Multiple myeloma (MM) is characterized by clonal expansion of malignant plasma cells in the bone marrow and their egress into peripheral blood with progression to plasma cell leukemia. Our previous study defined a functional role of CD40 activation in MM cell homing and migration. In this study, we examine signaling events mediating CD40-induced MM cell migration. We show that cross-linking CD40, using either soluble CD40L (sCD40L) or anti-CD40 monoclonal antibody (mAb), induces phosphatidylinositol 3–kinase (PI3K) activity and activates its downstream effector AKT in MM.1S cells. CD40 activation also activates the MAP kinase (MEK) pathway, evidenced by phosphorylation of extracellular signal-regulated mitogen-activated protein kinase (ERK), but not c-jun amino-terminal kinase (JNK) or p38, in a dose- and time-dependent manner. Using pharmacologic inhibitors of PI3K and MEK, as well as adenoviruses expressing dominant-negative and constitutively expressed AKT, we demonstrate that PI3K and AKT activities are required for CD40-induced MM cell migration. In contrast, inhibition of ERK/MEK phosphorylation only partially (10%-15%) prevents migration, suggesting only a minor role in regulation of CD40-mediated MM migration. We further demonstrate that CD40 induces nuclear factor (NF)–κB activation as a downstream target of PI3K/AKT signaling, and that inhibition of NF-κB signaling using specific inhibitors PS1145 and SN50 completely abrogates CD40-induced MM migration. Finally, we demonstrate that urokinase plasminogen activator (uPA), an NF-κB target gene, is induced by CD40; and conversely, that uPA induction via CD40 is blocked by PI3K and NF-κB inhibitors. Our data therefore indicate that CD40-induced MM cell migration is primarily mediated via activation of PI3K/AKT/NF-κB signaling, and further suggest that novel therapies targeting this pathway may inhibit MM cell migration associated with progressive MM.
Introduction
CD40, a member of the tumor necrosis factor receptor (TNFR) superfamily, was first identified and functionally characterized on B lymphocytes. It is activated as a trimer after interaction with CD40 ligand (CD40L) expressed on activated T cells. The interaction between CD40 and CD40L plays a central role in immune regulation, autoimmune diseases, and many human cancers, including multiple myeloma (MM).1,2 We and others have identified diverse biologic sequelae of CD40 activation in MM cells: up-regulation of cell-surface proteins (eg, B7, CD18, CD11a, CD49d, CD54)3; induction of interleukin (IL)–6 secretion3; and increased expression of adhesion molecules, including the Ku86 and Ku70 autoantigens, on the MM cell surface.4,5 Our studies show that triggering MM cells via CD40 ligation induces either proliferation or growth arrest and apoptosis of MM cells, depending upon their p53 status.6We recently further demonstrated that CD40 induces MM cell migration and vascular endothelial growth factor (VEGF) secretion, suggesting a functional role of CD40 activation in MM homing and angiogenesis.7 Interestingly, CD40 ligation–triggered VEGF secretion and angiogenesis have also been reported in endothelial cells,8 synovial fibroblasts,9 and Kaposi sarcoma cells.10 These studies not only define a mechanistic link between the immune response and angiogenesis, but also suggest that CD40 activation may promote tumor progression.
Studies of CD40 signal transduction have revealed induction of multiple mediators and pathways. Studies to date have focused on 3 mitogen-activated protein kinases (MAPKs): stress-activated protein kinase/c-jun amino-terminal kinase (SAPK/JNK), p38, and extracellular signal-regulated mitogen-activated protein kinase (ERK). The JNK and p38 pathways are predominantly activated by CD40 stimulation in multiple B-cell lines.11-14 Cross-linking CD40 rapidly activates p38 in human tonsillar B cells, whereas inhibition of p38 activity with specific inhibitor SB203580 inhibits CD40-induced gene expression and proliferation.14 Thus, p38 MAPK is required for CD40-induced gene expression and proliferation in B lymphocytes. In contrast, CD40 induces little, if any, activation of ERK.11,12 Other studies demonstrate that CD40 induces activation of src-type protein tyrosine kinases (lyn),15 phosphatidylinositol 3–kinase (PI3K),16 phospholipase Cγ2,16 and nuclear factor (NF)–κB/Rel.17 To date, however, the signaling cascades mediating the biologic sequelae of CD40 activation in human MM cells, including migration, are not delineated.
Several signaling molecules mediate cell migration, including PI3K,18-20 protein kinase B (AKT),21-23 and ERK.24,25 PI3K, consisting of a 110-kDa catalytic subunit and a tightly associated regulatory subunit encoded by thep85a gene, is a key intermediate in cellular responses induced by a vast array of divergent agonists.26Specifically, activation of PI3K is required for both insulin-like growth factor-1 (IGF-1)–induced vascular smooth muscle cell proliferation and migration27 and forTGF-β–mediated epithelial to mesenchymal cell transition and migration.28 In both endothelial and MM cells, VEGF stimulates PI3K activation and migration.23,29The AKT serine/threonine kinase is a core component of PI3K signaling and mediates MM cell growth, survival, and drug resistance.30,31 Recently, a major role for AKT in regulating migration/invasion in endothelial cells,21vascular pericytes,19 and a highly metastatic fibrosarcoma cell line32 has been shown. Specifically, AKT potently promotes fibrosarcoma cell HT1080 invasion by increasing cell motility and matrix metalloproteinase-9 production, in a manner highly dependent on its kinase activity and membrane-translocating ability.32 In contrast, multiple other studies have indicated that cell motility is modulated by the magnitude and the duration of ERK/MAPK activation,33 34 and that temporal and quantitative regulation of MAPK mediates tumor cell motility and invasion.
A large number of stimuli can activate NF-κB transcriptional factors. Ordinarily, NF-κB is sequestered in the cytoplasm by the inhibitory protein IκBα in its inactive form. Various stimuli induce phosphorylation of IκBα kinase (IKK) and ubiquitination of IκBα, with subsequent targeting and degradation by proteasomes. NF-κB then translocates into the nucleus, binds to appropriate consensus motifs, and regulates the transcription of various target genes. Induction of NF-κB via AKT reveals a direct link between AKT and IKKα. AKT mediates IKKα phosphorylation at threonine 23, whereas mutation of this amino acid blocks phosphorylation of AKT and activation of NF-κB by TNF in 293 cells; therefore, NF-κB activation by TNF requires AKT kinase.35 Upon platelet-derived growth factor (PDGF) stimulation, AKT transiently associates in vivo with IKK and induces IKK activation.36 NF-κB is a target of the antiapoptotic Ras/PI3K/AKT pathway induced by PDGF signaling.36 In addition, the effect of AKT on NF-κB induction is dependent upon cellular PI3K activity.37 Since CD40 activates NF-κB and its downstream targets, NF-κB may play a pivotal role in CD40-induced MM cell migration.
