Abstract
Studies associating the prothrombin 20210G>A (FII 20210A), factor V Leiden (FVL), and factor XIII Leu34 (FXIII-A Leu34) alleles with myocardial infarction (MI) have yielded conflicting results. Complicated gene-gene interactions, small sample sizes, and heterogeneous genetic and environmental backgrounds may contribute to opposing findings. Simultaneous analysis of multiple gene variants in a large sample size from a genetically isolated population may overcome these weaknesses. Genotyping was performed in 500 MI patients and 500 control subjects from the genetically isolated Newfoundland population to determine the prevalence of the FII 20210A, FVL, and FXIII-A Leu34 variants and their association with MI. Gene-gene interactions were also analyzed. The prevalence of the FII 20210A allele was higher in MI patients (3.2%) than in control subjects (1.0%;P = .015). The FII 20210A allele was also 5.6-fold higher in MI patients younger than 51 years than in age-matched control subjects (P = .04). FVL showed 3.9-fold higher prevalence in young patients than in patients older than 50 years (P = .004) and 2.7-fold higher than in age-matched control subjects (P = .007). Furthermore, the prevalence of combined carriers of the FXIII-A L34 and FII 20210A alleles was 12-fold higher in MI patients than in control subjects (P = .002) and with 92% penetrance. There was disequilibrium of the FXIII-A Leu34 allele to MI patients carrying the FII 20210A allele as a genetic background. Based on our data, we determined that (1) the FII 20210A allele is a risk factor for MI, possibly important for early onset; (2) FVL may predispose for early-onset MI; (3) the FXIII-A Leu34 allele predisposes for MI in males only; however, (4) interaction between the FII 20210A and FXIII-A Leu34 alleles forms a synergistic coeffect that strongly predisposes for MI, placing combined carriers at high risk for MI.
Introduction
The pathogenesis of myocardial infarction (MI) involves an interaction between environmental influences and genetic predisposition. Genetic factors involving blood coagulation may contribute to the pathogenesis of atherosclerosis and play a role in the clinical progression to plaque rupture and localized occlusive thrombus formation.1 The gene variants factor V Leiden (FVL, Arg506Gln) and prothrombin 20210G>A (FII 20210G>A) are the 2 most commonly recognized genetic prothrombotic risk factors for venous thrombosis.2-5 Based on the increased thrombotic tendency in venous thrombosis studies, these 2 gene variants have also been examined for possible association with arterial thrombosis in MI. Several studies have shown a higher prevalence of the FII 20210A allele in patients with MI than in healthy control subjects.6-9However, most of the results from these studies failed to achieve statistical significance, possibly because of the extremely low frequency of the FII 20210A allele in the studied population and the use of relatively small sample sizes. Nevertheless, a few studies have presented conflicting results.10,11 Although FVL strongly correlates with deep venous thrombosis (DVT), most previous studies have failed to show a correlation between FVL and MI.12-14Recently, a few studies have suggested that FVL may associate with early-onset MI15,16 and MI with normal findings on coronary angiography.17 In contrast, a common gene variant, factor XIII-A Val34Leu (FXIII-A Val34Leu), has recently been suggested to confer a protective role against MI based on a lower prevalence of the FXIII-A Leu34 allele in MI patients than in control subjects.18-20 However, conflicting results were also reported.21-24 Furthermore, results from function studies on the FXIII-A Leu34 allele do not support the hypothesis of a protective role against MI.25 26 Therefore, the roles of these 3 gene variants in the pathogenesis of MI remain unknown.
Because it is a multifactorial disorder, the genetic components in MI may be a combined effect of a number of genes with each playing only a small role. The predisposition imparted by individual genes may act independently or interact with other genes to result in an additive effect or a synergistic coeffect. Common challenges facing case control studies on possible gene-gene interactions include relatively small sample sizes, low frequency of gene variants, and ethnic heterogeneity of the investigated populations. To overcome these weaknesses, we simultaneously analyzed FVL, FII 20210G>A, and FXIII-A Val34Leu in 500 MI patients and 500 healthy controls of the genetically isolated Newfoundland population.
