affinity for the DBL1α domain of PfEMP1 but not for the CIDR1α or DBL2δ domains. Together, these findings prove that DBL1α is involved in cytoadherence through binding to an HS receptor, in addition to serving as a ligand in rosetting.
Our finding of HS as an endothelial receptor for pRBCs may at first glance seem to be in conflict with previous work that suggested that HS does not influence cytoadherence.34 The authors of the previous work argued that an HS receptor could not be an endothelial receptor for pRBCs because heparinase-treated target cells did not show decreased cytoadherence. Their study used heparinase I, an enzyme that is specific for IdoA2S within anN-sulfated sequence as found in highly sulfated sequences of HS and heparin yet rare in HS of human endothelial cells.35 Heparinase I treatment will therefore leave the HS chains on endothelial cells only partially digested, probably with enough sites remaining on the cell surface for the parasite to attach. The parasite lines used were HB3EC-6 and HB3C32, both known to bind CD36 but of an unknown heparin- or HS-binding status.
HS is expressed as protein-bound sugar chains in a tissue- and species-specific pattern, providing binding sites for a wealth of endogenous molecules32 and exogenous invaders such as protozoa, bacteria, or viruses.36 The biosynthetically controlled fine structure of HS creates a sophisticated tissue pattern as demonstrated by the tissue-staining pattern with the use of diverse anti-HS antibodies21,22,37,38 or growth factor molecules.39,40 GAGs such as heparin and CSA have previously been implicated in the reversion of sequestration in vitro and ex vivo by affecting either cytoadherence41,42 or rosetting13,16,23 25 of pRBCs. With this substantiation, it is important to investigate GAGs from different origins.
Both HS and heparin were found to block cytoadherence to HUVECs and HLECs. The higher concentrations of HS and heparin needed to affect adhesion to HLECs as compared with HUVECs (Figure 2A-B) are most likely due to the different receptor ensembles available on their cell surfaces. For example, CD36, a receptor involved in cytoadherence,5 is present on HLECs but is lacking on the HUVECs. HS was found to effectively block cytoadherence to HUVECs at an approximately 10-fold lower concentration than in rosette formation, whereas the opposite was true for heparin16 (Figure 2A,C). This may suggest a distinct binding specificity of DBL1α for endothelial and erythrocyte receptors. Another reason that a higher concentration of heparin versus HS is needed to block cytoadherence to HUVECs may be that heparin binds to cellular receptors on the endothelial cells and thereby enhances parasite adhesion at low concentrations of added heparin. This would also reduce the effective inhibitor concentration compared with HS, as suggested by our experiments and also as supported by experiments performed by McCormick and coworkers.43 These authors found that heparin could contribute to enhanced cytoadherence by acting as a bridge between PfEMP1 and CD36 on transfected CHO cells. Although this enhancing phenomenon was not found on the CD36-expressing HLECs, a similar effect was observed in our study with the use of HUVECs, even though these cells do not express CD36. When heparin was added at very low concentrations to normal HUVECs, the binding increased by approximately 20% (Figure 2A), whereas at higher concentrations heparin effectively inhibited binding. This might suggest that a heparin-binding cell surface protein is available on HUVECs, which after interaction with heparin would function as an “introduced” GAG receptor for pRBCs.
PECAM-1/CD31, another receptor that contributes to cytoadherence of FCR3S1.2,6 is present on HUVECs.44 In agreement with previous results, CIDR1α binds PECAM-1/CD31 (Figure3).15 This receptor is responsible for adhesion independent of HS. It was not competed with HS and remained after heparinase III treatment of HUVECs, yet could be completely inhibited by anti–PECAM-1/CD31 antibody. The total inhibition of cytoadherence achieved with high concentrations of either heparin or HS on the one hand and anti–PECAM-1/CD31 antibodies alone on the other hand is most likely due to steric hindrance of the respective domains in PfEMP1 in interactions with the cell surface. A potential mechanism for this 2-domain interaction could be a role for HS as a primary receptor at the periphery of the cells to attract the parasitic cells and then to provide opportunities for further contact with other endothelial receptors such as PECAM-1/CD31 and CD36 if these are present on the endothelial cell membrane. Adhesion would then be improved through simultaneous binding to several receptors. This process may be similar to lymphocyte rolling based on carbohydrate-receptor interactions.45
Fresh wild-type clinical P falciparum isolates have been demonstrated to bind to multiple receptors.23 The PfEMP1var1 expressed by the parasite used in this study, FCR3S1.2, has also been shown to mediate adherence to multiple receptors15 and is therefore a good model parasite for studying receptor recognition. The N-terminal head structure (DBL1α-CIDR1α) mediates binding to receptors, including the blood group A antigen, CD36, PECAM-1/CD31, IgM, and, importantly, an HS-like glycosaminoglycan. Further, the binding to DBL1α and CIDR1α domains are different since CIDR1α binds to PECAM-1/CD31, CD36, and IgM, whereas DBL1α has been shown to be a heparin-binding protein and also to interact with the blood group A antigen. The binding of pRBCs to HS-like GAGs on RBCs may well be a marker for vascular endothelial HS binding. Importantly, DBL1α has previously not been identified as being involved in endothelial cell binding.
