Interactions between the protein kinase C (PKC) activator/down-regulator bryostatin 1 and paclitaxel have been examined in human myeloid leukemia cells (U937) and in highly paclitaxel-resistant cells ectopically expressing a Bcl-2 phosphorylation loop–deleted protein (ΔBcl-2). Treatment (24 hours) of wild-type cells with paclitaxel (eg, 5 to 20 nM) in combination with 10 nM bryostatin 1 induced a marked increase in mitochondrial damage (eg, cytochrome c and Smac/DIABLO [second mitochondria-derived activator of caspases/direct IAP binding protein with low pI] release), caspase activation, Bid cleavage, and apoptosis; moreover, bryostatin 1 circumvented the block to paclitaxel-induced mitochondrial injury and apoptosis conferred by ectopic expression of the loop-deleted protein. Coadministration of tumor necrosis factor (TNF) soluble receptors, or ectopic expression of CrmA or dominant-negative caspase-8, abrogated potentiation of paclitaxel-induced mitochondrial injury and apoptosis by bryostatin 1, implicating the extrinsic apoptotic pathway in this process. Similar events occurred in HL-60 leukemia cells. Potentiation of paclitaxel-induced apoptosis in wild-type and mutant cells by bryostatin 1 was associated with increases in TNF-α mRNA and protein and was mimicked by exogenous TNF-α. Coadministration of the selective PKC inhibitor GFX (1 μM) blocked the increase in TNF-α mRNA levels and apoptosis in bryostatin 1/paclitaxel–treated cells. Lastly, synchronization of cells in G2M increased their sensitivity to TNF-α–associated lethality. Collectively, these findings indicate that in U937 cells, bryostatin 1 promotes paclitaxel-mediated mitochondrial injury and apoptosis, and circumvents resistance to cell death conferred by loss of the Bcl-2 phosphorylation domain, through the PKC-dependent induction of TNF-α. They further suggest that this process is amplified by paclitaxel-mediated arrest of cells in G2M, where they are more susceptible to TNF-α–induced lethality.
Introduction
Apoptosis represents a genetically regulated cell death program characterized by the engagement of a family of cysteine proteases termed caspases.1 Currently, two major apoptotic pathways have been identified: the intrinsic, or mitochondrial pathway, and the extrinsic, or receptor-related pathway.2 The former, which is typically initiated by cytotoxic drugs, involves perturbations in mitochondrial homeostasis, resulting in cytochromec release from the inner mitochondrial space. Once in the cytoplasm, cytochrome c binds to a cytoskeletal protein referred to as apoptotic protease-activating factor-1 (Apaf-1), which binds to and activates caspase-9. The resulting “apoptosome” complex activates the caspase cascade that induces cell death.3 Release of cytochrome c is regulated by complex interactions between proapoptotic (eg, Bid, Bax, Bak) and antiapoptotic (eg, Bcl-2, Bcl-xL) members of the Bcl-2 family.4 In contrast, the extrinsic apoptotic pathway is initiated by binding of ligands such as tumor necrosis factor (TNF) to members of the tumor necrosis factor receptor (TNFR) family, leading to recruitment of death domain–containing adaptor proteins such as the Fas-associated death domain (FADD). Death-effector domains (DEDs) on FADD interact with procaspase-8 prodomains, resulting in cleavage and activation by self-processing.5 Once activated, caspase-8 either directly cleaves apoptotic substrates or activates effector caspases (eg, procaspases-3, -6, and -7). Caspase-8 also cleaves and activates the proapoptotic protein Bid, a BH3-only domain member of the Bcl-2 family.6 Truncated Bid (tBid) translocates to the mitochondria, resulting in release of cytochrome c into cytosol. In this way, activation of the extrinsic pathway can serve to amplify apoptosis initiated by stimuli (eg, cytoxic drugs) primarily associated with mitochondrial injury.7 Significantly, apoptosis stemming from activation of the extrinsic pathway is relatively insensitive to blockade by Bcl-2 and related proteins.8
