Abstract
Elevated levels of mitogen-activated protein kinase/extracellular regulatory kinase (MAPK/ERK) activity are frequently found in some cancer cells. In efforts to reduce tumor growth, attempts have been made to develop cancer therapeutic agents targeting the MAPK. Here, by use of biologic, biochemical, and gene manipulation methods in human polymorphonuclear neutrophils (PMNs), we have identified a key pathway important in normal cell function involving MAPK/ERK in PMNs for growth inhibition of Candida albicans. Contact withC albicans triggered MAPK/ERK activation in PMNs within 5 minutes, and blocking of MAPK/ERK activation, either by the pharmacologic reagent PD098059 or by dominant-negative MAPK kinase (MEK) expression via vaccinia viral delivery, suppressed antimicrobial activity. Rac and Cdc42, but not Ras or Rho, were responsible for this MAPK/ERK activation. Expression of dominant-negative Rac (N17Rac) or Cdc42 (N17Cdc42) eliminated not only C albicans– mediated ERK phosphorylation but also phagocytosis and granule migration toward the ingested microbes, whereas dominant-negative Ras (N17Ras) and Rho (N19Rho) did not. PAK1 (p21-activated kinase 1) activation is induced by C albicans, suggesting that PAK1 may also be involved in the Rac1 activation of MAPK/ERK. We conclude from these data that Rac/Cdc42-dependent activation of MAPK/ERK is a critical event in the immediate phagocytic response of PMNs to microbial challenge. Therefore, use of MAPK pharmacologic inhibitors for the treatment of cancer may result in the interruption of normal neutrophil function. A balance between therapeutic outcome and undesirable side effects must be attained to achieve successful and safe anticancer therapy.
Introduction
Mitogen-activated protein kinases (MAPK), also referred to as extracellular regulatory kinases (ERK1/2), are crucial constituents of signaling pathways that are important in oncogenic transformation.1-4 There has been a dramatic increase in interest in the role of the MAPK pathway in governing neoplastic cell behavior in a variety of human tumors.5-10Much of this interest has focused on pharmacologic inhibition of cell growth by disruption of the MAPK pathway with either small molecules or by overexpression of proteins with a dominant-negative phenotype. Using a highly potent and selective inhibitor of MAPK kinase (MEK), which lies directly upstream of MAPK, inhibited tumor growth and enhanced tumor apoptosis in both mouse and human tumors.11 12However, because MAPK regulates multiple biologic activities in normal cells, including cell proliferation, differentiation, cell cycle traverse, and survival, it is important to assess potential undesirable effects of MEK inhibitors on normal physiological processes.
Human polymorphonuclear leukocytes or neutrophils (PMNs) constitute the body's foremost defense against invading microorganisms,13,14 and dysregulation of neutrophil antimicrobial activity has severe clinical consequences.15This is best exemplified in the infection rate observed in neutropenic cancer patients receiving cytotoxic chemotherapeutic agents or in patients with genetic defects in neutrophil function as seen in chronic granulomatous disease.16,17 The ability of PMNs to combat microbial pathogens is due to a number of specific activities, including (1) adherence of neutrophils to endothelium, (2) migration or chemotaxis to an inflammatory site, (3) ingestion or phagocytosis of pathogens into phagosomes, and (4) degranulation and killing of invading microorganisms.14 All of these components must act in concert for effective elimination of microbes. However, it is unclear how these events are achieved and what signals control them.
A precise understanding of the signaling mechanisms by which neutrophils mediate phagocytosis and microbicidal activity, however, has not been an easy task to pursue, primarily due to the fragility of freshly isolated neutrophils and the inability to develop long-term culture of neutrophils, which are terminally differentiated cells. Difficulty in gene delivery via plasmid transfection is another obstacle in the study of PMNs, preventing molecular analysis of the signaling pathways related to PMN function.18 Most of the data have been gathered from experiments where phagocytosis-related neutrophil receptors, such as FcR, CR3, and N-formyl-methionyl-leucyl-phenylalanine (fMLP) receptor, are transfected into either fibroblasts or macrophage tumor cell lines.19-21 By such manipulations, Syk activation has been shown to be required for immunoglobulin G (IgG)–mediated phagocytosis through the FcR.21 Furthermore, it has been reported that 2 distinct mechanisms of opsonized phagocytosis exist. Opsonized phagocytosis mediated by the FcR involves Rac and Cdc42, whereas the complement receptor signaling involves Rho.19In other cases, the use of pharmacologic agents in neutrophils has helped to identify the need for MAPK/ERK and Syk in microbicidal function.18,22-24 Studies of knock-out mice have also provided another means to establish phosphatidylinositol-3 kinase (PI-3K) as a critical signal molecule in neutrophil migration and function.25 How these molecules are integrated to control neutrophil function, however, has not been established.
In the present study, we show that human neutrophils are very receptive to gene transfer by recombinant vaccinia viruses, providing us with a potent tool to probe which pathways are triggered by microbes and what function they control in PMNs. Using Candida albicans as a model pathogen, we have identified a specific Rac/Cdc42-dependent but Ras/Rho-independent MAPK/ERK pathway that controls PMN function againstC albicans. Furthermore, we show that this control is at the level of lytic granule movement and phagocytosis within PMNs.
Materials and methods
Preparation of PMNs
Leukocyte buffy coats, obtained from Southwest Florida Blood Bank (Tampa) from healthy volunteers with no signs of allergy or asthma and on no medications, were diluted 1:2 in phosphate-buffered saline (PBS) and centrifuged over Ficoll-Hypaque (Pharmacia Fine Chemicals, Piscataway, NJ) at 400g for 30 minutes at room temperature. The PMN layer on the surface of the erythrocyte cell pellet was then collected, and contaminating erythrocytes were lysed by hypotonic shock with sterile distilled water for 30 seconds at room temperature. The PMNs were washed twice in PBS and then adjusted to the desired cell concentration. Careful washing of the neutrophil preparations (including centrifugation at 200g, aspiration of the supernatant followed by gentle resuspension with a pipette, and avoidance of sudden changes in temperature) allowed us to avoid PMN clumping and maintain the viability of PMNs for up to 24 hours in culture medium.26-29 Such processing yielded more than 99% viable PMNs with no mononuclear cell contamination, as determined by morphology with Giemsa staining.26 Dual staining of phycoerythrin-labeled anti-CD16+ neutrophil preparations with fluorescein isothiocyanate (FITC)–labeled anti-CD14 further indicated that monocytes were absent (data not shown).
Reagents and cell culture
All procedures used in this study were performed with endotoxin-free media and supplies to avoid nonspecific activation of PMNs. Cells were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum (FCS) (Flow Laboratories, McLean, VA) with 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 5 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (GIBCO Laboratories, Grand Island, NY). C albicans used in this study was a clinical isolate from a patient with chronic mucocutaneous candidiasis. The yeasts were grown by weekly transfer onto fresh Sabouraud agar plates at 28°C. The MEK inhibitor PD098059 and rabbit antiactive MAPK, generated against the phosphorylated TEY epitope of MAPK, were purchased from New England Biolabs (Beverly, MA). The monoclonal antibody against granzyme B (2C5) was generated as described previously.30 PAK1 polyclonal antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Vaccinia viral delivery of dominant-negative proteins
The advantages of vaccinia virus as a gene delivery vehicle are based on the fact that it has a high infection rate and infects a wide range of cell types including leukocytes. Additionally, the DNA replication cycle takes place in the cytoplasm of the host cells and uses the virus's own transcription system. Therefore, a short time is required for a significant amount of protein expression (2 to 4 hours), which is independent of the cellular biosynthetic capacity.31,32 Recombinant vaccinia viruses encoding dominant-negative MEK1, Rac (N17Rac), Cdc42 (N17Cdc42), and Rho (N19Rho) were constructed using the vector pSP11 in recombination with the WR strain of vaccinia.33 Vaccinia virus containing kinase-deficient PAK1 (KD) was kindly provided by Elizabeth Hong-Geller (Los Alamos National Laboratory, NM). Vaccinia virus expressing CD56, a large granular lymphocyte-specific surface marker, was used as a control. The procedure of vaccinia viral generation and infection has been described previously.34-37 Briefly, 5 × 106 PMN cells per treatment group were incubated with the vaccinia virus constructs for 2 hours at 37°C serum-free medium at a multiplicity of infection (MOI) of 4 to 6. The cells were washed and then further incubated in serum-containing medium for 2 hours at 37°C prior to the growth inhibition of C albicans assay, Western blotting, and in vitro kinase assays. Cell viability was evaluated by flow cytometric analysis after annexin V–phycoerythrin (PE) staining in some experiments.
