In approximately 5% to 10% of gastric mucosa–associated lymphoid tissue (MALT) lymphomas, evidence ofHelicobacter pylori infection is absent, and their pathogenesis is poorly understood. We reviewed the clinical data and histology, and we examined t(11;18)(q21;q21) and BCL10 expression pattern in 17 such cases. In each case, the absence of H pylori was confirmed by negative serology and histology/immunohistochemistry. Five cases with stage IEdisease were first treated with antibiotics, and none of them showed any endoscopic or histologic response. Review of the histology failed to identify any apparent difference between gastric MALT lymphomas with and without H pylori infection. Reverse transcription–polymerase chain reaction (RT-PCR) showed t(11;18)(q21;q21) in 9 (53%) of 17 cases, more frequent in lymphomas at stage IIE or above (5 of 6) than those at stage IE (3 of 10). Two t(11;18)(q21;q21)-positive lymphomas were treated by partial gastrectomy and more than 16 years later showed lymphoma relapse in the gastric stump with dissemination to other mucosal sites, poorly responsive to therapy. BCL10 nuclear expression was observed in 7 of 8 t(11;18)(q21;q21)-positive cases and 4 of 7 t(11;18)(q21;q21)-negative cases, including one case suspicious for a BCL10-involved chromosomal translocation. Our results show that t(11;18)(q21;q21) occurs at a high frequency in H pylori–negative gastric MALT lymphomas. Translocation-positive gastric MALT lymphomas tend to be aggressive, and patients with such lymphomas might benefit from prompt treatment and close follow-up.
Introduction
Most gastric mucosa-associated lymphoid tissue (MALT) lymphomas are associated with Helicobacter pyloriinfection. Colonization of the gastric mucosa by the organism induces lymphoid infiltration and formation of acquired MALT, from which the malignant clone arises. The lymphoma growth, particularly at early phases, critically depends on H pylori–specific tumor-infiltrating T cells, involving CD40 and CD40L costimulating molecules.1,2 Eradication of H pylori by antibiotics leads to complete regression of gastric MALT lymphoma in 70% of cases and is now used as first-line therapy for this tumor.3 4
Genetically, t(1;14)(p22;q32) and t(11;18)(q21;q21) are implicated in the development of H pylori–associated gastric MALT lymphoma. The t(1;14)(p22;q32) translocation occurs in around 5% of MALT lymphomas and causes deregulation of BCL105 that specifically relays the antigen receptor signaling to nuclear factor κB (NF-κB) pathway.6 Lymphoma cells with t(1;14)(p22;q32) show strong BCL10 expression in both the nucleus and cytoplasm, in contrast to normal B cells that express the protein only in the cytoplasm and at a much lower level.7 Nuclear expression of BCL10 at moderate intensity is found in MALT lymphomas without t(1;14)(p22;q32), particularly in those at advanced stages or with t(11;18)(q21;q21).8
The t(11;18)(q21;q21) translocation occurs in 30% of gastric MALT lymphomas. The translocation fuses the amino terminal of theAPI2 gene to the carboxyl terminal of the MALT1gene and generates a fusion product.9 TheAPI2-MALT1 fusion product activates NF-κB,10,11 a transcriptional factor for a number of survival-related genes. In line with this, gastric MALT lymphoma with t(11;18)(q21q;21) is often at advanced stages and does not respond toH pylori eradication.8,12 13
Study design
Patients and materials
Seventeen cases of H pylori–negative gastric MALT lymphoma were retrieved from surgical files of the authors' institutions. The diagnosis of gastric MALT lymphoma was made according to histologic criteria described by Isaacson et al.16 In each case, the absence of H pylori was confirmed by serology and histology/immunohistochemistry or by urease test (CLO) and histology/immunohistochemistry. In 4 cases, the absence of H pylori was further supported by bacterial culture. Formalin-fixed and paraffin-embedded tumor tissues from diagnostic biopsies were available in each case, and frozen tissues were available in 5 cases. In 6 cases, multiple formalin-fixed and paraffin-embedded tumor tissues were available, as patients underwent partial or total gastrectomy. Histology and clinical data were reviewed.