Paclitaxel (Taxol), a member of the taxane family, is a natural product that exhibits activity against a wide range of human hematopoietic and nonhematopoietic tumor cell types.9,10 Paclitaxel binds to microtubules, thereby promoting polymerization of tubulin heterodimers and inhibiting tubulin depolymerization, leading to α/β microtubular stabilization and the formation of nonfunctional microtubular bundles.11 The resulting disruption of the mitotic spindle assembly prevents chromosomes from segregating, leading to arrest of cells in the G2M phase of the cell cycle. However, the mechanism by which disruption of the spindle assembly process triggers the cell death program is uncertain and has been attributed to perturbations in signal transduction pathways and/or dysregulation of CDK1 (p34cdc2).12 In addition, there is accumulating evidence that paclitaxel-mediated lethality may be related to the Raf-dependent phosphorylation of Bcl-2 and resulting interference with the capacity of this antiapoptotic protein to prevent mitochondrial injury.13 The enhanced ability of a Bcl-2 mutant protein lacking the N-terminal phosphorylation loop domain (Bcl-2Δ32-80) to protect cells from paclitaxel-induced mitochondrial injury and apoptosis supports a functional role for Bcl-2 phosphorylation in the lethal response of cells to such agents.14 15
Bryostatin 1 is a macrocyclic lactone obtained from the marine bryozoanBugula neritina, which has shown significant preclinical antitumor activity16,17 and is currently undergoing phase 1 and 2 clinical evaluation in humans.18 On acute exposure, bryostatin 1 activates protein kinase C (PKC), whereas on chronic exposure, it induces down-regulation of PKC activity.19,20 In leukemic cells, bryostatin 1 has also been shown to lower the threshold for apoptosis induced by cytotoxic drugs (eg, ara-C).21-23 In view of evidence that PKC activation opposes apoptosis24 and that pharmacologic PKC inhibitors are potent inducers of cell death,25 it is tempting to relate the proapoptotic actions of bryostatin 1 to interruption of PKC cytoprotective actions.26-28
In previous studies, we29 and others30 have reported that bryostatin 1 potentiates the lethal effects of paclitaxel in a dose-dependent manner. Moreover, bryostatin 1 circumvents, at least in part, the capacity of ectopic expression of Bcl-xLto block paclitaxel-mediated apoptosis.31 However, the mechanism by which bryostatin 1 lowers the threshold for paclitaxel-mediated lethality remains unclear. The goal of the present studies was to gain insights into the factors responsible for this phenomenon, with an emphasis on characterizing the relative contributions of the intrinsic versus extrinsic pathways to bryostatin 1/paclitaxel lethality. In addition, parallel studies have been conducted in cells ectopically expressing a phosphorylation loop–deleted mutant to determine what role, if any, Bcl-2 phosphorylation might play in potentiation of lethality. The present results suggest that bryostatin 1–mediated induction of TNF-α, rather than down-regulation of PKC or modulation of Bcl-2 phosphorylation status, is primarily responsible for lowering the threshold for paclitaxel-related cell death and for circumventing the cytoprotective effects of Bcl-2.
Materials and methods
Cell lines
The human monoblastic leukemic U937, HL-60, and THP-1 cell lines were obtained from the American Type Culture Collection (Manassas, VA). Cells were maintained as previously described.32 For the generation of stable transfectants, the cDNAs encoding Bcl-2, loop deletant Bcl-2 (ΔBcl-2; Δ32-80), caspase-8 dominant negative (casp8-DN), or CrmA (obtained from Dr Kapil Bhalla, H. Lee Moffitt Cancer Center, Tampa, FL) were cloned into expression vectors (pSFFV or pcDNA3.1) containing a G418 resistance marker. U937 cells were transfected by electroporation as described previously.31These cells, designated U937/Bcl-2, U937/ΔBcl-2, U937/CrmA, and U937/casp8-DN, were maintained along with their empty-vector counterparts (U937/neo) in the presence of G418 (Geneticin; 400 μg/mL).
Drugs and chemicals
Bryostatin 1 was provided by Dr Anthony Murgo, Cancer Treatment and Evaluation Program, National Institutes of Health (Bethesda, MD); paclitaxel was from Sigma Chemical (St Louis, MO); 3,3-dihexyloxacarbocyanine (DiOC6) was purchased from Molecular Probes (Eugene, OR); recombinant human TNF-α (rhTNF-α) was from Calbiochem (San Diego, CA); recombinant human soluble TNF sRI/Fc chimera (sTNFR) was from R&D Systems (Minneapolis, MN); bisindoylmaleimide (GFX; GF 109203X) was purchased from Sigma Chemical.
Experimental protocols
Cells in logarithmic-phase growth were suspended at 2 × 105/mL and exposed to test agents for various intervals as indicated in a 37°C, 5% CO2 incubator. In the experimental paradigm, paclitaxel was added 15 to 30 minutes before the addition of bryostatin 1. In experiments involving kinase inhibitors and TNF soluble receptors, cells were pretreated with each agent 30 minutes before the addition of paclitaxel with or without bryostatin 1.