Western blotting analysis
Freshly purified PMNs were treated with either serum-free RPMI 1640 (5 × 106 cells per treatment), dimethyl sulfoxide (DMSO), or PD098059 for 2 hours at 37°C and then washed and mixed with paraformaldehyde-fixed C albicans at a ratio of 1:10 (PMNs/C albicans). The cells were pelleted rapidly at 1000 rpm in a microcentrifuge at 4°C followed by incubation for 0 to 5 minutes at 37°C. Then, the cells were solubilized by incubation at 4°C for 30 minutes in 1% Nonidet P-40 (NP-40), 10 mM Tris (tris(hydroxymethyl)aminomethane), 140 mM NaCl, 0.1 mM phenylmethylsulfonyl fluoride (PMSF), 10 mM iodoacetamide, 50 mM NaF, 1 mM EDTA (ethylenediaminetetraacetic acid), 0.4 mM sodium orthovanadate, 10 μg/mL leupeptin, 10 μg/mL pepstatin, and 10 μg/mL aprotinin. Cell lysates were centrifuged at 12000g for 15 minutes to remove nuclei and cell debris. The protein concentration of the soluble extracts was determined by using the Bio-Rad (Bradford, Hercules, CA) protein assay. For Western blots, 50 μg of the protein per lane was separated on 10% sodium dodecyl sulfate (SDS)–polyacrylamide gels, transferred to Immobilon membranes, and reacted with the desired antibody. The proteins were detected by the enhanced chemiluminescence detection system (ECL, Amersham, Piscataway, NJ).
In vitro kinase assay
Cell lysates from 5 × 106 PMNs were immunoprecipitated with 5 μg anti-PAK1 polyclonal antibody. The immunoprecipitated proteins were suspended in 30 μL kinase reaction buffer containing 10 mM Tris (pH 7.2), 10 mM MnCl2, 10 mM MgCl2, 10 μCi (0.37 MBq) γ-32P adenosine triphosphate (ATP) (Amersham), and 1 μM unlabeled ATP and then incubated at room temperature (RT) for 20 minutes with 4 μg myelin basic protein (MBP) (Upstate Biotechnology, Lake Placid, NY) as an exogenous substrate. The reaction was terminated by addition of sample buffer and quick centrifugation, and equal volume aliquots from each sample were analyzed by electrophoresis using 10% SDS-polyacrylamide gels. After transfer, phosphoproteins were detected by autoradiography.
Immunostaining
PMNs, untreated or pretreated with 50 μM PD098059 for 2 hours at 37°C, were added to C albicans prestained red by using the Sigma PKH26 red fluorescent cell linker kit at a 1:10 PMN/C albicans ratio in a total volume of 100 μL. The cells were spun rapidly at 1000 rpm for 1 minute in a cold microcentrifuge and then incubated for 0 to 5 minutes at 37°C. DMSO-treated PMNs were included as a control. The cells were then centrifuged onto microscope slides and fixed at −20°C with methanol-acetone (3:1) for 20 minutes. The slides were air-dried and rehydrated for 2 hours in several changes of PBS. All procedures were performed at room temperature. Monoclonal antigranzyme B was used to detect granule mobilization38 toward ingested C albicans in PMNs. The slides were washed several times with PBS and covered with coverslips in mounting media with antifade. Immunofluorescence was observed with a Leitz Orthoplan 2 microscope (Photometrics Ltd, Tucson, AZ), and images were captured by a CCD camera with the Smart Capture Program (Vysis, Downers Grove, IL).
Growth inhibition of C albicans
PMN function against C albicans was assessed by3H-glucose incorporation into residual C albicans after incubation with PMNs treated or untreated with PD098059 or infected with vaccinia viral constructs. PMNs were first serially diluted 2-fold from 6 × 105/mL to 7.5 × 104/mL in RPMI 1640 containing 2% FCS, and 50 μL of each cell suspension was added to triplicate wells of a 96-well flat-bottomed microtiter plate; 500 C albicans in 25 μL of medium was added to each well to achieve the effector-target (E/T) ratios of 60:1, 30:1, 15:1, and 7.5:1.C albicans was also added to empty wells to serve as controls. After incubation for 18 hours at 37°C, 50 μL of sterile water containing 10 μCi/mL of 3H-glucose (New England Nuclear Research Products, Boston, MA) was added for 3 hours at 37°C. The proliferating fungi that incorporated 3H-glucose were collected by adding 50 μL sodium hypochloride (bleach) immediately before processing through a harvester with distilled water onto glass fiber filters and counted on a β scintillation counter.39 The mean ± SE of the triplicate cultures was determined, and the percent growth inhibiton of C albicans was calculated as follows: % growth inhibition = cpm C albicans alone − cpm effector/C albicans × 100 cpm C albicans alone.
Each experiment was performed at least 3 times with different healthy donors, and only the 60:1 E-T ratios are reported in the figures for clarity.
