By retrospectively analyzing 288 patients with de novo myelodysplastic syndrome (MDS), we sought to determine the prevalence and clinical characteristics of bone marrow eosinophilia and basophilia that were detected at presentation. Bone marrow eosinophilia and basophilia were defined as a differential count of each cell type exceeding 5.0% and 1.0%, respectively. Of 288 patients with MDS, 36 (12.5%) fulfilled this criterion for bone marrow eosinophilia (MDS-Eos); 34 patients (11.8%) showed basophilia (MDS-Bas), and 11 (3.8%) satisfied both criteria (MDS-EosBas). The remaining 229 patients had neither eosinophilia nor basophilia in their bone marrow (MDS−/−) at presentation. Cytogenetic analysis was carried out on unstimulated bone marrow cells obtained from 264 patients. When the cytogenetic categorization of the IPSS (International Prognostic Scoring System) for MDS was applied, significantly higher numbers of MDS-Eos and MDS-Bas patients had chromosomal abnormalities carrying intermediate or poor prognosis, compared with the MDS−/− patients. Specific chromosomal abnormalities and complex karyotypes were associated with MDS-Eos and/or MDS-Bas. In accordance with these results, the overall survival rate was significantly lower, and the evolution to acute myelogenous leukemia (AML) occurred more frequently in the MDS-Eos and MDS-Bas than in the MDS−/− patients. Multivariate analysis demonstrated that bone marrow basophilia was an independent risk factor for evolution to AML. Our study indicates that bone marrow eosinophilia and basophilia in patients with MDS predict a poorer prognosis.
Introduction
Myelodysplastic syndromes (MDSs) are heterogeneous hematologic malignancies. Most patients die of progression to acute myelogenous leukemia (AML) or bone marrow failure leading to fatal infectious and bleeding complications. The classification for MDS, initially proposed by the French-American-British (FAB) cooperative group in 1982, is now widely used.1 Subsequently, several MDS scoring systems have been described, which can predict a patient's survival rate and the risk of evolution to AML.2-8 The International Prognostic Scoring System (IPSS) is one of these, and it classifies MDS patients into 4 subgroups (low, intermediate-1, intemediate-2, and high), depending on the grade of cytopenia, the percentage of blast cells in the bone marrow, and the type of chromosomal abnormalities.8 Given the heterogeneous nature of MDS, such a classification is important for helping to select the best therapeutic strategies for each patient.
We have previously proposed that bone marrow eosinophilia in patients with MDS may be a poor prognostic factor that is strongly associated with major karyotypic abnormalities.9 Bone marrow basophilia has also been reported in some MDS cases, although its clinical significance is currently unclear. Bone marrow eosinophilia and basophilia are not infrequently observed in myeloproliferative disorders. Although many patients with MDS display myeloproliferative features in their bone marrow, the prevalence and clinical characteristics of MDS patients with bone marrow eosinophilia and/or basophilia have not been extensively studied. In this report, we retrospectively analyzed 288 patients with de novo MDS to determine the clinical significance of bone marrow eosinophilia and basophilia, especially focusing on disease prognosis.
Patients and methods
Patients
We retrospectively investigated the clinical data at presentation obtained from 288 patients with de novo MDS (197 men and 91 women). The median age was 69 years (range, 15-93 years). They were diagnosed as having MDS between January 1982 and June 2001. Patients who had a previous history of exposure to mutagens or carcinogens (ie, suspected of secondary MDS) were excluded from this study. According to the FAB criteria, these patients were classified as refractory anemia (RA; 92 patients), RA with ringed sideroblasts (RARS; 15 patients), RA with excess of blasts (RAEB; 94 patients), RAEB in transformation (RAEBt; 62 patients), and chronic myelomonocytic leukemia (CMML; 25 patients). These patients were followed for 0.01 to 16.2 years (median, 1.2 years). Evolution to AML was diagnosed when the bone marrow blast percentage exceeded 30%. Following the initial diagnosis, 26 patients (RA, 1; RAEB, 9; RAEBt, 14; and CMML, 2) received combination chemotherapy or underwent allogeneic bone marrow transplantation (alloBMT), and 24 patients (RAEB, 10; RAEBt, 8; and CMML, 6) received low-dose cytarabine or hydroxyurea. The remaining patients (RA, 91; RARS, 15; RAEB, 75; RAEBt, 40; and CMML, 17) were given supportive care and then received cytotoxic agents or alloBMT when the disease progressed, such as when evolution to AML was observed.