Assessment of apoptosis
Cell morphology and apoptosis was monitored by examining cytocentrifuge preparations stained with the Diff-Quik stain set (Dade Behring, Deerfield, IL) as described previously23 or by annexin V and propidium iodide (PI) positivity. Briefly, following drug treatments, cells were costained with annexin V conjugated to fluorescein isothiocyanate (FITC) and PI per instruction provided by the manufacturer (BD PharMingen, San Diego, CA). The percentage of apoptotic (annexin V–positive and PI-positive) cells was determined by flow cytometric analysis.
Cytochrome c and Smac/DIABLO release assay
After treatment, cells were harvested by centrifugation at 600g for 10 minutes at 4°C. Cytosolic fractions were obtained by selective plasma membrane permeabilization with digitonin. Briefly, 2 × 106 cells were lysed 1 to 2 minutes in lysis buffer (75 mM NaCl, 8 mM Na2HPO4, 1 mM NaH2PO4, 1 mM EDTA [ethylenediaminetetraacetic acid], and 350 μg/mL digitonin). The lysates were centrifuged at 12 000g for 1 minute, and the supernatant was collected and added to an equal volume of 2 × sample buffer. The protein samples were quantified, separated by 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and subjected to immunoblot analysis as described in the next paragraph.
Western blot analysis
Western blot analysis was performed essentially as described previously.23 In brief, for each sample, 25 μg of protein per lane was separated by 4% to 20% SDS-PAGE (Invitrogen, Carlsbad, CA) and electroblotted to nitrocellulose (Schleicher & Schuell, Keene, NH). Subsequently, after incubation in phosphate-buffered saline (PBS)–Tween 20 (0.05%) supplemented with 5% nonfat dry milk for 1 hour at 22°C, the blots were incubated for 2 hours at 22°C in fresh blocking solution with an appropriate dilution of primary antibodies as follows: cytochrome c, caspase-3, and caspase-8, 1:1000 (BD PharMingen); and Smac/DIABLO (second mitochondria-derived activator of caspases/direct IAP binding protein with low pI), 1:500 (BIOMOL Research Laboratories, Plymouth Meeting, PA); blots were washed 3 times for 5 minutes in PBS–Tween 20 and then incubated with a 1:2000 dilution of horseradish peroxidase–conjugated secondary antibody for 1 hour at 22°C. Blots were washed 3 times for 5 minutes in PBS–Tween 20 and then developed by enhanced chemiluminescence (Amersham Biosciences, Braunschweig, Germany).
Caspase activity assay
Caspase activity was measured per instructions provided by the manufacturer using a colorimetric assay kit (Biovision, Palo Alto, CA). Caspase activity in cytosolic extracts from appropriate treatments was measured by spectrophotometric detection of the chromophoreP-nitroanilide (pNA) following its cleavage from the labeled substrate DEVD-pNA for caspase-3 and IETD-pNA for caspase-8.
Enzyme-linked immunosorbent assay (ELISA)
U937 cells (4 × 106) were exposed to drug treatment at various time intervals. Cell culture supernatants were collected by centrifugation. TNF-α protein levels in the supernatants were monitored by the ELISA OptEIA kit (BD PharMingen) and normalized against untreated controls.
Quantitative reverse transcription–polymerase chain reaction (RT-PCR)
U937 cells (4 × 106) were exposed to paclitaxel with or without bryostatin 1 for various time points, and total RNA was isolated using the Qiagen RNeasy Mini Kit; 10 ng RNA was used to conduct Taqman One-step RT-PCR (Applied Biosystems, Foster City, CA). Each tube contained both the TNF-α gene probe/primer and the human actin control probe/primer. Briefly, oligonucleotide probes were labeled at the 5′ end with FAM 6-carboxyfluorescein and at the 3′ end with the quencher dye N,N′,N′-tetramethyl-6 carboxyrhodamine. The TNF-α primers were (F) 5′-CCCCAGGACCTCTCTCAATC-3′; (R) 5′-CATGGGCTACAGGCTTGTCA-3′, and the probe sequence was 5′-CCCAGGCAGTCAGATCATCTTCTCGAA-3′. The results for the experimental gene were normalized to actin levels as specified by the manufacturer.