Results
Rapid stimulation of MAPK phosphorylation by C albicans
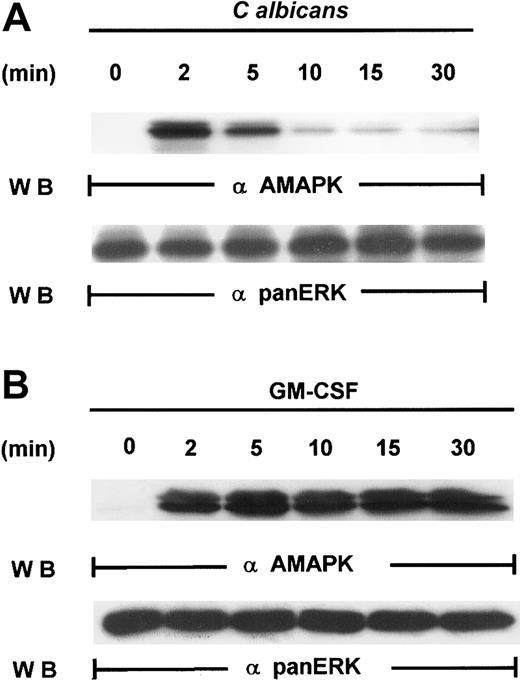
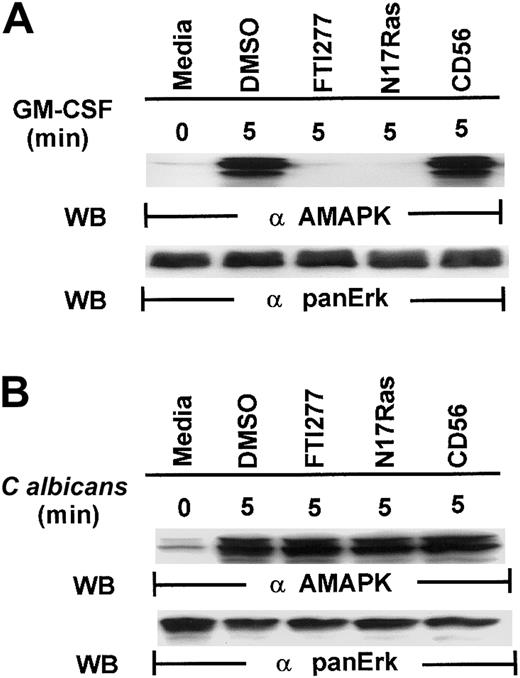
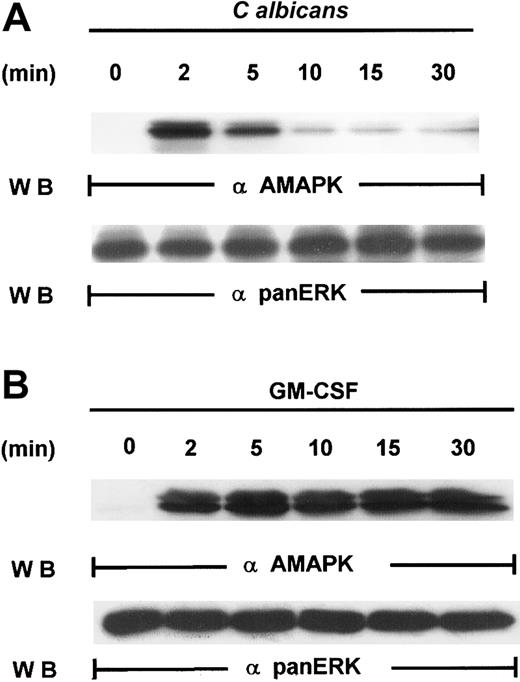
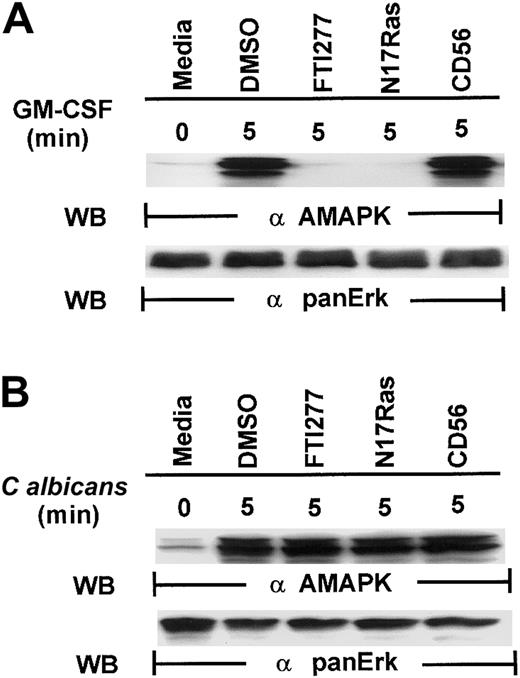
Our first attempt to investigate the signaling pathways required for microbial killing in PMNs was to examine if C albicanscould activate MAPK. Exposure of human PMNs to C albicansrapidly induced a transient activation of MAPK as detected by Western blotting with antiactive MAPK that recognized the phosphorylated TEY motif. MAPK activation peaked at 2 minutes and declined by 5 minutes (Figure 1A). This is in sharp contrast to MAPK activation by granulocyte-macrophage colony-stimulating factor (GM-CSF) (100 U/mL), a survival cytokine that consistently induced a long-lasting MAPK/ERK activity (Figure 1B), indicating that C albicans–induced MAPK/ERK activation may be associated with immediate PMN antifungal activity.
Induction of MAPK/ERK activation by GM-CSF and
C albicans. (A) Freshly isolated PMNs were pretreated with heat-killed C albicans at a PMN/C albicansratio of 1:10 for 0 to 30 minutes at 5 minutes at 37°C, and cell lysates were analyzed by immunoblotting with antiactive MAPK (upper panel) and then stripped and reprobed with anti-panERK to check for equal loading (lower panel). (B) PMNs, pretreated with GM-CSF (100 U/mL) at 37°C for 0 to 30 minutes, were lysed and sequentially probed for active-MAPK and anti-panERK. Results shown are representative of 3 separate experiments.
Induction of MAPK/ERK activation by GM-CSF and
C albicans. (A) Freshly isolated PMNs were pretreated with heat-killed C albicans at a PMN/C albicansratio of 1:10 for 0 to 30 minutes at 5 minutes at 37°C, and cell lysates were analyzed by immunoblotting with antiactive MAPK (upper panel) and then stripped and reprobed with anti-panERK to check for equal loading (lower panel). (B) PMNs, pretreated with GM-CSF (100 U/mL) at 37°C for 0 to 30 minutes, were lysed and sequentially probed for active-MAPK and anti-panERK. Results shown are representative of 3 separate experiments.
MAPK is associated with PMN killing of C albicans
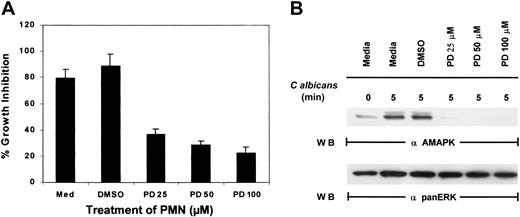
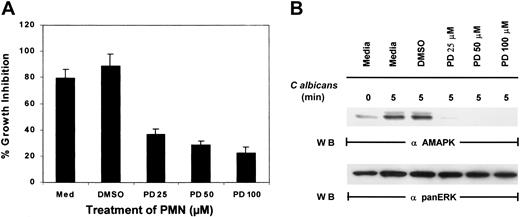
To examine if MAPK/ERK controls function in PMNs, an inhibitor of MEK, PD098059, was added to PMNs for 2 hours prior to the addition ofC albicans. While control PMNs cultured in medium alone or medium containing the inhibitor solvent DMSO significantly suppressed the growth of C albicans, increasing doses of PD098059 prevented PMN-mediated growth inhibition of C albicans(Figure 2A). As a control, whole-cell lysates prepared from the same pools of PMNs were evaluated for active MAPK/ERK, and the doses of PD098059 that inhibited PMN antimicrobial function also correspondingly inactivated MAPK/ERK phosphorylation (Figure 2B). Although phagocytosis mediated by fMLP has been demonstrated to involve p38 MAPK, addition of a p38 MAPK-selective inhibitor, SB203580, failed to reverse PMN-mediated growth suppression of C albicans (data not shown). These results suggest that MAPK/ERK activation and not p38 MAPK is required for the direct antimicrobial killing function of PMNs.
Effect of PD098059 on PMN function.
(A) PMNs were pretreated with medium, DMSO, or increasing doses of PD098059 for 2 hours at 37°C prior to testing for their ability to inhibit the growth of C albicans using3H-glucose incorporation. Results shown are representative of at least 3 separate experiments. (B) PMNs, pretreated with various doses of PD098059 for 2 hours at 37°C, were mixed withC albicans for 0 to 5 minutes at 37°C. Cell lysates were then analyzed by immunoblotting with antiactive MAPK (upper panel) or anti-panERK to check for equal loading (lower panel). Results shown are representative of 5 separate experiments.
Effect of PD098059 on PMN function.