Malaria parasites from patients are not clonal, yet a large number of patient blood samples contain GAG-binding parasites.17,23As many as 50% of rosettes formed by fresh isolates have been shown to be sensitive to the disruption by HS and heparin.13,16,17In another study using fresh wild-type isolates, it was found that more then 80% of the isolates tested (90 of 111 samples analyzed) bound to heparin, which could be associated to severity of disease.23 Clinical isolates in our study were analyzed for the use of HS receptor in cytoadherence. At least 2 of the 4 analyzed samples definitely contained parasites binding to HS for adhesion to HUVECs. For this reason, glycans are interesting candidates for the design of competitors against a broad range of strains also suggested by studies in which heparin was used as adjunct therapy in cerebral malaria.46,47 An improved understanding of the molecular mechanisms operative in sequestration may well provide a means to treat vascular clogging without affecting host tissues. The success of an antiadhesion drug therapy must be based on an action directed toward as many parasite determinants as possible. The finding that HS from different sources (highly sulfated from intestine, relatively poorly sulfated from endothelial cells) compete more potently than heparin in the cytoadhesion of infected erythrocytes opens an opportunity for selective design of competitors. Erythrocyte rosetting seems to have a preference forN-sulfated rather than O-sulfated groups, which differs from the specificity of many other endogenous and exogenous heparin/HS–binding molecules.17 These efforts should open novel possibilities in the development of carbohydrate-based drugs that could function as antiadhesive molecules in the treatment of malaria.
We thank Robert Kisilevsky for critical reading of the manuscript. Collecting of clinical isolates from Uganda by Berit Schmidt Aydin is very much appreciated.
Prepublished online as Blood First Edition Paper, November 14, 2002; DOI 10.1182/blood-2002-07-2016.
Supported by a grant from the program “Glycoconjugates in Biological Systems” (GLIBS) sponsored by the Swedish Foundation for Strategic Research, the Swedish Research Council, a grant from the European Commission (QLRT-PL-1999-30109), and the Swedish International Development Authority (Sida/SAREC).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mats Wahlgren, Microbiology and Tumor Biology Center (MTC), Karolinska Institutet, Box 280, SE-171 77 Stockholm, Sweden; e-mail: mats.wahlgren@smi.ki.se.

![Fig. 5. Binding of endothelial HS to DBL1α, CIDR1α, or DBL-2δ. / Human endothelial [35S]HS, bovine lung [3H]heparin, and human endothelial [35S]CS were incubated on columns with immobilized DBL1α-GST (panels A-C), CIDR1α-GST (panels D-F), and DBL2δ-GST (panels G-I) for 20 minutes followed by washing with TBS and elution with a stepwise gradient of increasing salt concentration in the same buffer. Then, 1-mL fractions were collected and analyzed for radioactivity. Control experiments carried out with GST NHS-sepharose did not show any binding (not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/6/10.1182_blood-2002-07-2016/4/m_h80634014005.jpeg?Expires=1768709369&Signature=z85njLiPBtJRzzkEneQeWe29-9EA1PF17WI1vv1iv3erlc7p21Gixa0uTuRy33nwKNQegW06~8Wmwm7bI42LliUaXMVB4138ttrtrGcIPKk2MlHmG0U2NZhl2~zZ~RESB27NdALXKBuEuu4oY6tWknrwgaC~CbvKO8OQToPQywYOnGojhpIvuVge9~gQ2QoS-T52PUmIOT6biAPDqpYeCPDdXzrF71vE5ZQCRmm27RQKh6AL2brzCRkUnFDHeXdmvrulZoV42pYy2-hjqKFk0ldFXDmN8sKUrfM7r2en2WS2Z2fX~zyRxMqXTj6vP2yfSSe8sq7VcMhj4a4AuMqwsg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