Reverse-transcription and polymerase chain reaction (RT-PCR) for detection of t(11;18)(q2l;q21)
RNA extraction, cDNA synthesis, and PCR amplification were carried out as described previously.13 In 5 cases in which fresh frozen tissues were available, total RNA was extracted from frozen tissues, and RT was carried out using SuperScript Preamplification System (Invitrogen, Paisley, United Kingdom) with oligo(dT) primer. The API2-MALT1 fusion transcript was amplified by PCR using a pair of primers (f-S and f-AS) that covered all the known breakpoints (Figure1).13 PCR products were analyzed on agarose gels.
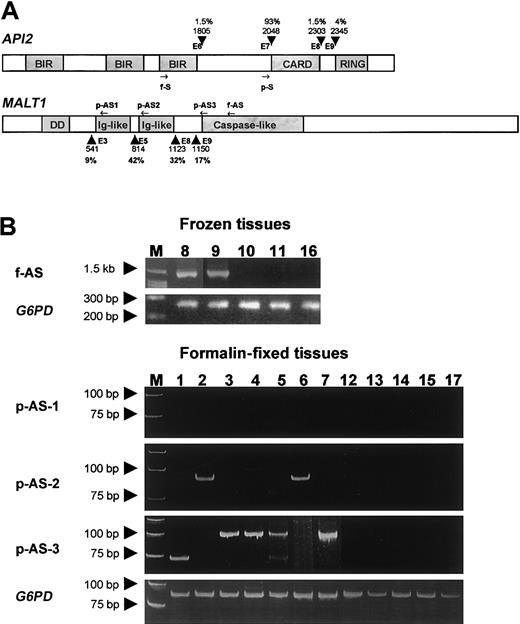
Gene structure, primer position, and RT-PCR results.
(A) Schematic representation of the API2 andMALT1 gene structure and primer position. Known breakpoints are indicated by arrowheads, and the nucleic acids are numbered according to the cDNA sequence of the API2 (GenBank no. NM001165) and MALT1 gene (GenBank no. AF130356). The incidences of these breakpoints were given in percentages. The position of primers used for frozen tissue are indicated by f-S and f-AS, whereas the position of primers used for fixed tissue are indicated by p-S, p-AS1, p-AS2, and p-AS3. (B) The upper panel shows the RT-PCR results from cases with frozen tissues. The lower panel displays the RT-PCR results from cases with fixed tissue. The numbers correspond to the case number in Table 1.
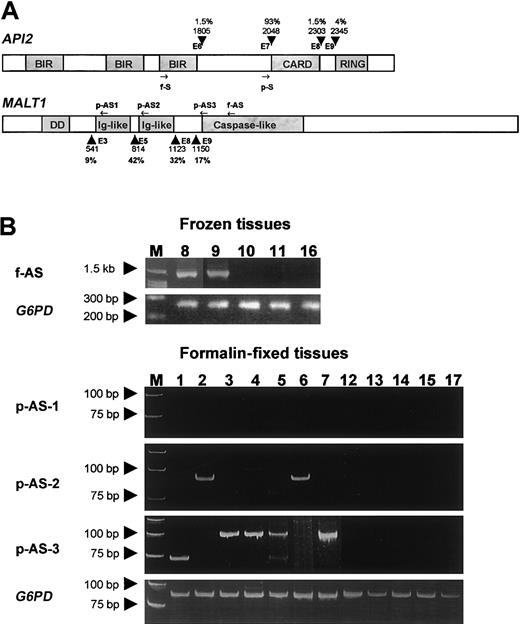
Gene structure, primer position, and RT-PCR results.
(A) Schematic representation of the API2 andMALT1 gene structure and primer position. Known breakpoints are indicated by arrowheads, and the nucleic acids are numbered according to the cDNA sequence of the API2 (GenBank no. NM001165) and MALT1 gene (GenBank no. AF130356). The incidences of these breakpoints were given in percentages. The position of primers used for frozen tissue are indicated by f-S and f-AS, whereas the position of primers used for fixed tissue are indicated by p-S, p-AS1, p-AS2, and p-AS3. (B) The upper panel shows the RT-PCR results from cases with frozen tissues. The lower panel displays the RT-PCR results from cases with fixed tissue. The numbers correspond to the case number in Table 1.