(A) PMNs were pretreated with medium, DMSO, or increasing doses of PD098059 for 2 hours at 37°C prior to testing for their ability to inhibit the growth of C albicans using3H-glucose incorporation. Results shown are representative of at least 3 separate experiments. (B) PMNs, pretreated with various doses of PD098059 for 2 hours at 37°C, were mixed withC albicans for 0 to 5 minutes at 37°C. Cell lysates were then analyzed by immunoblotting with antiactive MAPK (upper panel) or anti-panERK to check for equal loading (lower panel). Results shown are representative of 5 separate experiments.
Vaccinia viral expression of dominant-negative MEK1 but not Ras prevented PMN antimicrobial activity
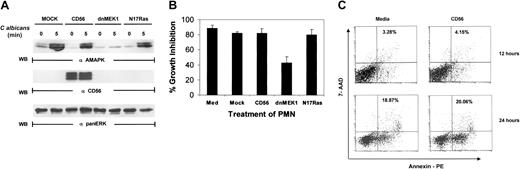
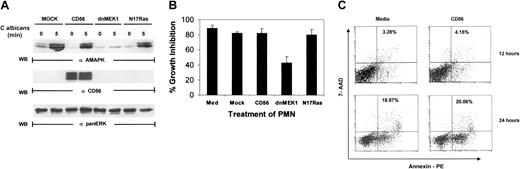
To confirm the involvement of MAPK/ERK in antimicrobial activity, PMNs were either mock infected or infected with vaccinia virus encoding dominant-negative MEK1 (dnMEK1). Infection was carried out for 4 hours, which provided sufficient time for ample production of proteins from viral delivery without loss of cell viability. CD56, which is not endogenously expressed in PMNs, was also introduced into a separate PMN pool as a nonspecific infection control. MAPK phosphorylation was induced by C albicans after 5 minutes in mock-infected PMNs. However, overexpression of dnMEK1 ablated MAPK/ERK phosphorylation in response to C albicans (Figure3A). Expression of the irrelevant gene, CD56, had no effect on MAPK/ERK activation. Because the Ras/Raf/MAPK pathway is thought to be the predominant signaling event linked to MAPK activation,40 we next examined whether Ras was involved in MAPK activation of PMNs and antifungal activity. Dominant-negative Ras (N17Ras) expression in PMNs, unlike dnMEK1, had no suppressive effects (Figure 3A).
Vaccinia viral expression of dominant-negative MEK 1 but not Ras blocks
C albicans–induced ERK activation. (A) PMNs were infected with recombinant vaccinia virus expressing dominant-negative MEK1, dominant-negative Ras (N17Ras), or an irrelevant protein (CD56) for 4 hours at 37°C. The infected PMNs were then incubated withC albicans, and samples were collected at 0 minutes (lanes 1, 3, 5, 7) and 5 minutes (lanes 2, 4, 6, 8). Cell lysates were then immunoblotted with antiactive MAPK (upper panel). The blot was then stripped and reprobed with the marker protein anti-CD56 to demonstrate that the viral infection was successful (middle panel). The blot was also reprobed with anti-panERK for equal loading (lower panel). (B) Aliquots of PMNs from the same experiment as in panel A were tested for growth inhibition of C albicans. Results shown are representative of 3 separate experiments. (C) PMNs either mock infected or CD56 infected at MOI of 1:6 for 12 hours or 24 hours were collected and analyzed for apoptosis by annexin V–PE binding. The percentage of apoptotic cells is indicated.
Vaccinia viral expression of dominant-negative MEK 1 but not Ras blocks
C albicans–induced ERK activation. (A) PMNs were infected with recombinant vaccinia virus expressing dominant-negative MEK1, dominant-negative Ras (N17Ras), or an irrelevant protein (CD56) for 4 hours at 37°C. The infected PMNs were then incubated withC albicans, and samples were collected at 0 minutes (lanes 1, 3, 5, 7) and 5 minutes (lanes 2, 4, 6, 8). Cell lysates were then immunoblotted with antiactive MAPK (upper panel). The blot was then stripped and reprobed with the marker protein anti-CD56 to demonstrate that the viral infection was successful (middle panel). The blot was also reprobed with anti-panERK for equal loading (lower panel). (B) Aliquots of PMNs from the same experiment as in panel A were tested for growth inhibition of C albicans. Results shown are representative of 3 separate experiments. (C) PMNs either mock infected or CD56 infected at MOI of 1:6 for 12 hours or 24 hours were collected and analyzed for apoptosis by annexin V–PE binding. The percentage of apoptotic cells is indicated.
Aliquots of cells from the same experiment were then examined for antimicrobial activity. Medium-cultured PMNs, mock-infected PMNs, and CD56-infected PMNs were all capable of suppressing the growth ofC albicans in vitro. Expression of dnMEK1, but not N17Ras, significantly down-regulated PMN antimicrobial activity (Figure 3B). Because PMNs rapidly undergo spontaneous cell death in vitro, we examined the PMN viability after mock infection and infection with CD56 vaccinia virus after 12 and 24 hours. Staining with annexin V–PE was used as an early indicator of apoptosis. A viability dye (7-aminoactinomycin D [7-AAD]) was also added to discriminate live and dead cells, and cells were analyzed by flow cytometry. We found that CD56 vaccinia virus infection did not affect the amount or rate of spontaneous cell death in this assay (Figure 3C). These results confirm that the reduced MAPK activity and antifungal activity was not caused by reduced viability due to the infection. Taken together, the findings with PD098059 and dnMEK1 reveal the necessity of MAPK activation in direct PMN antimicrobial function. However, this pathway is Ras independent.
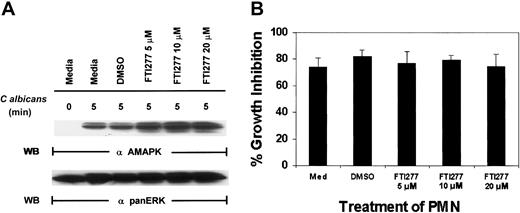
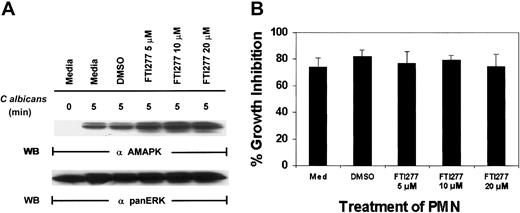
To support our observation with N17Ras, we next used a specific farnesyl-transferase inhibitor, FTI277, which blocks Ras function.41 FTI277 treatment of PMNs had no effect on MAPK activation induced by C albicans (Figure4A) and resulted in no inhibition of antifungal activity (Figure 4B). Importantly, FTI277 did not decrease PMN viability in these experiments as determined by annexin V–PE staining (data not shown). To ensure that FTI277 and N17Ras worked appropriately to block Ras activation in PMNs, we included GM-CSF as a control. GM-CSF (100 IU/mL) was added for 5 minutes in the presence of N17Ras and FTI277 (Figure 5). Both FTI277 (15 μM) and N17Ras were able to block GM-CSF–induced but not C albicans–induced MAPK activation. These results indicate that within the same PMNs, GM-CSF triggers a Ras-dependent MAPK pathway, whereas microbe-triggered MAPK is Ras independent.
C albicans –induced ERK activation in PMNs is Ras independent.
(A) Freshly isolated PMNs were treated with medium alone, DMSO, or various doses of the farnesyl-transferase inhibitor, FTI277, for 24 hours prior to the addition of C albicans for 0 to 5 minutes at 37°C. Cell lysates were immunoblotted with antiactive MAPK (upper panel) and then stripped and reprobed with anti-panERK for equal loading (lower panel). (B) The same aliquots of PMNs were also tested for their ability to inhibit C albicans growth. Results shown are representative of 4 separate experiments.
C albicans –induced ERK activation in PMNs is Ras independent.