The urokinase-type plasminogen activator (uPA) is a critical protease mediating tumor invasion and metastasis. uPA is up-regulated in a variety of solid malignancies, and its overexpression is induced by constitutive NF-κB/RelA activity.38-40 The NF-κB binding site located in the promoter of uPA gene controls its expression.41 Since uPA mediates conversion of plasminogen into plasmin, which degrades extracellular matrix (ECM) proteins such as fibronectin, laminin, and collagen IV, uPA may influence cancer progression.38,40 Proteolytic activity of uPA is necessary for both cell invasion and cell migration,38 suggesting its potential role in CD40-induced MM cell migration.
In this study, we examined PI3K/AKT/NF-κB and MAPK signaling mediating migration in MM.1S MM cells. Using specific pharmocokinetic inhibitors and adenoviruses expressing dominant-negative AKT, as well as constitutively expressed AKT, we show that the PI3/AKT/NF-κB pathway mediates MM cell migration induced by CD40. Furthermore, we found that uPA is a downstream target of CD40-induced PI3K/AKT/NF-κB signaling in MM cells.
Materials and methods
Cells and stimulation
MM.1S cells were kindly provided by Dr Steve Rosen (Northwestern University, Chicago, IL). The cells were cultured in RPMI1640 medium containing 10% fetal bovine serum, 2 mMl-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were serum starved overnight and were stimulated by CD40 cross-linking as previously reported.7 In brief, cells (2 × 106 or 20 × 106 per sample for total lysates or immunoprecipitations, respectively) were stimulated at 37°C with 5 μg/mL of G28.5 anti-CD40 monclonal antibody (mAb) unless otherwise indicated. In some cases, cells were CD40 cross-linked using 626.1 anti-CD40 mAb or 200 ng/mL of a recombinant CD40L–FLAG-tag fusion protein (sCD40L; Alexis Biochemicals, San Diego, CA) supplemented with 1 μg/mL CD40L enhancer (Alexis Biochemicals), according to the manufacturer's protocol.
Cell migration assay
MM.1S cells were serum starved overnight and then resuspended in 70 μL of RPMI1640 medium/0.5% fetal calf serum with or without indicated inhibitors. Cell migration was conducted in 24-well, 6.5-mm internal-diameter Transwell cluster plates (Corning Costar; Cambridge, MA). Briefly, cells (2.5 × 105/70 μL) pretreated with or without specific inhibitors were loaded onto polycarbonate membranes (8-μm pore size) separating 2 chambers of a transwell. Medium/0.1% FCS (500 μL) containing agonist anti-CD40 mAb (G28.5 or 626.1) or sCD40L (Alexis Biochemicals) was added to the lower chamber of the Transwell cluster plates. After 6 hours, cells migrating into the lower chamber were counted using a Coulter counter ZBII (Beckman Coulter, Miami, FL), as well as by hemacytometer. A mouse immunoglobulin G (IgG1) MOPC 21 was used as a control when anti-CD40 mAb was used as a stimulant.
Reagents
PI3K inhibitors LY 294002 (LY) and wortmannin (Wort; Sigma, St Louis, MO), as well as MEK 1/2 inhibitor PD98059 (Cell Signaling Technology, Beverly, MA), were dissolved in dimethyl sulfoxide and further diluted in RPMI1640 medium. Actinomycin D (act D) and cycloheximide (chx) were obtained from Sigma. Antibodies (Abs) for Western blotting were obtained from the following sources: anti-pERK and anti-IκBα were from Santa Cruz Biotechnology (Santa Cruz, CA); antiphosphorylated JNK, anti-JNK, anti-phosphorylated p38, anti-AKT, and anti-pAKT (detects phosphorylation on S-473 residue) were from Cell Signaling Technology; and anti-uPA mAb was from Oncogene Research Products (San Diego, CA).
Western Blotting and immunoprecipitation
Total cell lysates were subjected to 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride membranes, as previously reported.7
PI3K kinase activity assay
PI3K activity in antiphosphotyrosine (4G10) and anti-CD40 immunoprecipitates was performed as described.29Radioactive lipids were separated by thin-layer chromatography, using N-propanol: 2M HOAc (65/35, vol/vol). After drying, the plates were autoradiographed.
Analysis of AKT activity
AKT kinase assay was performed using the AKT kinase assay kit, according to the protocol provided by the manufacturer (Cell Signaling Technology). Briefly, 500 μg of total protein from MM cells was added to AKT Ab-coated beads and incubated at 4°C for 3 hours, followed by washing. Phosphorylation of GSK-3 was used as an indicator of phosphorylated AKT, since AKT negatively regulates GSK-3α/β kinase activity via phosphorylation of GSK-3 at Ser219. After the kinase reaction, the reaction mixture was electrophoresed on a 12% SDS-PAGE gel and Western blotted. The blots were probed with an antiphosphorylated GSK-3α/β (Ser219) Ab.
Nuclear NF-κB pull-down assay
MM.1S cells (5 × 106/time point) were incubated with G28.5 mAb after pretreatment with PS1145 or SN50, and nuclear extracts were prepared. Cells were pelleted and resuspended in 0.4 mL hypotonic lysis buffer (20 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), pH 7.9, 10 mM KCl, 1 mM EDTA (ethylenediaminetetraacetic acid), 0.2% Triton X-100, and 1 mM sodium orthovanadate plus protease inhibitors) and kept on ice for 20 minutes. After centrifugation at 14 000g for 5 minutes at 4°C, the nuclear pellet was extracted with 0.1 mL hypertonic lysis buffer (20 mM HEPES, pH 7.9, 0.4 M NaCl, 1 mM EDTA, and 1 mM sodium orthovanadate plus protease inhibitors) on ice for a further 20 minutes. After centrifugation at 14 000g for 5 minutes at 4°C, the supernatants were diluted to 100 mM NaCl and incubated with 25 μL of agarose beads conjugated to a consensus NF-κB binding oligonucleotide (Santa Cruz Biotechnology) for 1 hour at 4°C. After 3 washes, 25 μL of 2 × sample buffer was added and boiled for 5 minutes. The result was analyzed by SDS-PAGE and Western Blotting using an anti-p65 NF-κB Ab (Santa Cruz Biotechnology).