The island portion of the Canadian province of Newfoundland and Labrador is located in the Atlantic Ocean, off the eastern coast of Canada. The population of the island of Newfoundland consists mainly of descendants of English and Irish settlers who arrived in the 17th and 18th centuries.27 The geographic and social isolation of this island has ensured little inward migration for several hundred years28 and, thus, has led to a small population (530 000 residents; Statistics Canada 2001) with a relatively homogenous genetic background ideal for the study of complex multifactorial disease such as MI.
Our analysis not only attempted to correlate MI with each of the 3 gene variants, it examined for possible gene-gene interactions among the 3 gene variants in MI. High allele frequency of FXIII-A Leu34 in MI patients and healthy populations permits us to determine the distribution of coexistence of the FXIII-A Leu34 and either FII 20210A or FVL alleles in MI patient and control populations.
Patients, materials, and methods
Patients and control subjects
Blood samples were collected from 500 consecutive MI patients (221 men, 279 women) and 500 healthy control subjects (214 men, 286 women) of the genetically isolated Newfoundland population. Patients categorized in the MI group were those who sought treatment at the emergency department or within one of the Health Care Corporation of St John's hospitals with symptoms and biochemical evidence suggestive of MI. Only patients with cardiac troponin 1 values greater than 2.0 μg/L (Axsym; Abbott Diagnostics, Abbott Park, IL) or greater than 0.5 μg/L (Access II; Beckman-Coulter, Mississaugua, ON, Canada) were used in this group. Control subjects were selected from consecutive persons without prior history of MI or thrombosis brought to the emergency department for trauma, accidental injury, or other noncardiac and non–thrombosis-related events. Discarded blood samples collected for complete blood count were used for DNA extraction and analysis. Ethics approval for this study was granted by the Human Investigations Committee of Memorial University and by the Health Care Corporation of St John's.
Genotyping of FII 20210A, FVL, and FXIII-A Val34Leu
Genomic DNA was isolated from the peripheral blood using standard methods.29 Genotyping of FVL, FII 20210A, and FXIII-A Val34Leu was performed by PCR amplification of each of the target alleles from genomic DNA, followed by restriction digestion with each of corresponding enzymes MnlI, HindIII, and DdeI, respectively, as previously described.25 30 Digested PCR products were separated by electrophoresis in 10% polyacrylamide gels and were visualized by staining with ethidium bromide.
Prevalence determination and association study
The prevalence of each gene variant was calculated by counting the total carrier frequency including heterozygotes and homozygotes. Allele frequencies were determined by gene counting. Pearson χ2 statistical analysis was performed using SPSS version 10.0 to test the association between genotypes and the prevalence of MI. Odds ratio (OR) was calculated as a measure of the relative risk for MI and was given with 95% confidence intervals (CIs).
Analysis of gene-gene interaction
Gene-gene interactions were determined by comparing the prevalence of combined carrier for 2 of the 3 gene variants in patients and control subjects by analysis of the distribution of one chosen gene variant in subgrouped patients and control subjects who carry another gene variant as genetic background.
Results
Genotyping FII 20210A, FVL, and FXIII-A Val34Leu
Genotype distributions, carrier frequencies, and allele frequencies of FII 20210A, FVL, and FXIII-A Leu34 in the MI patient and control populations are given in Table 1. The FII 20210A allele was detected in 3.2% of patients, which was significantly higher than the 1% observed in control subjects (OR, 3.3; 95% CI, 2.6-4.0; P = .015). An identical prevalence of FVL was observed in patient and control populations (4.6% vs 4.6%). No homozygotes for either the FII 20210A or the FVL alleles were found in either population. In both populations, the FXIII-A Leu34 allele had similar prevalence (47.0% vs 47.8%) and allele frequency (27.7% vs 27.1%). The prevalence of homozygosity for the FXIII-A Leu34 allele was higher in patients than in control subjects (8.4% vs 6.4%), but the difference did not reach statistical significance.