(A) Freshly isolated PMNs were treated with medium alone, DMSO, or various doses of the farnesyl-transferase inhibitor, FTI277, for 24 hours prior to the addition of C albicans for 0 to 5 minutes at 37°C. Cell lysates were immunoblotted with antiactive MAPK (upper panel) and then stripped and reprobed with anti-panERK for equal loading (lower panel). (B) The same aliquots of PMNs were also tested for their ability to inhibit C albicans growth. Results shown are representative of 4 separate experiments.
FTI277 and Ras N17 inhibit GM-CSF– but not
C albicans–induced MAPK/ERK activation. Freshly isolated PMNs, pretreated with DMSO or with 15 μM FTI277 or infected with N17Ras, were stimulated with GM-CSF (100 U/mL) (A) or C albicans (B) for 0 to 5 minutes at 37°C. Cell lysates were immunoblotted with antiactive MAPK (upper panel) and reprobed with anti-panMAPK/ERK (lower panel) for equal loading. Results shown are representative of 3 separate experiments.
FTI277 and Ras N17 inhibit GM-CSF– but not
C albicans–induced MAPK/ERK activation. Freshly isolated PMNs, pretreated with DMSO or with 15 μM FTI277 or infected with N17Ras, were stimulated with GM-CSF (100 U/mL) (A) or C albicans (B) for 0 to 5 minutes at 37°C. Cell lysates were immunoblotted with antiactive MAPK (upper panel) and reprobed with anti-panMAPK/ERK (lower panel) for equal loading. Results shown are representative of 3 separate experiments.
C albicans–induced MAPK activation requires Rac and Cdc42 but not Rho
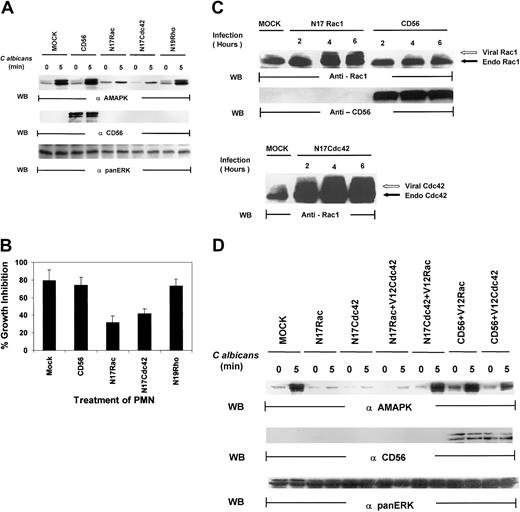
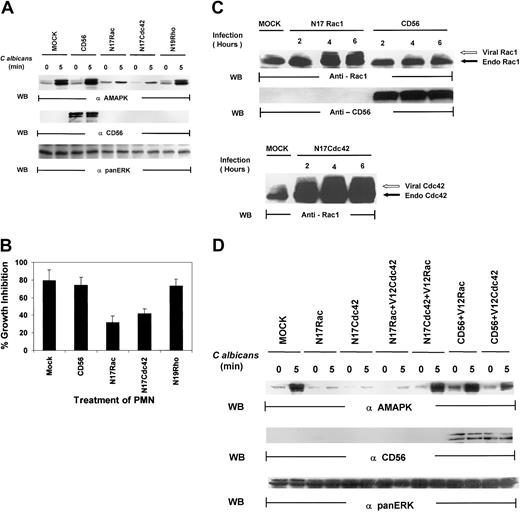
Several lines of evidence have suggested that the small guanosine triphosphatases (GTPases), Rac, Cdc42, and Rho, regulate phagocytosis.19,20 These proteins are key regulatory molecules that link surface receptors to cytoskeletal rearrangement.19 Rac has primarily been associated with MAPK–Jun N-terminal kinase (JNK) activation,42,43 but there is growing evidence that Rac and Cdc42 can also activate MAPK/ERK in a pathway that is independent of Ras.44-46 We therefore tested whether the Rho family of small guanosine triphosphate (GTP)–binding proteins might be upstream signals for MAPK activation in PMNs. As shown in Figure6A, PMNs, mock infected or infected with vaccinia viral constructs expressing CD56, demonstrated marked MAPK activation induced by C albicans. Dominant-negative Rac (N17Rac) and Cdc42 (N17Cdc42), but not Rho (N19Rho), expression in PMNs markedly reduced C albicans–induced MAPK phosphorylation. In addition, N17Rac and N17Cdc42, but not N19Rho, caused a significant decrease in PMN-mediated antifungal activity (Figure 6B). Thus, these results suggest that PMN function against C albicans is controlled by a Ras/Rho-independent but Rac/Cdc42-dependent MAPK/ERK signaling pathway. Importantly, we found that significant amounts of CD56, which is not endogenously expressed in PMNs, are present in these PMNs (Figure 6C). Western blot analysis of whole-cell lysates of PMNs revealed that significant amounts of Rac1 and Cdc42 were expressed in the appropriate samples as evidenced by the slower gel mobility of the recombinant proteins N17Rac and N17Cdc42, which contain myc-tag epitopes, compared with the faster migration of the respective endogenous proteins (Figure 6C). This was further confirmed by vaccinia vector containing green fluorescent protein (GFP) that demonstrated approximately 50% to 60% of cells are positive (data not shown). All these data indicate that the dominant-negative constructs introduced into PMNs are not only highly expressed but also are able to block specific PMN function against C albicans.
Both N17Rac and N17Cdc42 block
C albicans–induced MAPK/ERK activation and PMN activity against C albicans. (A) PMNs were infected with recombinant vaccinia virus expressing dominant-negative Rac (N17Rac), Cdc42 (N17Cdc42), Rho (N19Rho), or an irrelevant protein (CD56) for 4 hours at 37°C. (A) The infected PMNs were treated withC albicans, and samples were collected at 0 minutes (lanes 1, 3, 5, 7, 9) and at 5 minutes (lanes 2, 4, 6, 8, 10). Cell lysates were probed with antiactive MAPK (top panel) and reprobed with the marker protein anti-CD56 to demonstrate successful viral infection (middle panel). The blot was again stripped and reprobed with anti-panMAPK/ERK for equal loading (lower panel). (B) Aliquots from the same PMNs were also tested for their ability to inhibit C albicans growth. Results shown are representative of 3 separate experiments. (C) PMNs were infected with CD56, N17Rac, or N17Cdc42 for 2 to 6 hours at 37°C. The infected PMNs were then collected at each time point, and cell lysates were probed with anti-Rac1 (top panel), CD56 (middle panel), or Cdc42 (bottom panel) as indicated. The myc-tagged virally expressed proteins were differentiated from endogenous Rac1 and Cdc42 by the appearance of a slower migrating band (indicated by open arrow). (D) PMNs were infected with CD56, N17Rac, or N17Cdc42 for 15 minutes at 37°C and then reinfected with either constitutively active V12Rac1 or V12Cdc42 for 4 hours at 37°C. The infected PMNs were then treated with C albicans,and samples were collected at 0 minutes and at 5 minutes, and cell lysates were probed with antiactive MAPK (top panel) or CD56 for expression of the marker protein (middle panel) or anti-panERK for equal loading (bottom panel).