Morphology
The differential counts of the circulating leukocytes and bone marrow cells were evaluated at each institution on 200 leukocytes and 500 nucleated cells, respectively. As for the basophil and eosinophil counts in the peripheral blood and the bone marrow, 3 hematologists (T.M., H.H., and A.Y.) independently re-evaluated 288 samples. The reviewers were blinded to the clinical data and the other reviewers' determinations. Among the re-evaluated samples, highly significant (P < .0001, r > 0.8) concordances among reviewers was found by correlation analysis.
Cytogenetics
Cytogenetic analysis was carried out on unstimulated bone marrow cells obtained from 264 patients at presentation. For each patient, 10 to 22 metaphases were examined. According to the IPSS for MDS,8 the karyotypes of the patients were categorized into good (normal, del(5q) alone, del(20q) alone, or −Y alone), poor (≥ 3 abnormalities [designated as a complex karyotype] or chromosome 7 abnormalities), and intermediate (abnormalities other than those mentioned earlier) risk groups.
Statistics
All analyses were performed with Statview 5.0 software (SAS Institute, Cary, NC). Comparisons between qualitative variables were carried out using chi-square tests. With the quantitative variables, differences in the distribution among the cytogenetic subgroups were analyzed by the Bonferroni/Dunn test using one-way analysis of variance (ANOVA). The survival rates and evolution to AML were estimated by the Kaplan-Meier product limit method and compared with the log-rank test. The Cox proportional hazards model was used for estimates of univariate and multivariate prognostic significance. The survival time was defined as the time interval from the hematologic diagnosis to death, or to the latest contact with the patient. All tests were 2-tailed, and P < .05 was considered statistically significant.
Results
Bone marrow eosinophilia and basophilia
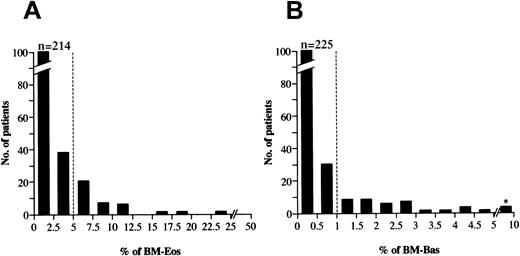
The percentages of eosinophils and basophils in the bone marrow from the 288 patients were distributed as shown in Figure1. The median percentages for eosinophils and basophils were 1.4% (range, 0%-24.3%) and 0.2% (range, 0%-8.9%), respectively. The bone marrow eosinophil and basophil percentages in healthy subjects were previously described as less than 5% and 1%, respectively.10 We, therefore, defined bone marrow eosinophilia and basophilia as more than 5.0% and 1.0%, respectively. By this criterion, 36 of 288 patients (12.5%) were found to have bone marrow eosinophilia (MDS-Eos) at presentation. Thirty-four patients (11.8%) had bone marrow basophilia (MDS-Bas). Among these, 11 had both eosinophilia and basophilia. The remaining 229 patients had neither of these conditions (MDS−/−). Table1 shows the clinical profiles of the patients in the MDS-Eos, MDS-Bas, and MDS−/− groups. There were no significant differences in mean age, polymorphonuclear leukocyte counts, or platelet counts among the 3 groups. However, the distribution of the FAB classification and male-female ratio was significantly different among the 3 groups (P < .05, chi-square test). The difference in the hemoglobin concentration between MDS-Eos and MDS−/− was also significant. The circulating eosinophil counts were significantly higher in MDS-Eos and MDS-Bas patients compared with those in MDS−/− patients. The prevalence of patients with circulating eosinophil counts exceeding 0.5 × 109/L was 18%, 9%, and 2% in the MDS-Eos, MDS-Bas, and MDS−/− groups, respectively. The circulating basophil counts were significantly higher in MDS-Bas patients compared with MDS-Eos and MDS−/− patients. Absolute circulating basophil counts exceeding 0.1 × 109/L were observed in 24%, 42%, and 7% in MDS-Eos, MDS-Bas, and MDS−/−patients, respectively.