Recombinant adenovirus
Replication-defective adenovirus vectors expressing dominant-negative (Ad dnAKT) and constitutively active forms of AKT (Ad myrAKT) driven by CMV promoter were kindly provided by Dr Kenneth Walsh (St Elizabeth's Medical Center, Boston, MA).42 The Ad dnAKT containing 3 amino acid mutations at 3 critical phosphorylation sites functions as a dominant negative for endogenous AKT. The Ad myrAKT has an in-frame fusion of the c-src myristoylation sequence to the N-terminus of the wild-type AKT coding sequence, thereby targeting the fusion protein to the membrane. Ad β-gal recombinant adenoviruses43 were used as a negative control. All viruses were produced in 293 cells and purified by 2 runs of ultracentrifugation through CsCl gradient, as published previously.43 To obtain transduction efficiencies of more than 85%, multiplicities of infection of 200 were used to infect MM.1S cells, without any significant toxicity. Cells were typically infected with adenoviruses for 2 hours, followed by replacement with fresh medium overnight, and then treatment with or without test reagents (Wort, LY, PS1145, SN50) for 1 to 2 hours. Transmigration assay was performed as described in “Cell migration assay.”
uPA secretion
Supernatants from control or CD40-activated MM.1S cells incubated in serum-free RPMI1640 were collected after 12 hours. Supernatants from CD40-activated MM.1S cells with or without PI3K inhibitors (Wort, 0.2 μM; LY, 30 μM) and NF-κB inhibitors (PS1145, 10 μM; SN50, 1 μM) were collected after 48 hours. The medium was concentrated 10-fold by using a VIVASPIN concentrator (VIVASCIENCE, Cambridge, MA). Secretion of uPA was detected by Western blotting analysis of supernatants with anti-uPA mAb (Oncogene Research Products, Cambridge, MA).
Statistical analysis
Statistical significance of differences observed in CD40-activated versus control cells was determined using the Studentt test. The minimal level of significance was aP value less than .05.
Results
CD40 activation induces transmigration of MM.1S cells
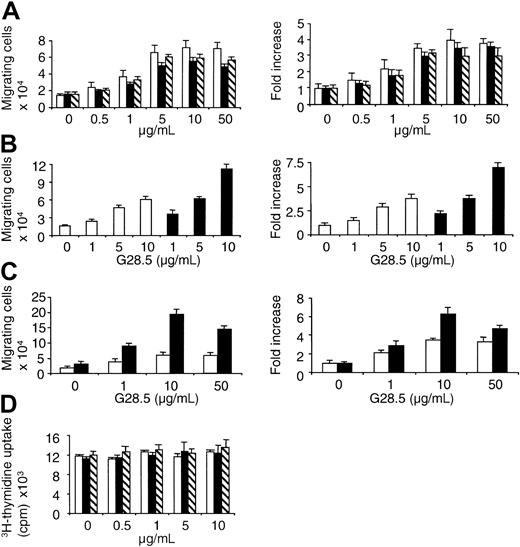
We recently demonstrated that CD40 activation increases MM cell migration in a transwell migration assay in which anti-CD40 mAb or soluble human CD40L (sCD40L) is applied in the lower chamber of a transwell system.7 Since we have not studied the biologic sequelae of CD40 activation in the CD40-expressing MM.1S MM cell line, we first assayed transmigration triggered by CD40. MM.1S (CD38+CD45−) cells express high levels of CD138 (syndecan-1) and are Epstein-Barr virus–independent. In addition, IL-6 induces AKT and NF-κB activation in MM.1S cells, as in primary myeloma patient cells.30 When 250 000 MM.1S cells were applied to the top chamber of transwells, approximately 13 250 ± 3994.3 cells migrate to the lower chamber of transwells following 6 hours of incubation (Figure 1A-C). Thus, the baseline migration of MM.1S cells, without any stimulants added to the lower chamber in the transmigration assay, is approximately 4% to 7%. As shown in Figure1A, CD40 activation, either by anti-CD40 mAb (clone G28.5 or 626.1) or sCD40L, induces MM.1S cell migration in a dose-dependent manner within 6 hours, with peak migration at 50 μg/mL of stimulant. When anti-CD40 mAb G28.5 was added to both upper and lower chambers in the transwells, an even more prominent dose-dependent increase in migrating MM.1S cells was observed (Figure 1B). Similar results were also obtained when sCD40L was added to the upper and lower chambers of the transwells (data not shown). Since adhesion plays a role in cell migration, we compared MM cell migration in a transmigration assay using filters separating 2 chambers in the transwell, either coated with or without fibronectin (40 μg/mL). In the presence of fibronectin, the fold increase in migrating cells was significantly increased at 10 and 50 μg/mL of G28.5 (P = .01 and .025, respectively; Figure1C). These results confirm our previous finding that cell migration is induced by CD40 activation in human MM cells.7Interestingly, CD40 activation of MM.1S cells, even at concentrations as high as 10 μg/mL, did not significantly alter DNA synthesis (P = .15; Figure 1D), even though the cells proliferated in response to 50 ng/mL IL-6 (cpm, 29 609 ± 1230).
CD40 activation induces transmigration of MM.1S MM cells.
(A) Serum-starved MM.1S cells were plated on a polycarbonate membrane (8-μm pore size) in the transwell cluster plate and activated using 0 to 50 μg/mL of anti-CD40 mAb (G28.5 or 626.1) or sCD40L added to the lower chamber. After 6 hours of incubation, migrating cells in the lower chamber were counted. G28.5 (■); 626.1 (▪); sCD40L (▧). (B) Serum-starved MM.1S cells were plated at the upper chamber in the transwell cluster plate and activated using 0 to 10 μg/mL G28.5 anti-CD40 mAb added in the lower chamber (■) or added both in the upper and lower chambers (▪). Migrating cells into the lower chamber were collected and quantitated. (C) Membranes separating upper from lower chambers in the transwell were coated with (▪) or without (■) fibronectin (40 μg/mL) overnight. Results are mean ±SE of 3 independent experiments. (D) MM.1S were incubated with CD40 stimulants (μg/mL): G28.5 (■); 626.1 (▪); sCD40L (▧). After 36 hours, the cells were pulsed with 3H-thymidine, and DNA synthesis was measured. Results are mean ±SE of 3 independent experiments.
CD40 activation induces transmigration of MM.1S MM cells.
(A) Serum-starved MM.1S cells were plated on a polycarbonate membrane (8-μm pore size) in the transwell cluster plate and activated using 0 to 50 μg/mL of anti-CD40 mAb (G28.5 or 626.1) or sCD40L added to the lower chamber. After 6 hours of incubation, migrating cells in the lower chamber were counted. G28.5 (■); 626.1 (▪); sCD40L (▧). (B) Serum-starved MM.1S cells were plated at the upper chamber in the transwell cluster plate and activated using 0 to 10 μg/mL G28.5 anti-CD40 mAb added in the lower chamber (■) or added both in the upper and lower chambers (▪). Migrating cells into the lower chamber were collected and quantitated. (C) Membranes separating upper from lower chambers in the transwell were coated with (▪) or without (■) fibronectin (40 μg/mL) overnight. Results are mean ±SE of 3 independent experiments. (D) MM.1S were incubated with CD40 stimulants (μg/mL): G28.5 (■); 626.1 (▪); sCD40L (▧). After 36 hours, the cells were pulsed with 3H-thymidine, and DNA synthesis was measured. Results are mean ±SE of 3 independent experiments.