Both N17Rac and N17Cdc42 block
C albicans–induced MAPK/ERK activation and PMN activity against C albicans. (A) PMNs were infected with recombinant vaccinia virus expressing dominant-negative Rac (N17Rac), Cdc42 (N17Cdc42), Rho (N19Rho), or an irrelevant protein (CD56) for 4 hours at 37°C. (A) The infected PMNs were treated withC albicans, and samples were collected at 0 minutes (lanes 1, 3, 5, 7, 9) and at 5 minutes (lanes 2, 4, 6, 8, 10). Cell lysates were probed with antiactive MAPK (top panel) and reprobed with the marker protein anti-CD56 to demonstrate successful viral infection (middle panel). The blot was again stripped and reprobed with anti-panMAPK/ERK for equal loading (lower panel). (B) Aliquots from the same PMNs were also tested for their ability to inhibit C albicans growth. Results shown are representative of 3 separate experiments. (C) PMNs were infected with CD56, N17Rac, or N17Cdc42 for 2 to 6 hours at 37°C. The infected PMNs were then collected at each time point, and cell lysates were probed with anti-Rac1 (top panel), CD56 (middle panel), or Cdc42 (bottom panel) as indicated. The myc-tagged virally expressed proteins were differentiated from endogenous Rac1 and Cdc42 by the appearance of a slower migrating band (indicated by open arrow). (D) PMNs were infected with CD56, N17Rac, or N17Cdc42 for 15 minutes at 37°C and then reinfected with either constitutively active V12Rac1 or V12Cdc42 for 4 hours at 37°C. The infected PMNs were then treated with C albicans,and samples were collected at 0 minutes and at 5 minutes, and cell lysates were probed with antiactive MAPK (top panel) or CD56 for expression of the marker protein (middle panel) or anti-panERK for equal loading (bottom panel).
Because both N17Rac and N17Cdc42 blocked MAPK and antimicrobial activities, we next speculated that they must belong in the same pathway, particularly based on recent evidence that one could lie downstream of the other.47 We examined if constitutively active V12Cdc42 could rescue PMN function blocked by N17Rac or, alternatively, if V12Rac could rescue PMN function blocked by N17Cdc42. After infection with either N17Rac or N17Cdc42 for 15 minutes, PMNs were infected with constitutively active V12Rac or V12Cdc42. V12Cdc42 had no effect on N17Rac-mediated inhibition of MAPK activation, but V12Rac was able to rescue the blockade effect of N17Cdc42 on MAPK activation (Figure 6D). Virally expressed V12Rac and V12Cdc42 were both equally active in triggering MAPK activation in the presence of an irrelevant CD56 vector. Thus, Rac acts as a downstream effector of Cdc42 in PMNs.
C albicans–induced MAPK activation involves PAK1 activation
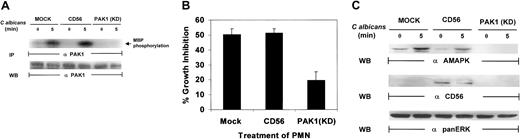
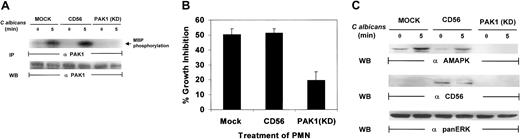
Recent studies have indicated that p21-activated kinase 1 (PAK1) is able to regulate cytoskeletal changes and phagocytic uptake in human neutrophils in response to chemoattractant stimuli.48 PAK1 also serves as a downstream target for both Rac1 and Cdc42.49,50 Many insights into the mechanism of chemotaxis have been made using Dictyostelium cells, which exhibit chemotaxis responses with many similarities to mammalian macrophage and neutrophils. In both mammalian leukocytes and Dictyosteliumcells, translocation and phosphorylation of PAK1 is essential for proper cell polarity and chemotaxis.51 Recent data from our laboratory have indicated that PAK1 may act as a specific mediator for target-induced MAPK activation in natural killer (NK) cells.35 Therefore, it is essential to explore the role of PAK1 in C albicans–induced PMN activation. PAK1 protein was immunoprecipitated from the whole-cell lysates of PMNs after C albicans stimulation and analyzed for its kinase activity. PAK1 kinase activity was significantly increased following C albicans stimulation for 5 minutes (Figure7A). To further examine the role of PAK1 in C albicans–induced killing, we utilized a kinase-defective, dominant-negative PAK1 mutant that was expressed in recombinant vaccinia virus.52 This mutant was used to inhibit PAK1-mediated, Cdc42-dependent responses in PMNs. Expression of kinase-deficient PAK1 markedly suppressed PAK1 kinase activity (Figure7A). The same PMNs were then tested for antifungal activity. Kinase-deficient PAK1 expression inhibited PMN activity against C albicans growth, whereas PMNs infected with control CD56 viral vector remained highly active against C albicans, equal to that in mock-infected control PMNs (Figure 7B). Examination of whole-cell lysates from the same PMNs demonstrated that kinase-deficient PAK1, unlike control CD56, also markedly inhibited MAPK activation induced by C albicans (Figure 7C). Altogether, these data suggest that PAK1 may act as an upstream effector in C albicans–induced MAPK activation, which drives PMN antifungal activity.
PAK1 is required for
C albicans–induced MAPK activation in PMNs. (A) Kinase-deficient PAK1 and CD56-expressing PMNs were stimulated withC albicans for 0 to 5 minutes at 37°C. Whole-cell lysates were immunoprecipated with anti-PAK1 for analysis of PAK1 activity by an in vitro kinase assay with MBP as the phosphorylation substrate. After autoradiography, the same membrane was stripped and reprobed with anti-PAK1 to check for equal loading. (B) PMNs from the same aliquots were also tested for their ability to inhibit C albicansgrowth. (C) PMNs from panel B were also probed with antiactive MAPK, anti-CD56, or anti-panERK.
PAK1 is required for
C albicans–induced MAPK activation in PMNs. (A) Kinase-deficient PAK1 and CD56-expressing PMNs were stimulated withC albicans for 0 to 5 minutes at 37°C. Whole-cell lysates were immunoprecipated with anti-PAK1 for analysis of PAK1 activity by an in vitro kinase assay with MBP as the phosphorylation substrate. After autoradiography, the same membrane was stripped and reprobed with anti-PAK1 to check for equal loading. (B) PMNs from the same aliquots were also tested for their ability to inhibit C albicansgrowth. (C) PMNs from panel B were also probed with antiactive MAPK, anti-CD56, or anti-panERK.