Patient distribution.
Distribution of the percentages of bone marrow eosinophils (A) and basophils (B) of 288 patients with de novo MDS in this study. We defined bone marrow eosinophilia and basophilia as more than 5% and 1%, respectively (dotted lines). *Includes 3 patients having bone marrow basophilia with 7.8%, 7.9%, and 8.9%, respectively.
Patient distribution.
Distribution of the percentages of bone marrow eosinophils (A) and basophils (B) of 288 patients with de novo MDS in this study. We defined bone marrow eosinophilia and basophilia as more than 5% and 1%, respectively (dotted lines). *Includes 3 patients having bone marrow basophilia with 7.8%, 7.9%, and 8.9%, respectively.
Cytogenetics
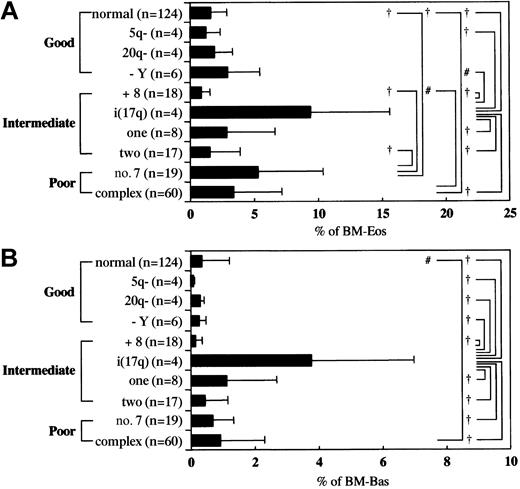
Cytogenetic analysis was performed in 264 patients. Chromosomal abnormalities were observed in 140 patients (53%). Depending on the type of chromosomal abnormalities in IPSS, the patients were categorized into good, intermediate, and poor risk groups. As shown in Table 2, the majority of patients with MDS-Eos and MDS-Bas (79.5% and 81%, respectively) were classified into the intermediate or poor risk groups. In contrast, 60% of the MDS−/− patients were in the good risk group (P < .0001, chi-square test). Thus, an increase in bone marrow eosinophils and basophils was highly associated with cytogenetic abnormalities predicting a poorer prognosis. A significant association of bone marrow basophilia and eosinophilia with high-risk IPSS was also noted (Table 2).
We next examined whether specific chromosomal abnormalities were associated with bone marrow eosinophilia and basophilia. In previous reports, bone marrow eosinophilia or basophilia in MDS was observed in patients having complex karyotypes,9,11 a chromosome 7 abnormality,12 i(17q),13t(6;9),14-16 inv16,17-19 or t(5;12).20 In our study, t(6;9) was observed in only 2 patients and neither inv16 nor t(5;12) was detected. In the 10 cytogenetic patterns shown in Figure 2, abnormalities in chromosome 7, complex karyotypes, and i(17q) were significantly associated with an increase in bone marrow eosinophils by one-way ANOVA. Likewise, the complex karyotype and i(17q) were found to be associated with an increase in bone marrow basophils. Thus, specific chromosomal abnormalities were associated with bone marrow eosinophilia and basophilia. However, neither circulating eosinophil counts nor basophil counts was significantly associated with specific chromosomal abnormalities (data not shown).