In the remaining cases, formalin-fixed and paraffin-embedded tissues from diagnostic biopsies were used for RNA extraction.13Reverse-transcription was carried out using a mixture of gene-specific primers, including glucose-6-phosphate dehydrogenase (G6PD)primers, which were specially designed for formalin-fixed paraffin-embedded tissues. Three sets of PCR primers with one common sense primer covering 93% of the known breakpoints on theAPI2 gene and 3 antisense primers targeting all 4 breakpoints on the MALT1 gene were used for PCR of theAPI2-MALT1 fusion transcript (Figure 1).13 TheG6PD was amplified in parallel as a control. PCR was performed separately with each primer set at least in duplicate, and the PCR products were analyzed on 10% polyacrylamide gels.
Where indicated, PCR products were gel purified (QIAquick Gel Extraction kit; Qiagen) and sequenced in both directions using dRhodamine dye terminators on an ABI Prism 377 sequencer (PE Applied Biosystems, Foster City, CA).
BCL10 immunohistochemistry
BCL10 was immunostained with a mouse monoclonal antibody (clone 151) on formalin-fixed and paraffin-embedded tissues as described previously.7
Results and discussion
The cases used in this study were selected purely based on their absence of H pylori infection as confirmed by negative serology and histology/immunohistochemistry or urease test (CLO) and histology/immunohistochemistry. The enzyme-linked immunosorbent assay for detection of serum H pylori immunoglobulin G (IgG) antibody is sensitive, capable of detecting 95% of cases.17 In 7 cases, patient's sera were also tested for CagA antibodies and none of them were positive. CagA is highly immunogenic, and antibodies to CagA are frequently detected in patient's sera that are negative for H pylori IgG antibody.18H pylori detection by histologic and immunohistochemical examination is less sensitive and is often affected by the quality and nature of biopsies, but nonetheless it can diagnose around 70% of cases.19 In the present series, multiple biopsies obtained at various stages of patient's treatment were available in each case, and all biopsies consistently showed absence of H pylori infection. Thus, the cases included in this study were most likely negative for H pyloriinfection.
The cases studied were also unlikely positive for non–H pylori helicobacters, such as Helicobacter heilmannii andHelicobacter felis, which are also associated with human diseases, including gastric MALT lymphoma.20 Histologic examination of multiple biopsies in each case failed to reveal any direct evidence of presence of bacteria in the gastric mucosa. Gastric MALT lymphomas associated with H heilmannii infection have been shown to respond to antibiotic therapy.21 Of the cases studied, 5 cases with stage IE lymphoma were first treated with antibiotics, and none of them showed any endoscopic and histologic response during 4.5 to 12 months of follow-up.
Of the 17 cases studied, clinical staging was available in 16 cases (Table 1), with 10 cases at stage IE and the remaining 6 cases at stage IIE or above. As mentioned earlier, 5 of the 10 cases with stage IE disease were first treated with antibiotics but failed to respond. They were subsequently treated by total gastrectomy or radiotherapy. Of the remaining patients, cases 1 and 2 were first treated by partial gastrectomy because of gastric ulcer, and a review of histology of the gastrectomy specimens showed existence of MALT lymphoma in each case. More than 16 years later, both patients showed occurrence of MALT lymphoma in the gastric stump and in additional mucosal sites (lung in case 1; colon and terminal ileum in case 2). Clonal analysis of the rearranged immunoglobulin heavy chain gene showed that the recurrent tumors were clonally related to the original gastric MALT lymphoma in each case. Both patients were treated with rituximab, with one patient showing only partial response and the other stable disease. All the remaining patients were treated with chemotherapy or partial gastrectomy on diagnosis of MALT lymphoma.