MAPK activity is required for phagocytosis and intracellular granule mobilization in PMNs
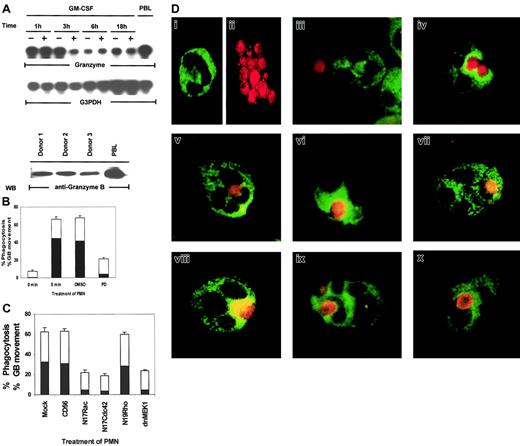
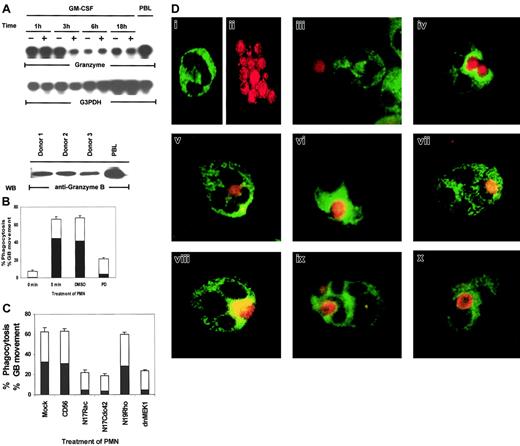
In the above experiments, we utilized candidal growth inhibition as an assay for PMN biologic function. However, this assay records both intracellular and extracellular microbicidal effects—that is, death by phagocytosis and by exocytosis of antimicrobial products. For death of ingested microbes, mobilization of lytic granules into phagosomes is a critical event.13,14 Both the production of oxygen intermediates and the release of antimicrobial enzymes contained in lytic granules determine the intracellular killing capacity of PMNs.13 It has been reported that granular proteins such as perforin and granulysin have broad effects against intracellular pathogenic bacteria, fungi, and parasites in vitro.53 But another key granular protein, granzyme B, has not been reported to be expressed in human PMNs, and its antimicrobial activity has not been defined. As shown in Figure 8A, granzyme B is expressed in PMNs, as demonstrated by mRNA expression. Western blot analysis and immunostaining of PMNs with a monoclonal antibody specific for granzyme B demonstrated that granzyme B is also present at the protein level (Figure 8A, bottom panel). We then used granzyme B as a specific marker for lytic granules in PMNs to examine if the Rac/Cdc42-dependent MAPK pathway controls phagocytosis or granule mobilization. We employed 2-color staining whereby C albicans was prelabeled with a red flourescent cell linker and the lytic granules in PMNs were stained with FITC-labeled antigranzyme B. By visual assessment of stained PMNs and C albicans, MAPK was found to control not only phagocytosis but also granule polarization toward the ingested microbe (Figure 8B). Phagocytosis was evaluated by counting the number of PMNs that showed ingested microbes out of 100 total PMNs on each slide. When the phagocytizing PMNs were located, the position of the granzyme B molecules within the PMNs was also recorded. Granule movement was then evaluated by counting the number of PMNs with rings of granzyme B clearly visible around their ingested microbes out of 100 PMNs containing phagocytized C albicans. At 0 minutes of exposure to C albicans,phagosomes were visible in 7.6% ± 1.2% of PMNs, whereas by 5 minutes, 66% ± 2.9% of PMNs had ingested microbes. The solvent control DMSO had little effect on phagocytosis (67.6% ± 2.4%), whereas the MEK inhibitor PD098059 greatly reduced the ability of the treated PMNs to phagocytize microbes (21% ± 1.6%).
Rac, Cdc42, MEK1, and ERK control phagocytosis and granule localization as monitored by immunostaining of granzyme B in PMNs.
(A) Constitutive expression of granzyme B mRNA in PMNs. Total RNA (20 μg) was purified from PMNs cultured in 100 U/mL GM-CSF or medium for the indicated times and analyzed by Northern blotting for granzyme B mRNA (top and middle panels). The PMN lysates from 3 blood donors were Western blotted with antigranzyme B antibody to show the protein expression of granzyme B in neutrophils (bottom panel). Cell lysates from peripheral blood lymphocytes (PBLs) were used as a positive control for this experiment. (B) Percentages of inhibition of phagocytosis (■) or granzyme B (▪) mobilization by PD098059. PMNs pretreated with medium, DMSO, or 50 μM PD098059 were stimulated with C albicans for 0 to 5 minutes at 37°C, and the number of PMNs showing ingested C albicans of a total of 100 PMNs were counted and expressed as a percent of total PMNs. The number of PMNs with ingested C albicansthat also contained clearly visible granzyme B surrounding the phagosome were also counted and expressed as percent of the total PMNs. (C) Percentages of inhibition of phagocytosis (■) or granzyme B (▪) mobilization by dominant-negative Rac, Cdc42, Rho, and MEK1. PMNs expressing CD56, N17Rac, N17Cdc42, N17Rho, or MEK1 were also evaluated for phagocytosis and granule movement. (D) Freshly isolated PMNs were preincubated with DMSO or 50 μM PD098059 for 2 hours at 37°C. The cells were then mixed with fixed C albicans for 0 to 5 minutes at 37°C and cytospun onto microscope slides. Control PMNs stained with granzyme B–FITC (i), C albicans stained red with PKH67-GL (ii), PMNs incubated with C albicans at 0 minutes (iii), DMSO-treated (iv), PD098059-treated PMNs (v), PMNs expressing CD56 (vi), dominant-negative MEK1 (vi), N19Rho (viii), N17Rac (ix), or N17Cdc42 (x).
Rac, Cdc42, MEK1, and ERK control phagocytosis and granule localization as monitored by immunostaining of granzyme B in PMNs.
(A) Constitutive expression of granzyme B mRNA in PMNs. Total RNA (20 μg) was purified from PMNs cultured in 100 U/mL GM-CSF or medium for the indicated times and analyzed by Northern blotting for granzyme B mRNA (top and middle panels). The PMN lysates from 3 blood donors were Western blotted with antigranzyme B antibody to show the protein expression of granzyme B in neutrophils (bottom panel). Cell lysates from peripheral blood lymphocytes (PBLs) were used as a positive control for this experiment. (B) Percentages of inhibition of phagocytosis (■) or granzyme B (▪) mobilization by PD098059. PMNs pretreated with medium, DMSO, or 50 μM PD098059 were stimulated with C albicans for 0 to 5 minutes at 37°C, and the number of PMNs showing ingested C albicans of a total of 100 PMNs were counted and expressed as a percent of total PMNs. The number of PMNs with ingested C albicansthat also contained clearly visible granzyme B surrounding the phagosome were also counted and expressed as percent of the total PMNs. (C) Percentages of inhibition of phagocytosis (■) or granzyme B (▪) mobilization by dominant-negative Rac, Cdc42, Rho, and MEK1. PMNs expressing CD56, N17Rac, N17Cdc42, N17Rho, or MEK1 were also evaluated for phagocytosis and granule movement. (D) Freshly isolated PMNs were preincubated with DMSO or 50 μM PD098059 for 2 hours at 37°C. The cells were then mixed with fixed C albicans for 0 to 5 minutes at 37°C and cytospun onto microscope slides. Control PMNs stained with granzyme B–FITC (i), C albicans stained red with PKH67-GL (ii), PMNs incubated with C albicans at 0 minutes (iii), DMSO-treated (iv), PD098059-treated PMNs (v), PMNs expressing CD56 (vi), dominant-negative MEK1 (vi), N19Rho (viii), N17Rac (ix), or N17Cdc42 (x).
We next evaluated whether granule movement toward the ingested microbe was also subject to control by MAPK/ERK. On further visual examination of the PMNs that had phagocytized C albicans, PD098059 also adversely affected granzyme B mobilization toward the ingested microbes. As shown in Figure 8B, only 4% ± 0.5% PD098059-treated PMNs showed granzyme B mobilization as compared with 44.6% ± 4.6% medium- or 41.5% ± 5.0% DMSO-treated control PMNs. Expression of N17Rac, N17Cdc42, and dnMEK1 had similar inhibitory effects on both phagocytosis and granule mobilization (Figure 8C).
Two-color staining clearly depicted dispersed FITC-labeled granzyme B–containing granules (Figure 8Di) in control PMNs and at 0 minutes of contact with C albicans (Figure 8Diii). After 5 minutes ofC albicans exposure, granzyme B rapidly gathered in PMNs, surrounding the ingested microbe (Figure 8Div). In PD098059-treated PMNs, granzyme B retained its dispersed intracellular localization (Figure 8Dv). Furthermore, in comparison to CD56 expression (Figure 8Dvi), dnMEK1 in PMNs also suppressed granzyme B intracellular mobilization toward ingested C albicans (Figure 8Dvii). N17Rac and N17Cdc42, but not N19Rho, had similar effects (Figure 8Dviii-x). These results indicate that phagocytosis and granule mobilization are simultaneously regulated by the Cdc42→Rac→MEK1→ERK pathway in PMNs.