Association between cytogenetic abnormalities and bone marrow eosinophilia or basophilia.
The mean value of the percentages of bone marrow eosinophils (A) and basophils (B). Each patient was categorized into 1 of 10 cytogenetic groups. Each value indicates the mean ± SD. # and † indicate that the difference is significant between indicated groups with P < .05 and P < .01, respectively.
Association between cytogenetic abnormalities and bone marrow eosinophilia or basophilia.
The mean value of the percentages of bone marrow eosinophils (A) and basophils (B). Each patient was categorized into 1 of 10 cytogenetic groups. Each value indicates the mean ± SD. # and † indicate that the difference is significant between indicated groups with P < .05 and P < .01, respectively.
Prognosis of patients with MDS-Eos and MDS-Bas
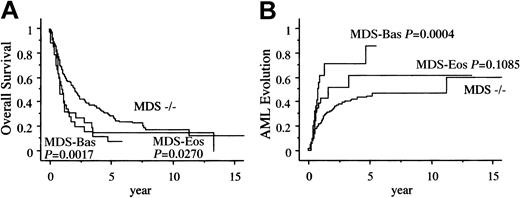
A total of 193 (67%) patients died during the study period. Among the 36 patients with MDS-Eos, 13 patients died after evolution to AML and 12 patients died of MDS-related bone marrow failure. Of the 34 patients with MDS-Bas, 14 patients died after evolution to AML and 11 patients died of bone marrow failure (Table3). Figure3A shows the unadjusted survival curves of those with bone marrow eosinophilia and basophilia. The overall survival (OS) in the MDS-Eos and MDS-Bas patients was significantly shorter than that of the MDS−/− patients (median, 1.1, 0.9, and 2.1 years, respectively). Figure 3B shows the Kaplan-Meier curve for development of AML among the 3 groups. The intervals for 25% evolution to AML in the MDS-Eos and MDS-Bas patients were significantly shorter than that in the MDS−/− patients (0.6, 0.5, and 1.1 years, respectively).
Kaplan-Meier estimates of AML evolution.
Panel A shows the overall survival and panel B shows the probability of AML evolution.
Kaplan-Meier estimates of AML evolution.
Panel A shows the overall survival and panel B shows the probability of AML evolution.
Univariate analysis of prognostic predictors
The univariate analysis of the prognostic factors is shown in Table 4. In this analysis, we treated hematologic indices as continuous variables. The clinical characteristics associated with a significantly longer survival were younger age (≤ 60 years); female sex; a high hemoglobin (Hb) concentration; low percentages of blasts, eosinophils, and basophils in the bone marrow; and low peripheral blood basophil counts. The cytogenetic, FAB, and IPSS classifications were also significant indicators of MDS prognosis. The Hb concentration, bone marrow blast and basophil percentages, cytogenetics, IPSS, and FAB classifications were good predictors for evolution to AML.
Multivariate analysis of prognostic predictors
Multivariate analysis confirmed the significant effects on survival of age, sex, and IPSS. The bone marrow basophil percentage and IPSS were independent predictors for AML evolution (Table5).
Discussion
Anecdotal reports have described a relatively infrequent association of MDS with bone marrow eosinophilia and/or basophilia.11,13 21-27 In each case, researchers have attempted to delineate the specific clinical features of these cases. However, the lack of any large-scale study has made it difficult to actually determine the prevalence and clinical significance of bone marrow eosinophilia and basophilia in patients with MDS. In this report, we retrospectively analyzed 288 patients with de novo MDS. We found that a substantial portion of the patients with MDS exhibited MDS-Eos and MDS-Bas at presentation. Regarding their clinical outcomes, the MDS-Eos and MDS-Bas patients had a poorer survival rate and were at a higher risk of AML evolution than the MDS−/− patients. Our study also confirmed prior reports that indicated the importance of a variety of clinical parameters, including age, sex, and IPSS. Multivariate analysis suggests that bone marrow basophilia is an independent predictive factor for evolution to AML.