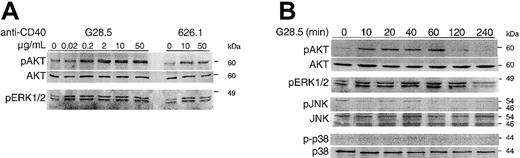
CD40 activation selectively phosphorylates AKT and ERK
CD40-induced signaling in MM cells is not well characterized. We therefore next investigated whether CD40 activation induces phosphorylation of AKT. Since MAPK JNK, p38, and ERK are the focus of studies of CD40 signaling in many B-lymphoma lines, we also determined whether CD40 triggering in MM.1S cells activates these kinases. CD40 activation by anti-CD40 mAb stimulates phosphorylation of AKT and ERK in a dose- (Figure 2A) and time- (Figure2B) dependent manner in MM.1S cells. Peak activation of AKT is maximum at more than 2 μg/mL of anti-CD40 mAb G28.5, whereas activation of ERK is induced by 0.02 μg/mL of G28.5 (Figure 2A). The phosphorylation of both AKT and ERK induced by CD40 occurs within 10 minutes and persists for at least 60 minutes after CD40 activation by G28.5 (2 μg/mL; Figure 2B). Although phosphorylation of AKT returns to baseline within 2 hours, activation of ERK is sustained (Figure 2B). These results therefore indicate that CD40 activation selectively activates AKT and ERK/MAPK pathways in MM.1S cells.
CD40 induces phosphorylation of AKT and ERK in a dose- and time-dependent manner.
Serum-starved MM.1S cells were activated by CD40 by incubation with anti-CD40 mAb (G28.5 or 626.1) at the indicated concentrations for 15 minutes (A) or with G28.5 (2 μg/mL) for 0 to 240 minutes (B). Samples were collected and analyzed by Western blotting with antiphosphorylation-specific Abs. Detection of total AKT on the same blots was used to demonstrate equal loading of samples. Lysates were also probed for pJNK and p38 using specific antiphospho Abs.
CD40 induces phosphorylation of AKT and ERK in a dose- and time-dependent manner.
Serum-starved MM.1S cells were activated by CD40 by incubation with anti-CD40 mAb (G28.5 or 626.1) at the indicated concentrations for 15 minutes (A) or with G28.5 (2 μg/mL) for 0 to 240 minutes (B). Samples were collected and analyzed by Western blotting with antiphosphorylation-specific Abs. Detection of total AKT on the same blots was used to demonstrate equal loading of samples. Lysates were also probed for pJNK and p38 using specific antiphospho Abs.
PI3K and AKT activity mediate MM.1S cell migration induced by CD40
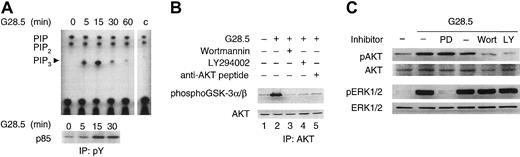
Since phosphatidylinositol (3,4,5)P3 (PIP3) regulates cell migration and PI3K can activate AKT, we next asked whether CD40 activation induces PI3K activity in MM.1S cells. Specifically, we performed PI3K and AKT kinase activity assays using cell lysates of CD40-activated versus unstimulated MM.1S cells. As shown in Figure 3A (upper panel), PI3K kinase activity in antiphosphotyrosine immunoprecipitates prepared from CD40-activated cell lysates is significantly induced 5 minutes following CD40 activation, peaks at 15 minutes, and declines thereafter. A time-dependent increase in p85 immunoreactivity is also observed in these antiphosphotyrosine immunoprecipitates following CD40 activation (Figure 3A, lower panel), confirming that the increased PI3K activity in the antiphosphotyrosine immunoprecipitates is due, at least in part, to p85/p110 PI3K. Moreover, AKT kinase activity measured by phosphorylation of GSK-3α/β, was abrogated by PI3K inhibitors Wort (0.2 μM) and LY (30 μM) (Figure 3B). These results confirm that CD40 activation induces PI3K activity and triggers AKT activity in MM.1S cells. Additionally, as shown in Figure 3C, activation of AKT and ERK is blocked by PI3K inhibitors Wort (0.2 μM) or LY (30 μM) and MEK1/2 inhibitor PD098959 (30 μM), respectively, but neither Wort (0.2 μM) nor LY (30 μM) inhibits CD40-induced phosphorylation of ERK. These data indicate that activation of ERK by CD40 is mediated via MEK, without PI3K/AKT cross-talk.
CD40 triggers AKT activities via PI3K activation.
(A) Serum-starved MM.1S cells were activated by CD40 with 2 μg/mL G28.5 anti-CD40 mAb and collected at indicated time intervals. PI3K kinase assay was performed using equal amounts of lysates immunoprecipitated with antiphosphotyrosine 4G10 mAb, and immunocomplexes were assayed for the ability to phosphorylate PIP2. Equal amounts of PI and PIP2 produced in each sample demonstrate a specific induction of PI3K by CD40 activation. Control (c) indicates a PI3K kinase assay performed on protein A alone. (B) Serum-starved MM.1S cells, with or without pretreatment with PI3K inhibitors Wortmannin (0.2 μM) and LY294002 (30 μM) or anti-AKT peptide (16 μg/mL), were incubated with 2 μg/mL G28.5 anti-CD40 mAb for 30 minutes. Cell lysates were prepared from each sample and immunoprecipitated with an anti-AKT Ab with (lane 5) or without (lanes 1-4) competitor peptide. Kinase activity was measured with GSK-3α/β as a substrate and visualized by Western blotting with an antiphospho GSK-3 antibody, according to manufacturer's protocol. Western blotting with an anti-AKT Ab (lower panel) served as a loading control. (C) Serum-starved MM.1S cells, with or without pretreatment with indicated inhibitors, were activated by CD40 as described in “Materials and methods.” Cells were collected 30 minutes following CD40 activation, and cell lysates were prepared and subjected to Western blotting using anti-pAKT and anti-pERK Abs. Total AKT and total ERK were detected using anti-AKT and anti-ERK Abs on the same blots, demonstrating equal loading of each sample. PD indicates PD098959 (30 μM); Wort, Wortmannin (0.2 μM); and LY, LY294002 (30 μM).
CD40 triggers AKT activities via PI3K activation.