The distribution of the 3 gene variations was further analyzed by subgrouping patients and control subjects according to age (Table2). MI patients were divided into those with an early age of onset (50 years or younger) and those with a later age of onset (older than 50 years). The control population was also divided into the 2 corresponding age groups. Interestingly, a disequilibrium distribution of the FVL allele was observed in the early-onset patient group. The FVL allele was detected in 13.0% of patients with early age of onset, which was significantly higher than the 3.8% in patients with a later age of onset (OR, 3.9; 95% CI, 3.3-4.4; P = .004) and the 4.8% in the age-matched control subjects (OR, 3.0; 95% CI, 2.2-3.7; P = .007). The prevalence of the FII 20210A allele was also significantly higher in the early-onset group of MI patients (4.3%) than in control subjects younger than 50 (0.8%). Although the prevalence of FII 20210A was slightly higher in the early-onset group than in the late-onset group, this difference did not achieve statistical significance. The FXII Leu34 allele showed a slight but statistically insignificant difference in prevalence between the patients with early- and late-onset age.
The distribution of the 3 gene variations was further analyzed by subgrouping patients and control subjects based on sex (Table3). The sex ratios in the 500 MI patients and the 500 control subjects were similar. The distribution of the 3 gene variations in control subjects showed no significant difference between men and women. However, there were significant differences in the distribution of FVL and the FXIII-A Leu34 allele between sexes in MI patients. The prevalence of the FVL and FXIII-A Leu34 alleles was significantly higher in male than in female patients. The prevalence of the FII 20210A allele was not significantly different between sexes among MI patients.
Gene-gene interactions
FII 20210A and FXIII-A Leu34 alleles.
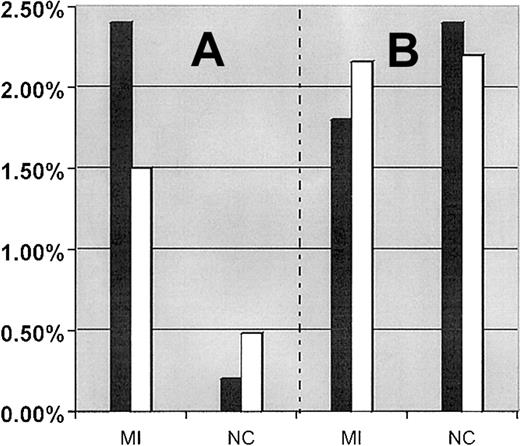
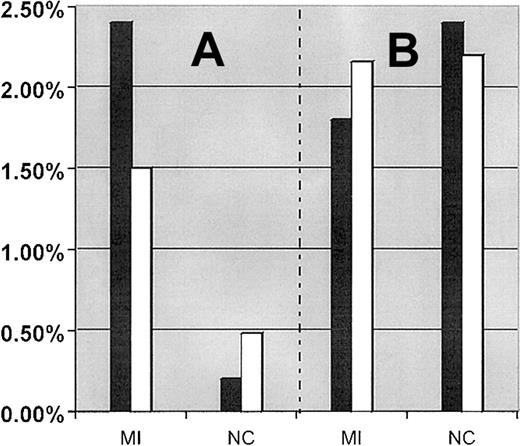
Interaction between the FXIII-A Leu34 allele and the FII 20210A allele was first analyzed by comparing the prevalence of combined carriers (persons carrying both FII 20210A and FXIII-A Leu34 alleles) in the total patient and control populations, with their corresponding theoretical prevalence of combined carriers. Using carrier frequencies described in Table 1, the theoretical prevalence for combined carriers of the FII 20210A and FXIII-A Leu34 alleles is 1.5% (47.0% × 3.2%) in MI patients and 0.48% (47.8% × 1%) in control subjects (Figure 1A). The observed prevalence of combined carriers in the MI patient population (2.4%; 12 of 500) was 1.6-fold higher than its theoretical prevalence (1.5%), and in the healthy control population (0.2%; 1 of 500) it was 2.4-fold lower than its theoretical expected prevalence (0.48%). The observed prevalence of combined carriers was 12-fold higher in MI patients than in the control population (P = .002). Of the 46 MI patients 50 years or younger, 2 (4.4%) were combined carriers of the FXIII-A Leu34 and FII 20210A alleles compared with 10 (2.2%) combined carriers older than 50 years.
Comparison between the expected and observed prevalence of combined carriers of mutations in MI patients and control subjects.
(A) Comparison between the expected and observed prevalence of combined carrier for the FII 20210A + FXIII-A Leu34 alleles in MI patients and control subjects, respectively. (B) Comparison between the expected and observed prevalence of combined carrier for FVL + FXIII-A Leu34 alleles in MI patients and control subjects, respectively. Filled bar (▪) represents the observed prevalence of combined carriers. Empty bar (■) represents the expected prevalence of combined carriers.