Because MDS is a neoplastic disorder of pluripotent stem cells, a question arises whether or not the abnormally increased eosinophils and basophils are part of a neoplastic clone. In some cases, clonal cytogenetic abnormalities were demonstrable directly in the eosinophils and basophils, suggesting that they were neoplastic.28,29However, this is not always the case.30 31 Eosinophil and basophil differentiation is promoted by many cytokines, which may be secreted by neoplastic myeloid cells (reactive eosinophilia and basophilia). Cytologic features are generally unreliable in distinguishing between reactive and neoplastic conditions. Thus, cytogenetic and/or molecular genetic analysis would be required for unequivocal evidence of neoplastic transformation.
A number of cytogenetic abnormalities have been described in MDS patients with bone marrow eosinophilia. These abnormalities included t(5;12)(q33;p13),20inv16(p13q22),17-19 and other abnormalities in chromosomes 7 or 16.9,23,32,33 The t(5;12)(q33;p13) abnormality is less common but appears to be relevant for the pathogenesis of the eosinophilia. It has been postulated that a translocation involving chromosome 5 might generate the rearrangement of cytokine genes that regulate eosinophil differentiation. The inv16(p13q22) abnormality is another example, in which the relationship with eosinophilia has been well established in the case of M4Eo. Monosomy 7 has been reported in cases of acute leukemia in association with more than 5% bone marrow eosinophils.34,35 An increased basophil count was also observed in MDS patients with specific cytogenetic abnormalities, such as i(17q) and t(6;9)(q23;q34.3).11,24-26 Four case reports have described MDS with prominent basophilia and complex karyotypes. Two of these patients developed AML within a relatively short time.13 28
In our study of a Japanese population with MDS, neither t(5;12)(q33;p13) nor inv16(p13q22) was observed. In contrast, an abnormal chromosome 7, complex karyotypes, and i(17q) were associated with an increase in bone marrow eosinophils. Meanwhile, i(17q) and complex karyotypes were significantly associated with bone marrow basophilia. Because we have not performed cytogenetic analysis directly on the eosinophils and basophils, the pathogenic role of these chromosomal abnormalities remains uncertain.
In the literature, 40% to 50% of patients with MDS died because of evolution to AML. Other causes of death include infection, bleeding, and therapy-related complications. In our study, 40% of the patients eventually developed acute leukemia, which was the main cause of death. Relatively young patients with MDS, categorized as a high-risk group, can be treated with allogeneic stem cell transplantation, and 20% to 40% of these patients may be cured. Therefore, the evaluation of prognostic factors for MDS is important for deciding the best therapeutic strategy. Kaplan-Meier analysis revealed that MDS-Eos and MDS-Bas both indicate a poorer survival and a high risk of development of AML. Although the effect of bone marrow eosinophilia is not significant on multivariate analysis, this finding might be due to the significant correlation of MDS-Eos with a chromosome 7 abnormality, an important factor indicating a poorer prognosis within the IPSS. However, bone marrow basophilia is associated with specific cytogenetic abnormalities and a complex karyotype, a shorter survival and frequent evolution to AML. The effect of bone marrow basophilia on evolution to AML is independent of IPSS. Taken together, our study indicates that bone marrow eosinophilia and basophilia predict a poorer prognosis in patients with MDS.
Prepublished online as Blood First Edition Paper, December 27, 2002; DOI 10.1182/blood-2002-03-0947.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Takafumi Matsushima, Third Department of Internal Medicine, Gunma University School of Medicine, Showa-machi 3-39-15, Maebashi, Gunma 371-8511, Japan; e-mail:tmatsu@showa.gunma-u.ac.jp.