(A) Serum-starved MM.1S cells were activated by CD40 with 2 μg/mL G28.5 anti-CD40 mAb and collected at indicated time intervals. PI3K kinase assay was performed using equal amounts of lysates immunoprecipitated with antiphosphotyrosine 4G10 mAb, and immunocomplexes were assayed for the ability to phosphorylate PIP2. Equal amounts of PI and PIP2 produced in each sample demonstrate a specific induction of PI3K by CD40 activation. Control (c) indicates a PI3K kinase assay performed on protein A alone. (B) Serum-starved MM.1S cells, with or without pretreatment with PI3K inhibitors Wortmannin (0.2 μM) and LY294002 (30 μM) or anti-AKT peptide (16 μg/mL), were incubated with 2 μg/mL G28.5 anti-CD40 mAb for 30 minutes. Cell lysates were prepared from each sample and immunoprecipitated with an anti-AKT Ab with (lane 5) or without (lanes 1-4) competitor peptide. Kinase activity was measured with GSK-3α/β as a substrate and visualized by Western blotting with an antiphospho GSK-3 antibody, according to manufacturer's protocol. Western blotting with an anti-AKT Ab (lower panel) served as a loading control. (C) Serum-starved MM.1S cells, with or without pretreatment with indicated inhibitors, were activated by CD40 as described in “Materials and methods.” Cells were collected 30 minutes following CD40 activation, and cell lysates were prepared and subjected to Western blotting using anti-pAKT and anti-pERK Abs. Total AKT and total ERK were detected using anti-AKT and anti-ERK Abs on the same blots, demonstrating equal loading of each sample. PD indicates PD098959 (30 μM); Wort, Wortmannin (0.2 μM); and LY, LY294002 (30 μM).
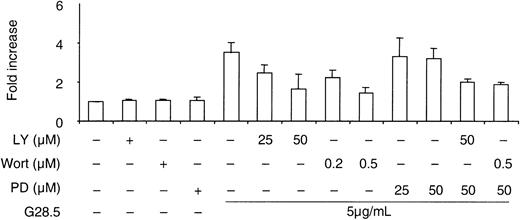
We next studied whether PI3K or ERK mediates CD40-induced MM cell migration. As demonstrated in Figure 4, Wort and LY inhibit CD40-triggered MM cell migration in a dose-dependent manner, whereas PD98059 (30μM), even at concentrations that inhibit ERK activation, only partially (10%-15%) blocks MM cell migration induced by CD40. In the presence of LY (50 μM) or Wort (0.5 μM), PD98059 did not further inhibit CD40-induced MM cell migration. These data indicate that PI3K activity, but not ERK activity, mediates MM cell migration stimulated by CD40.
Effects of PI3K and ERK inhibitors on CD40-induced MM.1S migration.
A transmigration assay was performed as described in “Materials and methods.” Serum-starved MM.1S cells, with and without pretreatment with inhibitors for 1 hour, were seeded in the upper chamber in the transwell cluster plate. At 6 hours after CD40 activation by G28.5 anti-CD40 mAb (5 μg/mL) in lower chamber, migrating cells in the lower chamber were collected and counted. Data are the means ± SD of triplicate determinations.
Effects of PI3K and ERK inhibitors on CD40-induced MM.1S migration.
A transmigration assay was performed as described in “Materials and methods.” Serum-starved MM.1S cells, with and without pretreatment with inhibitors for 1 hour, were seeded in the upper chamber in the transwell cluster plate. At 6 hours after CD40 activation by G28.5 anti-CD40 mAb (5 μg/mL) in lower chamber, migrating cells in the lower chamber were collected and counted. Data are the means ± SD of triplicate determinations.
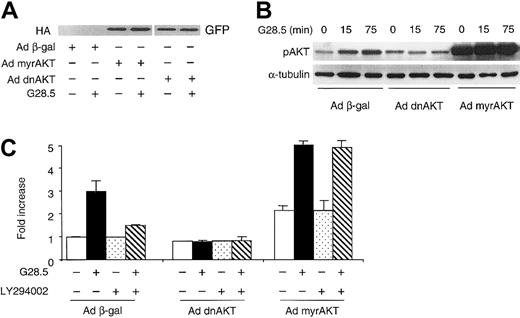
In order to define the functional role of AKT activity mediating CD40-induced MM cell migration, MM.1S cells were first transduced with adenovirus vectors expressing either dominant-negative AKT mutant (Ad dnAKT) or Ad myrAKT. The expression of dnAKT and myrAKT was confirmed by Western blotting using green fluorescent protein (GFP) and hemagglutinin (HA) Abs, respectively (Figure5A). Phosphorylation of AKT in adenovirus Ad myrAKT MM.1S transfectants is observed even without CD40 stimulation (Figure 5B), confirming constitutive activation by Ad myrAKT. The activation of AKT was further enhanced in Ad myrAKT–transduced MM.1S cells at 15 minutes after CD40 stimulation (clearly seen in the films with shorter exposure). In contrast, CD40 stimulation did not induce AKT phosphorylation in MM.1S cells transduced with dominant-negative Ad dnAKT, compared with cells transduced with control Ad β-gal viruses (Figure 5B, center). The temporal sequelae of AKT activation by CD40 were similar in Ad βgal–transduced cells (Figure 5B, right), as in nontransduced MM.1S cells (Figure 2).
AKT mediates CD40-induced MM cell migration in a PI3K-dependent manner.
(A) MM.1S cells were infected with Ad dnAKT or Ad myrAKT for 2 hours, and fresh medium was then added. Cell lysates from infected cells, with or without CD40 stimulation, were probed using an anti-HA mAb for Ad myrAKT and an anti-GFP Ab for Ad dnAKT. (B) MM.1S cell were transduced with indicated adenoviruses overnight and stimulated with G28.5 mAb for indicated time intervals. Total cell lysates were prepared and phosphorylation of AKT was detected. (C) Infected MM.1S cells were pretreated with or without LY294002 (30 μM) for 1 hour, and then seeded in the upper chamber in the transwell cluster plate. Transmigration assays were performed as described in “Materials and methods.”
AKT mediates CD40-induced MM cell migration in a PI3K-dependent manner.
(A) MM.1S cells were infected with Ad dnAKT or Ad myrAKT for 2 hours, and fresh medium was then added. Cell lysates from infected cells, with or without CD40 stimulation, were probed using an anti-HA mAb for Ad myrAKT and an anti-GFP Ab for Ad dnAKT. (B) MM.1S cell were transduced with indicated adenoviruses overnight and stimulated with G28.5 mAb for indicated time intervals. Total cell lysates were prepared and phosphorylation of AKT was detected. (C) Infected MM.1S cells were pretreated with or without LY294002 (30 μM) for 1 hour, and then seeded in the upper chamber in the transwell cluster plate. Transmigration assays were performed as described in “Materials and methods.”