PKC activity
A minor modification of a previously described method33 was employed to assay membrane, cytosolic, and total PKC activity in U937 cells exposed to bryostatin 1 with or without paclitaxel. Briefly, cell pellets were homogenized in 20 mM Tris (tris(hydroxymethyl)aminomethane), 0.5 mM EDTA, 0.5 mM EGTA (ethylene glycol tetraacetic acid), pH 7.5, containing 25 μg/mL protease inhibitors. The homogenate was incubated on ice for 30 minutes and centrifuged for 30 minutes at 100 000g to yield a cytosolic supernatant and a membrane pellet, which was homogenized in the same buffer containing 0.5% Triton X-100. After incubation on ice for 30 minutes followed by centrifugation for 5 minutes at 12 000g, the supernatant, representing the membrane fraction, was isolated. PKC activity in each fraction was monitored using a commercially available kit (Signa TECT PKC assay system; Promega, Madison, WI). For both fractions, normalized quantities of protein were added to coactivation buffer (2.5 × 10−5 Ci/mL [9.3 × 10−3MBq/mL] [γ-32P]ATP, 2 × 10−5 M adenosine triphosphate [ATP], and 5 × 10−5 M biotinylated peptide substrate) in the presence or absence of activation buffer (0.32 mg/mL phosphatidylserine, 0.032 mg/mL diacylglycerol, 20 mM Tris-HCl, pH 7.5, 10 mM MgCl2). After a 5-minute incubation at 30°C, reactions were terminated by the addition of guanidine hydrochoride (2.5 M) and spotted on SAM2 membrane (Promega). The membranes were washed thoroughly, and radioactivity was measured by liquid-scintillation counting. Purified PKC was used as a positive control, and myristoylated PKC peptide inhibitor was used to indicate specificity. The enzyme activity of PKC was determined by subtracting the activity of the enzyme in the absence of phospholipids (control buffer) from that of the enzyme in the presence of phospholipids (activation buffer). Kinase activity was calculated as picomoles of [γ-32P]ATP incorporated per minute per microgram of protein. Values are expressed as the percentage of PKC activity relative to those of untreated cell extracts (100%).
Cell synchronization
To assess further the effects of bryostatin 1–mediated signaling pathway on cell cycle traverse, aphidicolin block was employed to synchronize cells in the S phase. In brief, U937 cells were exposed to 100 nM aphidicolin for 24 hours and washed 3 times in drug-free medium. At various times after washout of aphidicolin, cells were treated with rhTNF (0.025 ng/mL). Cell death was determined by annexin V and PI positivity as described in “Assessment of apoptosis.”
MPM-2/propidium iodide bivariate flow cytometry
Following treatment of U937 cells with paclitaxel with or without bryostatin 1 for 18 hours, cells were harvested and fixed with 1% paraformaldehyde and 70% ethanol. After washing with PBS containing 0.05% Tween 20 and 1% fetal bovine serum, cells were labeled with MPM-2 antibody (final concentration of 6 μg MPM-2 antibody per milliliter; Upstate Biotechnology, Lake Placid, NY) for 1 hour at 4°C. Cells were washed twice with PBS and incubated with goat antimouse-FITC (Boehringer Mannheim, Mannheim, Germany) for 1 hour at room temperature in the dark. Cells were washed twice with PBS and resuspended in 5 μg/mL PI containing 50 μg/mL RNase A. Samples were analyzed on a Becton Dickinson FACScan, and data were analyzed using CellQuest software.
Statistical analysis
The significance of differences between experimental groups was determined using the Student t test for unpaired observations.