Discussion
Most research to date has suggested that signaling by the MAPK/ERK pathway is intimately involved in the ability of growth factors to stimulate proliferation.40,54 The currently accepted view of signaling through the MAPK/ERK cascade is based on studies with established fibroblastoid and transformed cell models that link MAPK/ERK activation with proliferation, whereby the greater the activation, the greater is the proliferative response.55,56 In agreement with increased MAPK/ERK activity correlating with increased transformation and proliferation, elevated levels of MAPK/ERK activity are frequently found in some cancer cells. Indeed, in efforts to reduce tumor growth, attempts have been made to develop cancer therapeutic agents targeting the MAPK/ERK pathway.11 However, emerging evidence indicates that the MAPK/ERK pathway controls normal cell functions, including secretion and reduced nicotinamide adenine dinucleotide phosphate (NADPH) regulation.18 More defined studies from our laboratory have shown that the MAPK pathway is also a key regulator of essential lymphocytic functions in addition to those associated with cell growth and proliferation.38 Depending upon the cell type and stimulus, MAPK activation can, therefore, modulate proliferation/differentiation or particular biologic functions.
Here, by use of biologic, biochemical, and gene manipulation methods in human PMNs, we have uncovered yet another function of the MAPK pathway, the ability to control direct microbicidal capacity against C albicans. Our studies demonstrated that C albicansinduced rapid MAPK activation in PMNs. Inhibition of MAPK activation, either by the pharmacologic agent PD098059 or by overexpression of dominant-negative MEK1, blocked overall PMN function against C albicans, as monitored by fungal growth inhibition. This antimicrobial activity requires both phagocytosis and granule mobilization toward the ingested microbe, and blockade of MAPK by PD098059 or dnMEK1 sharply reduced not only PMN phagocytic capacity but also the directed targeting of lytic granules.
In contrast to the role of MAPK/ERK that we have described here, distinct mechanisms of activation have implicated different signaling pathways to be involved in phagocytosis and degranulation. PD098509 has been reported to affect phagocytosis mediated by fMLP but not degranulation.18,23 24 We believe that the difference in our results and those published using fMLP most likely lies in the mechanism of activation. fMLP induces receptor-mediated signaling through a heterotrimeric G protein that leads to the activation of Src family kinases and p38 MAPK. Of note, we used a unique system of PMN activation directly mediated by C albicans. The exact mechanism for direct microbial stimulation of PMNs has not been elucidated but may involve a unique receptor as well as a unique signaling pathway.
Until now, events downstream from Rac and Cdc42 have not been elucidated, probably due to the problems inherent in the previous experimental systems that used receptor-transfected fibroblasts or tumor cell lines, which may not possess the full complement of kinases normally present in neutrophils. Indeed, direct phagocytosis of microbes, not aided by FcR or complement receptors, is not well studied. In the present study, however, using human PMNs we have identified that Rac and Cdc42 critically control phagocytosis and granule movement toward ingested microbes and that this regulation is mediated through MAPK/ERK. Of the small GTPases, N17Rac or N17Cdc42, but not N19Rho, suppressed MAPK/ERK activation triggered by microbe exposure in PMNs and at the same time inactivated phagocytosis and granule movement. On the other hand, neither N17Ras nor the Ras inhibitor FTI277 could interfere with MAPK activation or microbicidal function in PMNs, thus pinpointing a specific Rac/Cdc42-dependent, Ras-independent MAPK pathway for PMN function. It is of significance that biologic function against microbes in PMNs is mediated via a Ras-independent MAPK pathway, similar to that controlling tumor lysis in natural killer cells.34 Whether Ras usage is a key strategy by which cells signal gene expression and cell growth, but not mediate cell function, needs to be further explored.
Rac is a required component for NADPH oxidase function in human neutrophils.57 In other cellular systems, Rac has been shown to be involved in cytoskeletal alterations and membrane ruffling.42,58 Of note, both Rac and Cdc42 are required for actin reorganization, which is a critical step in PMN phagocytosis of microorganisms.20,59 Indeed, for IgG-mediated phagocytosis, the ingestion of IgG-coated particles is able to activate both Rac and Cdc42.20 Using Swiss 3T3 cells, Cdc42-mediated actin assembly can be blocked by expression of N17Rac, suggesting that Rac is downstream of Cdc42.47 The impact of our work is the discovery that both Rac and Cdc42 not only control phagocytosis but also direct granule movement within PMNs toward the phagosome. Another important finding is that, although Rac has been primarily associated with MAPK-JNK activation in other cell systems,43 Rac preferentially utilizes MAPK/ERK to mediate PMN function. Support for this alternate Rac-MAPK pathway is becoming evident, as reported recently in fibroblasts and epithelial cells.44-46
Involvement of PAK1 in cytoskeletal rearrangement has been reported. Furthermore, PAK1 has been demonstrated to colocalize with actin to form phagocytic cups in activated human PMNs stimulated with fMLP.48 We and others have identified PAK1 as a possible intermediate between Rac and MAPK.44,48 58 Here, we show that PAK-1 is also activated by direct conjugation with C albicans. We have utilized a dominant-negative (kinase-deficient) mutant of PAK1 to further investigate the role of this enzyme in PMN antimicrobial activity. The inhibition of MAPK activation suggests that PAK1 activation is upstream of MAPK. However, the kinase-deficient PAK1 mutant contains an intact CRIB domain for interaction with Rac and Cdc42. Therefore, we cannot completely eliminate the possibility that the kinase-deficient PAK1 may still sequester Cdc42 or Rac1 nonspecifically. Further experiments will be needed to resolve the role of PAK1 in neutrophil-mediated antifungal activity.
Recently, several reports have demonstrated that PI-3K plays a key role in the early stages of neutrophil chemotaxis.25,60-62 Indeed, fMLP has been shown to activate a PI-3K–dependent Rac/Cdc42 pathway in neutrophils and differentiated HL-60 cells.63 We have recently shown that PI 3-kinase works upstream of Rac in NK cells to control lytic function.35 Activation of PI 3-kinase by an unknownC albicans–induced cell surface receptor(s) may lead to the activation of Rac and Cdc42 in our system. However, there are likely to be some differences in the signal cascades that control PMN microbicidal function and NK lytic function, and one distinction we have already observed is the lack of involvement of Cdc42 in NK cells. NK cells utilize a PI 3-kinase→Rac→PAK→MEK→ ERK pathway, whereas PMNs prefer a Cdc42→Rac→MEK→ERK pathway. Whether these distinct pathways are triggered via distinct receptors—that is, tumor ligands for NK cells and microbial ligands for PMNs—is an important subject for further investigation.
More importantly, in this study we try to address the question proposed by Duesbery et al.64 They have raised the question of whether MAPK inhibitors, used as a strategy to treat cancer, would potentially lead to undesirable effects on normal physiological processes. Data from our studies indicate that use of MAPK pharmacologic inhibitors for the treatment of cancer may result in the interruption of normal neutrophil function. Because MAPK is a regulator of diverse intracellular signaling pathways involved in normal physiologic processes, a balance between therapeutic outcome and undesirable side effects must be attained to achieve successful and safe anticancer therapy.
We thank the Analytical Microscopy Core, Flow Cytometric Core, and the Molecular Imaging Core facilities of the H. Lee Moffitt Cancer Center.
Prepublished online as Blood First Edition Paper, January 2, 2003; DOI 10.1182/ blood-2001-12-0180.
Supported by American Heart Association grant AHA0256422B and U.S. Public Health Service grant CA83146.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sheng Wei, Immunology Program, H. Lee Moffitt Cancer Center & Research Institute, 12902 Magnolia Dr, Tampa, FL 33612; e-mail: wei@moffitt.usf.edu.