We next performed transmigration assays using MM.1S cells transduced with these adenoviruses, in the presence or absence of PI3K inhibitors. Adenovirus infection of MM.1S cells was performed overnight, followed by serum starvation before addition of cells to the upper chamber of transwells. As shown in Figure 5C, CD40 activation induced migration in MM.1S cells transduced with control Ad β-gal, whereas CD40-induced MM migration was blocked in Ad dnAKT MM.1S transfectants. Pretreatment with LY (30 μM) for 1 hour inhibited CD40-induced migration of control Ad β-gal–transduced cells. Interestingly, transduction of Ad myrAKT stimulated baseline migration of MM.1S cells, compared with MM.1S cells transduced with control Ad β-gal, suggesting that constitutively active AKT, per se, can initiate cell migration. CD40 activation further enhanced (2-fold) migration of Ad myrAKT–transduced MM.1S cells; moreover, migration triggered by CD40 activation was not inhibited by LY (30 μM) in Ad myrAKT–transduced cells (Figure 5C). To rule out the possibility that cell death related to Ad dnAKT or LY treatment contributed to the inhibitory effects on cell migration, we assayed cell viability of Ad dnAKT– or LY-treated cells using trypan blue exclusion. All cells were viable at 24 hours of incubation (data not shown); therefore, the observed inhibition of CD40-induced migration by Ad dnAKT or LY294002 was not due to cell death. These data indicate that AKT activity mediates MM cell migration induced by CD40 activation and further confirm that AKT is activated by CD40 through PI3K.
Inhibition of NF-κB signaling inhibits MM.1S migration induced by CD40 activation
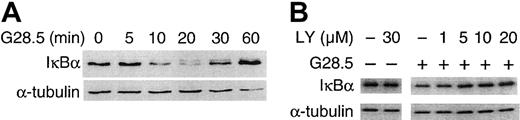
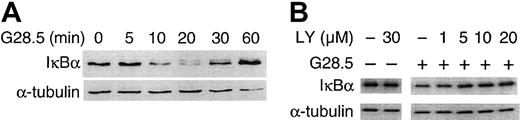
We next determined whether CD40-induced NF-κB activation in MM.1S cells mediates CD40-induced MM cell migration. As shown in Figure6A, IκBα is rapidly degraded upon CD40 activation of MM.1S cells via G28.5 (2 μg/mL; Figure 6A); degradation of IκBα was also seen uponCD40 activation of MM.1S cells with sCD40L (2 μg/mL; data not shown). Degradation of IκBα peaked at 20 minutes after CD40 stimulation and returned to baseline within 1 hour (Figure 6A). PI3K inhibitor LY (0-30 μM), in a dose-dependent manner, blocked the degradation of IκBα (Figure 6B), with complete inhibition of degradation by LY (30 μM). These results confirm that CD40 activates NF-κB in MM.1S cells and suggest that CD40-induced NF-κB activation is mediated via PI3K/ AKT signaling.
CD40 induces NF-κB activation in a PI3K-dependent manner.
(A) Serum-starved MM.1S cells were stimulated with 2 μg/mL G28.5 anti-CD40 mAb, and total cell lysates were made at the indicated time points. Western blotting was performed using an anti-IκBα Ab. The same blot was stripped and probed for tubulin to confirm equal loading. (B) Serum-starved MM.1S cells pretreated with PI3K inhibitor LY (0-30 μM) were stimulated with CD40, and Western blotting using an anti-IκBα Ab was performed. Again immunoblotting with anti–α-tubulin served as a control loading.
CD40 induces NF-κB activation in a PI3K-dependent manner.
(A) Serum-starved MM.1S cells were stimulated with 2 μg/mL G28.5 anti-CD40 mAb, and total cell lysates were made at the indicated time points. Western blotting was performed using an anti-IκBα Ab. The same blot was stripped and probed for tubulin to confirm equal loading. (B) Serum-starved MM.1S cells pretreated with PI3K inhibitor LY (0-30 μM) were stimulated with CD40, and Western blotting using an anti-IκBα Ab was performed. Again immunoblotting with anti–α-tubulin served as a control loading.
To directly define the role of CD40-induced AKT activation in downstream NF-κB signaling, we used MM.1S Ad dnAKT transfectants to block activation of AKT and MM.1S Ad myrAKT transfectants to constitutively activate AKT. MM.1S cells transduced with either virus were incubated with G28.5 anti-CD40 mAb (2 μg/mL), and Western blotting using an anti-IκBα Ab was performed on whole cell lysates at indicated time points. As seen in Figure 6A, CD40 induced IκBα degradation in MM.1S cells at 10 to 30 minutes. The expression of kinase-dead AKT by Ad dnAKT blocks CD40-induced IκBα degradation (Figure 7A), whereas the expression of constitutively active AKT by Ad myrAKT accelerates IκBα degradation (Figure 7B). These results indicate that NF-κB is a downstream target of AKT activation induced by CD40.
Effects of Ad dnAKT and Ad myrAKT on CD40-induced IκBα degradation.
Adenoviruses expressing kinase-dead AKT (Ad dnAKT) (A) and constitutively active AKT (Ad myrAKT) (B) were transduced into MM.1S cells, followed by serum starvation for 2 hours. Serum-starved adenovirus-transduced cells were incubated with 2 μg/mL G28.5 anti-CD40 mAb, and collected at the indicated time points. Cell lysates were subjected to Western blotting using anti-IκBα, and with anti–α-tubulin mAb as a loading control.
Effects of Ad dnAKT and Ad myrAKT on CD40-induced IκBα degradation.
Adenoviruses expressing kinase-dead AKT (Ad dnAKT) (A) and constitutively active AKT (Ad myrAKT) (B) were transduced into MM.1S cells, followed by serum starvation for 2 hours. Serum-starved adenovirus-transduced cells were incubated with 2 μg/mL G28.5 anti-CD40 mAb, and collected at the indicated time points. Cell lysates were subjected to Western blotting using anti-IκBα, and with anti–α-tubulin mAb as a loading control.
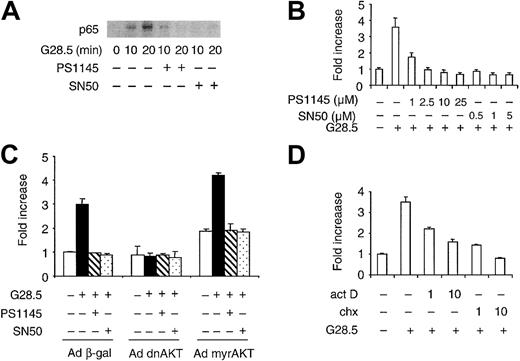
We next examined CD40-induced nuclear translocation of NF-κB in MM.1S cells using nuclear NF-κB pull-down assays. Nuclear extracts prepared from control and CD40-treated MM.1S cells were incubated with agarose beads conjugated to consensus NF-κB oligomers, and nuclear NF-κB was assayed by Western blotting using anti-p65 Ab. As shown in Figure 8A, CD40 activation induced increased nuclear NF-κB, which was inhibited in a time-dependent manner by pretreatment with either PS1145 (10 μM) or SN50 peptide (1 μM) (Figure 8A). The novel IKK inhibitor PS114544 blocks activation of IκBα, thereby interfering with NF-κB translocation, whereas cell-permeable SN50 peptide inhibits nuclear translocation of NF-κB. In order to assess the role of the NF-κB signaling in CD40-mediated MM cell migration, we used these 2 inhibitors in transmigration assays. As shown in Figure 8B, pretreatment (1 hour) with PS1145 (1-25 μM) or SN50 (0.5-5 μM) markedly reduces CD40-induced cell migration, with complete inhibition by PS1145 (2.5 μM) and SN50 (0.5 μM), conditions that do not cause cell death.44 45 Moreover, NF-κB inhibitors PS1145 and SN50 did not block CD40-induced migration of MM.1S cells expressing constitutively active AKT (Figure 8C). These results confirm that CD40-induced AKT-dependent NF-κB activation mediates CD40-triggered MM cell migration.