Results
Bryostatin 1 potentiates paclitaxel-induced apoptosis in human leukemia cells
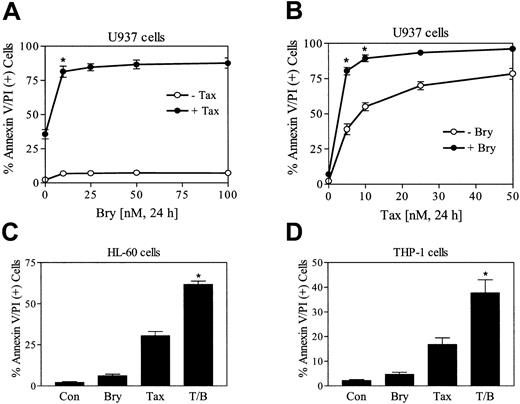
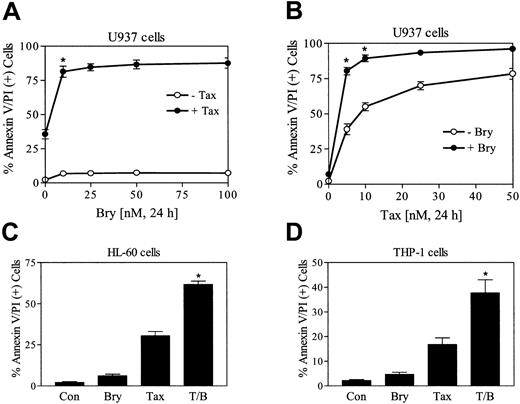
To define the effects of bryostatin 1 on paclitaxel-induced apoptosis, U937 cells were exposed to 5 nM paclitaxel in combination with increasing concentration of bryostatin 1 (10 to 100 nM). As shown in Figure 1A, bryostatin 1 treatment alone exerted minimal toxicity (6% to 10% apoptosis) but dramatically enhanced paclitaxel-induced apoptosis. An increase in paclitaxel-induced apoptosis by a minimally toxic concentration of bryostatin 1 (10 nM) was also observed at higher paclitaxel concentrations (eg, 10 to 20 nM) although potentiatiation was most marked at paclitaxel concentrations of about 5 nM (Figure 1B). Bryostatin 1 also increased paclitaxel lethality in HL-60 and THP-1 leukemia cells (Figure 1C-D) as well as in primary blast cells obtained from the peripheral blood of a patient with acute myeloid leukemia (AML) (data not shown). In each of the cell lines examined, the effects of combined treatment with bryostatin 1 and paclitaxel was significantly greater than the sum of the effects of each agent administered alone (P < .05 in each case).
Bryostatin 1 potentiates paclitaxel-induced apoptosis in leukemic cell lines.
(A) U937 cells were exposed to 5 nM paclitaxel (Tax) in combination with increasing concentrations of bryostatin 1 (Bry; 10 to 100 nM) for 24 hours, after which the percentage of apoptotic cells was determined by flow cytometric analysis of annexin V/PI–stained cells as described in “Materials and methods.” (B) U937 cells were treated with 10 nM bryostatin 1 combined with the indicated concentrations of paclitaxel for 24 hours, after which apoptosis was monitored as described in “Materials and methods.” (C) HL-60 cells were treated with 10 nM paclitaxel with or without 10 nM bryostatin 1 for 24 hours, after which apoptosis was determined as described in “Materials and methods.” (D) THP-1 cells were treated with 10 nM paclitaxel with or without 10 nM bryostatin 1 for 24 hours. Values for panels A-D represent the means for 3 separate experiments (± SE). *Significant difference compared with the sum of the effects of each agent administered alone (P < .05 in each case).
Bryostatin 1 potentiates paclitaxel-induced apoptosis in leukemic cell lines.
(A) U937 cells were exposed to 5 nM paclitaxel (Tax) in combination with increasing concentrations of bryostatin 1 (Bry; 10 to 100 nM) for 24 hours, after which the percentage of apoptotic cells was determined by flow cytometric analysis of annexin V/PI–stained cells as described in “Materials and methods.” (B) U937 cells were treated with 10 nM bryostatin 1 combined with the indicated concentrations of paclitaxel for 24 hours, after which apoptosis was monitored as described in “Materials and methods.” (C) HL-60 cells were treated with 10 nM paclitaxel with or without 10 nM bryostatin 1 for 24 hours, after which apoptosis was determined as described in “Materials and methods.” (D) THP-1 cells were treated with 10 nM paclitaxel with or without 10 nM bryostatin 1 for 24 hours. Values for panels A-D represent the means for 3 separate experiments (± SE). *Significant difference compared with the sum of the effects of each agent administered alone (P < .05 in each case).