Comparison between the expected and observed prevalence of combined carriers of mutations in MI patients and control subjects.
(A) Comparison between the expected and observed prevalence of combined carrier for the FII 20210A + FXIII-A Leu34 alleles in MI patients and control subjects, respectively. (B) Comparison between the expected and observed prevalence of combined carrier for FVL + FXIII-A Leu34 alleles in MI patients and control subjects, respectively. Filled bar (▪) represents the observed prevalence of combined carriers. Empty bar (■) represents the expected prevalence of combined carriers.
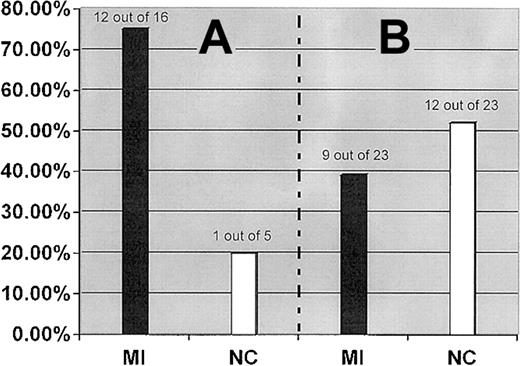
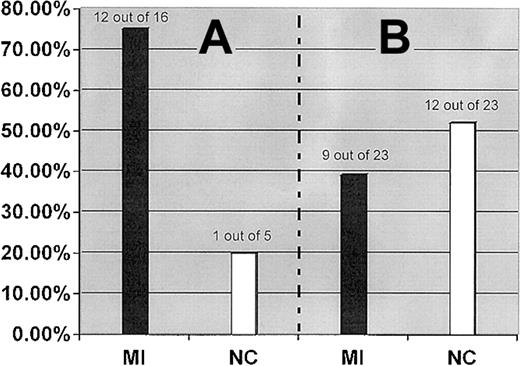
The interaction between the FII 20210A and the FXIII-A Leu34 alleles was further examined by analysis of the distribution of the FXIII-A Leu34 allele in subgrouped patients and control subjects who carry the FII 20210A allele as a genetic background. Although the FXIII-A Leu34 allele showed an almost equal distribution in our MI patient and control populations, it was detected in 75.0% (12 of 16) patients with a genetic background of the FII 20210A allele but only in 20.0% (1 of 5) of control subjects with the same genetic background (OR, 3.7; 95% CI, 2.4-5.1; P = .013) (Figure2).
FXIII-A Leu34 allele.
Prevalence of the FXIII-A Leu34 allele in MI patients and healthy control subjects (NC) who carry the FII 20210A allele (A) and FVL (B).
FXIII-A Leu34 allele.
Prevalence of the FXIII-A Leu34 allele in MI patients and healthy control subjects (NC) who carry the FII 20210A allele (A) and FVL (B).
Of 13 combined carriers of the FII 20210A and FXIII-A Leu34 alleles identified from the studied population (500 patients and 500 control subjects), 12 (92.3%) subjects, including 7 men and 5 women, belonged to the MI patient population, but only 1 (0.7%) man was part of the control population. The coexistence of these 2 gene variants imparts a strong predisposition for MI with high penetrance.
FVL and FXIII-A Leu34 alleles.
The prevalence of combined carriers of FVL and the FXIII-A Leu34 allele were similar in MI patient (9 of 500, 1.8%) and control subject (12 of 500, 2.4%) groups and was consistent with their expected frequencies (2.16% in patients and 2.2% in control subjects) (Figure1B). We further analyzed the prevalence of the FXIII-A Leu34 allele in patients and control subjects who carry the FVL allele as a genetic background. The prevalence of the FXIII-A Leu34 allele was slightly higher in the subgrouped control subjects (12 of 23, 52.17%) than in the subgrouped patients (9 of 23, 39.1%), but the difference was not statistically significant.
FII 20210A and FVL alleles.
There were no combined carriers of the FII 20210A allele and FVL in the MI patient or control populations. This was to be expected considering a calculated frequency of combined carriers of 0.13% in MI patients and 0.01% in healthy control subjects, respectively.