Inhibition of NF-κB signaling blocks CD40-mediated MM.1S migration, which is dependent on transcription and new protein synthesis.
(A) Serum-starved MM.1S cells were pretreated with either 10 μM PS1145 for 90 minutes or 1 μM SN50 peptides for 30 minutes, and then incubated with 2 μg/mL G28.5 mAb for the indicated intervals. Nuclear extracts from each sample were prepared, and oligonucleotides containing the consensus binding sequence of NF-κB bound to agarose beads were used to pull down nuclear NF-κB. The resulting samples were analyzed by Western blotting using an anti-p65 NF-κB Ab. (B) Serum-starved MM.1S cells were either left intact or pretreated with PS1145 and SN50 at the indicated concentration for 90 minutes and 30 minutes, respectively, and then added to the upper chamber in a transwell cluster plate for a transmigration assay. (C) MM.1S cells were transduced with Ad dnAKT, Ad myrAKT, or control Ad β-gal adenoviruses. Cells were preincubated with or without PS1145 (10 μM) and SN50 (1 μM), and a transmigration assay then performed triggered by G28.5 (5 μg/mL) anti-CD40 mAb. (D) Serum-starved cells were treated for 1 hour with act D or chx (1 and 10 μg/mL), and transmigration was then determined as described in “Materials and methods.” Data are the mean ±SD of triplicate determinations. Similar results were obtained in at least 2 additional experiments.
Inhibition of NF-κB signaling blocks CD40-mediated MM.1S migration, which is dependent on transcription and new protein synthesis.
(A) Serum-starved MM.1S cells were pretreated with either 10 μM PS1145 for 90 minutes or 1 μM SN50 peptides for 30 minutes, and then incubated with 2 μg/mL G28.5 mAb for the indicated intervals. Nuclear extracts from each sample were prepared, and oligonucleotides containing the consensus binding sequence of NF-κB bound to agarose beads were used to pull down nuclear NF-κB. The resulting samples were analyzed by Western blotting using an anti-p65 NF-κB Ab. (B) Serum-starved MM.1S cells were either left intact or pretreated with PS1145 and SN50 at the indicated concentration for 90 minutes and 30 minutes, respectively, and then added to the upper chamber in a transwell cluster plate for a transmigration assay. (C) MM.1S cells were transduced with Ad dnAKT, Ad myrAKT, or control Ad β-gal adenoviruses. Cells were preincubated with or without PS1145 (10 μM) and SN50 (1 μM), and a transmigration assay then performed triggered by G28.5 (5 μg/mL) anti-CD40 mAb. (D) Serum-starved cells were treated for 1 hour with act D or chx (1 and 10 μg/mL), and transmigration was then determined as described in “Materials and methods.” Data are the mean ±SD of triplicate determinations. Similar results were obtained in at least 2 additional experiments.
Since NF-κB can activate many target genes, we speculated that gene transcription and protein synthesis may modulate CD40-induced MM migration. MM.1S cells were pretreated with act D and chx for 1 hour, and transmigration was determined as described in “Materials and methods.” Both act D (1 and 10 μg/mL) and chx (1 and 10 μg/mL) significantly inhibit CD40-induced MM.1S migration in a dose-dependent manner (Figure 8D), suggesting that cell migration is dependent on transcription and de novo protein synthesis. These data demonstrate that NF-κB plays a pivotal role in mediating MM cell migration stimulated by CD40.
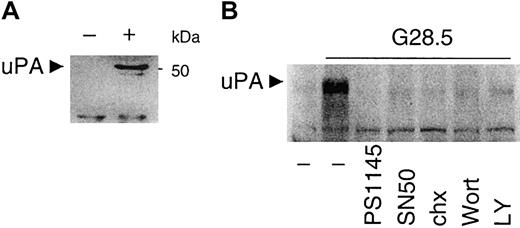
CD40-induced uPA secretion is inhibited by PI3K and NF-κB blockade
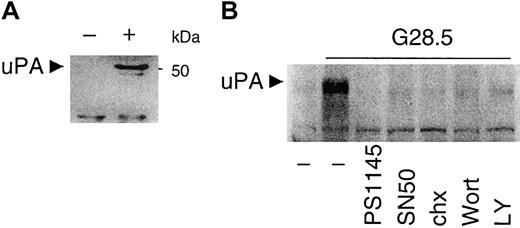
We previously showed that CD40 induces VEGF secretion and migration in MM cells7; however, NF-κB–responsive elements in the VEGF promoter have not yet been unidentified. We next examined a potential downstream target of PI3K/AKT-dependent NF-κB activation by CD40 signaling in MM.1S cells, specifically testing whether uPA is induced by CD40 in MM.1S cells. To address this hypothesis, conditioned media from control and CD40-activated MM.1S cells incubated for 12 hours in serum-free RPMI1640 media were collected, concentrated, and subjected to Western blotting analysis with anti-uPA mAb. As seen in Figure 9A, uPA is not detectable in control MM.1S cell supernatants, but uPA secretion is induced by CD40 activation. Treatment with NF-κB inhibitors PS1145 (10 μM) or SN50 (1 μM) inhibits the secretion of uPA (Figure 9B). The PI3K inhibitors LY (30 μM) and Wort (0.2 μM) also inhibit CD40-induced uPA expression and secretion from CD40-stimulated MM.1S cells (Figure 9B). Inhibition of protein synthesis by chx (5 μg/mL) has a similar effect. These data indicate that uPA is induced and secreted in CD40-activated MM.1S cells, and that uPA expression is dependent on CD40-induced PI3K and NF-κB activity.
CD40 stimulates uPA secretion, which is blocked by PI3K and NF-κB inhibitors.
(A) Media from untreated (−) and CD40-activated (+) MM.1S cells cultured in serum-free medium for 12 hours were collected and concentrated as described in “Materials and methods.” Secretion of uPA was detected by Western blot analysis with an anti-uPA mAb. (B) Media from untreated or CD40-activated MM.1S cells, in the presence or absence of PI3K inhibitors (0.2 μM Wortmannin, Wort; 30 μM LY294002, LY), NF-κB inhibitors (10 μM PS1145; 1 μM SN50), or 5 μg/mL chx for 48 hours, were concentrated for assay of uPA secretion as described in “Materials and methods.”
CD40 stimulates uPA secretion, which is blocked by PI3K and NF-κB inhibitors.