Histology was reviewed by 2 hematopathologists (P.G.I. and A.C.) to identify whether there are differences between MALT lymphoma with and without H pylori infection. As tissue biopsies were small and often distorted, we focused on the 6 cases with gastrectomy specimens, in which multiple formalin-fixed and paraffin-embedded tissues were available. In essence, H pylori-negative gastric MALT lymphomas showed similar histologic features to those associated with H pylori. Characteristic lymphoepithelial lesions, similar extents of reactive lymphoid follicles, and tumor-infiltrating T cells were seen in H pylori–negative gastric MALT lymphomas. These observations suggested that these lymphomas like H pylori–positive cases were most likely arising from acquired MALT. However, the agent that induced the acquired MALT in these cases remains enigmatic.
The frequency of t(11;18)(q21;q21) in H pylori–positive gastric MALT lymphoma has been reported to range from 30% to 50%.22-26 The number of cases studied in these reports was relatively small, and the investigation was commonly based on those treated by gastrectomy, which was often biased toward the advanced cases. Thus, these studies may have overestimated the incidence of t(11;18)(q21;q21) in gastric MALT lymphoma. We recently screened for t(11;18)(q21;q21) by RT-PCR in 135 cases of H pylori–associated gastric MALT lymphoma randomly selected and found the translocation in 32 (23.7%) cases (M.-Q. D. et al, unpublished data, October 2002). By using the same RT-PCR methodology followed by sequencing confirmation of suspicious PCR products (2 from frozen tissue and one from fixed tissue), we showed that the translocation was detected in 9 (53%) of the 17 H pylori–negative gastric MALT lymphomas (Table 1). Thus, the incidence of t(11;18)(q21;q21) in H pylori–negative gastric MALT lymphoma is much higher than that of H pylori–positive cases. This finding is in line with the previous finding that the translocation has been found in 2 of 2 H pylori–negative gastric MALT lymphomas.14
Similar to H pylori–positive gastric MALT lymphoma, the occurrence of t(11;18)(q21;q21) in H pylori–negative cases was significantly associated with more advanced cases, being detected in 3 of 10 cases at stage IE but in 5 of 6 cases at stage IIE or above (P < .05, chi-square test). Notably, 2 patients with t(11;18)(q21;q21)-positive gastric MALT lymphoma (nos. 1 and 2) were initially treated by partial gastrectomy, and more than 16 years later both patients showed lymphoma relapse in the gastric stump and disseminated lesions in additional mucosal sites, which were poorly responsive to therapy with rituximab. A further case (no. 9) underwent total gastrectomy and 36 months later showed lymphoma relapse in lung. The findings highlight 2 important issues. First, it further questions the role of gastrectomy in treatment of gastric MALT lymphoma because the lymphoma cells are widely disseminated in the gastric mucosa and cannot be completely cleared by a partial gastrectomy.27 Second, t(11;18)(q21;q21)-positive cells are capable of surviving and remain dormant for a long period before forming relapse and disseminated lesions, which could impose a challenge for clinical treatment. Thus, it is tempting to speculate that effective systemic treatment administered at an earlier time could be beneficial in such patients.
Of the 15 cases in which immunohistochemistry for BCL10 was performed, one case (no. 17) showed strong BCL10 nuclear expression in most of the tumor cells, similar to that seen in t(1;14)(p22;q32)-positive MALT lymphoma. In the remaining 14 cases, 7 of the 8 t(11;18)(q21;q21)-positive cases and 3 of the 6 translocation-negative cases displayed a BCL10 nuclear expression at a moderate level. The remaining cases exhibited BCL10 expression in the cytoplasm. These findings are similar to those observed in H pylori–positive gastric MALT lymphomas.8
In conclusion, t(11;18)(q21;q21) occurs at a high frequency in H pylori–negative gastric MALT lymphomas. Patients with H pylori–negative gastric MALT lymphoma should be treated with chemotherapy or radiotherapy on diagnosis. The t(11;18)(q21;q21)-positive tumors may require close follow-up.
Prepublished online as Blood First Edition Paper, November 27, 2002; DOI 10.1182/blood-2002-10-3167.
Supported by research grants from the Leukemia Research Fund, United Kingdom.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ming-Qing Du, Department of Histopathology, Royal Free and University College Medical School, University College London, Rockefeller Building, University Street, London WC1E 6JJ, United Kingdom; e-mail: m.du@ucl.ac.uk.