Discussion
Our results showed that the prevalence of FVL and the FXIII-A Leu34 allele in the Newfoundland population is consistent with that reported from white populations elsewhere. However, the 1% prevalence of the FII 20210A allele determined from the Newfoundland population was lower than that reported from most other white populations (2.0%-2.5%).31
Although of lower prevalence in the Newfoundland population, the FII 20210A allele was still shown to be 3.3-fold higher in MI patients than in the control population. The prevalence of FII 20210A is even higher among MI patients with early-onset age (5.6-fold higher) than in age-matched control subjects. This may suggest that the FII 20210A allele is important for early age of onset of MI. The low frequency of the FII 20210A allele in white populations and the extremely low frequencies in other populations32-35 indicate that the FII 20210A allele may only contribute to the pathogenesis of MI in a small portion of MI patients.
FVL is commonly recognized as a strong risk factor in the pathogenesis of venous thrombosis; however, most studies have thus far failed to show an association between FVL and MI.12-14 Recently, some studies have suggested that FVL may be associated with early-onset MI15,16 and MI with normal findings on coronary angiography.17 Although there was identical prevalence of FVL (4.6%) in our patient and control populations, the prevalence of FVL in patients with early-onset age was 3.4-fold higher than in patients with later onset age and 2.7-fold higher than in age-matched control subjects. These data suggest a possible thrombotic predisposition of FVL for early onset of MI. Furthermore, the prevalence of FVL was higher in male MI patients than in female MI patients by almost 3-fold. Nevertheless, the proportion of male MI patients with FVL was not significantly different from the proportion of male control subjects (P = .110). It is not possible, with these data, to suggest FVL as a sex-specific risk factor for MI. Because only a relatively small number of early-onset MI patients have been studied, further efforts focusing on FVL in other early-onset MI patients and on possible sex differences will be of value.
The FXIII-A Leu34 allele has been proposed as a protective factor against MI because of the reduced prevalence observed in MI patients. We observed a slightly but nonsignificantly lower prevalence of the FXIII-A Leu34 allele in MI patients (47.0%) than in the control population (47.8%). However, homozygosity for the L allele was more frequently observed in MI patients than in control subjects (8.4% vs 6.4%), and the gene frequency for FXIII-A Leu34 was similar in patient and control populations. These data suggest that the FXIII-A Leu34 allele does not confer a protective role against MI. Comparison of male MI patients with female MI patients showed an almost 2-fold higher prevalence of the FXIII-A Leu34 allele in men. Comparison of male MI patients carrying the FXIII-A Leu34 allele with male control subjects showed a significantly higher prevalence among MI patients (P = .012). These data suggest that the FXIII-A Leu34 allele may impart an increased risk for MI that is influenced by sex.
Genetic predisposition for MI can result from an additive effect of several genes or a synergistic effect from interactions between multiple genes. Each of these genes may only make a small contribution to the global pathogenesis of MI. In our study, an interaction between the FII 20210A and FXIII-A Leu34 alleles was identified based on the distribution disequilibrium of combined carriers among MI patients. The prevalence of combined carriers in MI patients was higher than expected, but it was lower than expected in control subjects. The FXIII-A Leu34 allele shows equal carrier frequency in MI patients and control subjects, and it does not seem to independently predispose for MI. Furthermore, the FII 20210A allele imparted only a 3.2-fold increased risk for MI. However, combined carriers of the FII 20210A and FXIII-A Leu34 alleles showed a 12-fold increased risk for MI. These results indicate that the interaction of the FII 20210A and FXIII-A Leu34 alleles forms a synergistic rather than an additive effect in the pathogenesis of MI. In our study, the penetrance of MI in persons carrying the FII 20210A and FXIII-A Leu34 alleles was 92.3%. Therefore, we conclude that the coexistence of the FII 20210A allele with the FVIII Leu34 allele is a strong predisposing factor for MI.