(A) Media from untreated (−) and CD40-activated (+) MM.1S cells cultured in serum-free medium for 12 hours were collected and concentrated as described in “Materials and methods.” Secretion of uPA was detected by Western blot analysis with an anti-uPA mAb. (B) Media from untreated or CD40-activated MM.1S cells, in the presence or absence of PI3K inhibitors (0.2 μM Wortmannin, Wort; 30 μM LY294002, LY), NF-κB inhibitors (10 μM PS1145; 1 μM SN50), or 5 μg/mL chx for 48 hours, were concentrated for assay of uPA secretion as described in “Materials and methods.”
Discussion
We and others have defined the role of PI3K, AKT, and NF-κB signaling in mediating MM cell proliferation and antiapoptosis.30,31,44 45 In the present study, we have shown that PI3K/AKT signaling mediates CD40-induced transmigration of MM.1S MM cells. We first showed a significant induction of AKT and PI3K activity by CD40 stimulation; and, conversely, we demonstrated a concentration-dependent inhibition of MM migration by PI3K inhibitors Wort and LY. Using adenoviruses expressing dominant-negative AKT and constitutively expressed AKT, we confirmed the role of AKT in MM migration. Specifically, expression of dominant-negative AKT blocks CD40-induced MM migration, whereas expression of constitutively active AKT overcomes inhibition of CD40-induced migration by LY. In addition, we found that constitutive AKT activity induces MM migration. We show that constitutively active AKT promotes IκBα degradation, whereas expression of dominant-negative AKT inhibits IκBα degradation, and went on to identify NF-κB as a downstream target of PI3K/AKT signaling. Finally, the blockade of NF-κB activation and transmigration using PS1145 and SN50, respectively, abrogates CD40-induced MM cell migration. These studies confirm that PI3K/AKT/NF-κB activation mediates CD40-induced MM cell migration. CD40-induced PI3K/AKT/NF-κB activity also induces expression and secretion of uPA, suggesting a role of uPA in this migratory response.
CD40 activation in human MM.1S cells, either by anti-CD40 mAb or sCD40L treatment, results in activation of PI3K, AKT, and NF-κB. CD40 activation also induces ERK/MAPK, without significant activation of JNK and p38. Since CD40 activation induces either p38 or JNK in B cells, these data suggest distinct molecular sequelae of CD40 signaling in MM cells versus normal B cells. An important sequelae of CD40 activation in MM.1S cells is migration, and we defined the signaling pathways mediating this response. The MEK inhibitor PD098059 only partially (10%-15%) blocked MM migration induced by CD40, suggesting that ERK activation plays only a minor role. Importantly, PI3K activity is significantly induced in MM.1S cells following CD40 activation; and conversely, CD40-induced MM cell migration is inhibited by Wort and LY in a dose-dependent manner. Therefore, PI3K activity mediates CD40-induced MM cell migration.
Since CD40 activation also induces sustained AKT activity in MM.1S cells, we next defined whether AKT mediated MM cell migration following CD40 activation. CD40-induced migration was not observed in MM.1S transfectants overexpressing dnAKT, confirming that induction of AKT activity is required for MM.1S cell migration stimulated by CD40. Conversely, overexpression of AKT in Ad myrAKT-transduced MM.1S induced migration but did not overcome the inhibitory effect of PI3K inhibitor LY, indicating that AKT is downstream of PI3K and mediates CD40-triggered migration. Our results are consistent with the recent demonstration that overexpression of constitutively active AKT in bovine lung microvascular endothelial cells stimulates cytokinesis and migration in the absence of VEGF.21 Our prior study shows that AKT activation in MM cells stimulates proliferation,30 whereas the present results suggest that AKT activation also mediates MM migration. The present study therefore indicates that AKT activation mediates migration even in primary patient MM cells before onset of secondary plasma cell leukemia.
We next show that CD40-induced AKT activation mediates IκBα degradation and NF-κB activation in MM.1S cells. Using adenovirus transduction and 2 different classes of NF-κB inhibitors, PS1145 and SN50, our results confirm that the mechanisms whereby CD40 activates NF-κB involve consecutive activation of AKT and IKK, consistent with the observed phosphorylation and degradation of IκBα and translocation of NF-κB p65 into the nucleus. Since both PS1145 and SN50 completely inhibit CD40-induced migration in control as well as in Ad myrAKT–transduced MM.1S transfectants, NF-κB is downstream of AKT in CD40-induced signaling mediating MM cell migration.
The role of NF-κB activity in controlling cell survival and conferring protection against drug-induced apoptotic stimuli is well documented.44-47 Our prior studies have identified NF-κB as a novel target whereby PS341 (proteosome inhibitor) and IMiDs (thalidomide analog immunomodulatory drugs) target MM cells to inhibit growth, induce apoptosis, and overcome drug resistance in the bone marrow microenvironment.44,45 To our knowledge, however, the present study is the first demonstration of the involvement of NF-κB in cell migration. Coupled with evidence that many tumor cells have constitutive NF-κB site binding and transactivation activity,46 47 the current results suggest that enhanced NF-κB activity may potentiate tumor cell migration and invasion.
Little is known about the role of uPA in MM pathogenesis; however, uPA and uPA receptor are expressed in MM cells.48 uPA is only weakly expressed in unstimulated MM.1S cells, as reported recently in U266 MM cells.49 However, uPA is induced in U266 as well as MM cells from patients following binding to vitronectin (VN) and fibronectin (FN) ECM proteins.49 uPA induced by interaction with VN and FN interaction may enhance the ability of cells to invade via stroma and subendothelial basement membrane. In the current study, we observed significant induction of uPA by CD40 activation in MM.1S cells. This is the first report of uPA induction by CD40 and further supports a functional role of CD40 activation in MM invasion and spreading. In addition, our data showed that induced uPA secretion is dependent on NF-κB activity and, to a lesser extent, PI3K/AKT activity. These data confirm that uPA promoter contains NF-κB binding sites that mediate the induction of uPA expression. Furthermore, these results suggest a possible role of uPA in MM cell invasion and proteolytic digestion of bone matrix.
In summary, our data suggest that CD40-induced MM transmigration is mediated by PI3K/AKT-induced transactivation of NF-κB and related induction of expression and secretion of uPA. These provide the framework for novel therapeutic strategies targeting PI3K/AKT/NF-κB signaling and uPA secretion to inhibit migration and progressive disease in MM.
We thank Dr Kenneth Walsh at St Elizabeth's Medical Center (Boston, MA) for valuable reagents.
Prepublished online as Blood First Edition Paper, November 14, 2002; DOI 10.1182/ blood-2002-09-2813.
Supported by a Multiple Myeloma Research Foundation Fellow Research Award (Y.-T.T.); National Institutes of Health grants RO-1 50947 and PO1-78378; and the Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth C. Anderson, Department of Adult Oncology, Dana-Farber Cancer Institute, M557, 44 Binney St, Boston, MA 02115; e-mail:kenneth_anderson@dfci.harvard.edu.