Given that the Newfoundland population is descended from a relatively small number of founders, it is possible that a closely linked gene rather than the FXIII-A Leu34 allele may be the causative mutation. This, however, is a considerably less likely possibility for a number of reasons. First, there is a close biochemical functional relationship between FXIII and FII. The 2 factors are closely related sequentially in the clotting cascade. Activated FII (thrombin) is responsible for the generation of fibrin from fibrinogen. Fibrin monomers quickly polymerize into larger polymers. These are relatively loosely held together. Activated FII also activates FXIII, which helps strengthen the clot by cross-linking fibrin chains. The reinforced clot is more resistant to fibrinolysis by plasmin. Second, the FII 20210A allele is observed to correlate with elevated levels of prothrombin in plasma, which may enhance the activation of FXIII and lead to a greater tendency toward thrombosis. Third, the FXIII-A Leu34 allele has been clearly shown to be more prone to activation by thrombin than the wild-type (V34) allele. The FXIII-A Val34Leu variant results in an amino acid substitution 4 residues from the thrombin cleavage site that enhances the rate of activation by thrombin.26Heterozygotes and homozygotes for the FXIII-A Leu34 allele display increased sensitivity to activation by thrombin,25,26whereas a more significant increase in FXIII activation occurs in homozygotes for the FXIII-A Leu34 allele.25,26 No doubt the higher levels of the FXIII-A Leu34 enzyme can create a greater risk for plasmin-resistant clots. Our data suggest that the increased FXIII activity from the FXIII-A Leu34 allele is not in itself sufficient to impart a significant effect on the development of MI. However, the coexistence of the FII 20210A and FXIII-A Leu34 alleles results in a synergistic enhancement of prothrombotic tendency through interaction of the 2 gene products at the biochemical level.25Specifically, the higher tendency for clot formation by higher levels of FII with the FII 20210A allele is coupled with a tendency for greater resistance to fibrinolysis because of greater FXIII activity by the FXIII-A Leu34 allele. This enhanced prothrombotic tendency could result in a greater risk for MI in persons carrying both variants.
Typical of disease of later onset, 90% of our patients experienced MI at age 50 years or older. Only 25% of our control subjects were in this older age group. It is possible that some of our control subjects younger than 50 years will have MI later in life. This may result in an underestimation of the true risk associated with certain alleles. The positive associations described here would only be strengthened by such an underestimation. Other alleles found to impart no significant risk for MI may in fact be weak risk factors. We also recognize the small number of persons in the control and patient groups carrying the FII 20210A allele. Although the difference in carrier frequency for this allele was significantly greater in the younger MI patient group, this observation would be strengthened by examination of a larger number of younger persons with MI. Additional studies examining more MI patients and age-matched control subjects in the Newfoundland population and in other populations will be of even greater value to more clearly establish the magnitude of risk imparted by the presence of these genetic risk factors. These studies are currently under way.
In summary, we analyzed 3 gene variants, FII 20210A, FVL, and FXIII Val34Leu in 500 patients with MI and 500 healthy control subjects from a genetically isolated Newfoundland population.
The FII 20210A allele was associated with MI, and, possibly more important for early age of onset of MI, FVL was only associated with MI in patients with early-onset age. A strong predisposition for MI results from the interaction between the FII 20210A and FXIII-A Leu34 alleles, as was suggested by a 12-fold increased prevalence of combined carriers in MI patients compared with control subjects. There was also a high (92%) penetrance of MI in combined carriers. Therefore, we conclude that (1) the FII 20210A allele is a genetic risk factor for MI, possibly for early onset; (2) FVL predisposes for early onset of MI; (3) the FXIII-A Leu34 allele may independently predispose to MI in males only; (4) interaction between the FII 20210A and FXIII-A Leu34 alleles results in a synergistic coeffect that strongly predisposes to MI. Combined carriers of the FII 20210A and FXIII-A Leu34 alleles are at very high risk for MI.
We thank Drs Ban Younghusband, Bruce Sussex, and Guang Sun for critically reading this manuscript.
Prepublished online as Blood First Edition Paper, December 12, 2002; DOI 10.1182/blood-2002-09-2888.
Supported by grants from the Janeway Children's Hospital Foundation (P.P., Y.-G.X.) and the Faculty of Medicine Research and Development Fund, Memorial University of Newfoundland (Y.-G.X.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ya-Gang Xie, Discipline of Laboratory Medicine, Rm 418, Janeway/HSC, Memorial University of Newfoundland, 300 Prince Philip Dr, St John's, NF, Canada A1B 3V6; e-mail:yxie@mun.ca.