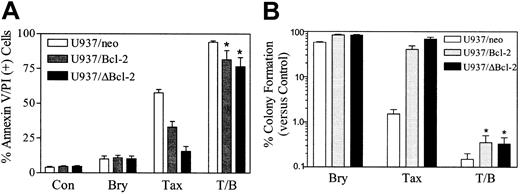
Addition of bryostatin 1 circumvents leukemic cell resistance to paclitaxel conferred by ectopic expression of full-length as well as an N-terminal phosphorylation loop–deleted Bcl-2 protein
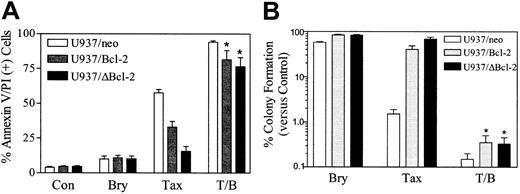
To investigate the role of Bcl-2 and its phosphorylation in the response of leukemic cells to the bryostatin 1/paclitaxel regimen, U937/neo, U937/Bcl-2, and U937/ΔBcl-2 cells were exposed to paclitaxel (10 nM) with or without bryostatin 1 (10 nM) for 24 hours. As we have previously reported, ectopic expression of full-length Bcl-2 significantly protected cells from paclitaxel lethality, and deletion of loop region, which contains the major phosphorylation sites,14 conferred a very high degree of protection (Figure 2A). In marked contrast, ectopic expression of either full-length Bcl-2 or the loop-deleted Bcl-2 failed to protect cells from apoptosis induced by the combination of bryostatin 1 and paclitaxel. In parallel studies, bryostatin 1 also significantly reduced the clonogenic survival of paclitaxel-treated U937/Bcl-2 and U937/ΔBcl-2 cells (eg, from 5.82% and 7.66% to 0.42% and 0.41% respectively; Figure 2B). Similar results were obtained when cells were exposed to 5 nM paclitaxel (data not shown). These findings indicate that administration of bryostatin 1 can circumvent blockade of paclitaxel-mediated apoptosis conferred by the full-length and loop-deleted Bcl-2 protein and argue against a role for modulation of Bcl-2 phosphorylation in this process. They also indicate that the ability of these agents to inhibit the clonogenic growth of leukemia cells may far exceed their capacity to induce apoptosis, at least at early exposure intervals, and raise the possibility that other mechanisms of reproductive cell death may be involved in the paclitaxel/bryostatin 1 interaction.
Coadministration of bryostatin 1 overcomes the protective effects of Bcl-2 against paclitaxel-induced apoptosis.
(A) U937/neo (■), U937/Bcl-2 (░), and U937/ΔBcl-2 (▪) cells were treated with paclitaxel (Tax; 10 nM) with or without bryostatin 1 (Bry; 10 nM) for 24 hours. Apoptotic cells were quantified by annexin V and PI positivity as described in “Materials and methods.” (B) Cells were treated as in panel A, after which they were washed free of drug and plated in soft agar, after which colonies consisting of at least 50 cells were scored after 12 days. Values were expressed as a percentage relative to untreated controls (Con). Values for panels A and B represent the means ± SE for 3 separate experiments involving different U937/Bcl-2 and U937/ΔBcl-2 clones. *Significant difference compared with treatment with paclitaxel or bryostatin 1 alone (P < .001).
Coadministration of bryostatin 1 overcomes the protective effects of Bcl-2 against paclitaxel-induced apoptosis.
(A) U937/neo (■), U937/Bcl-2 (░), and U937/ΔBcl-2 (▪) cells were treated with paclitaxel (Tax; 10 nM) with or without bryostatin 1 (Bry; 10 nM) for 24 hours. Apoptotic cells were quantified by annexin V and PI positivity as described in “Materials and methods.” (B) Cells were treated as in panel A, after which they were washed free of drug and plated in soft agar, after which colonies consisting of at least 50 cells were scored after 12 days. Values were expressed as a percentage relative to untreated controls (Con). Values for panels A and B represent the means ± SE for 3 separate experiments involving different U937/Bcl-2 and U937/ΔBcl-2 clones. *Significant difference compared with treatment with paclitaxel or bryostatin 1 alone (P < .001).
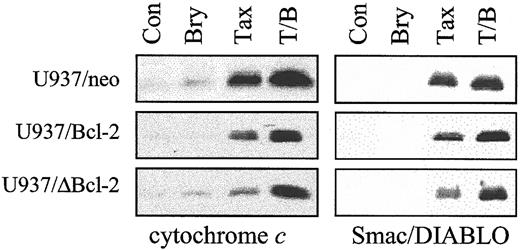
Addition of bryostatin 1 overcomes the ability of full-length or loop-deleted Bcl-2 to protect leukemic cells from paclitaxel-induced mitochondrial dysfunction
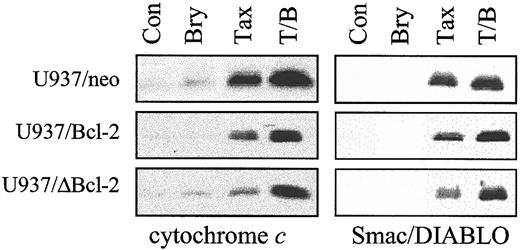
As shown in Figure 3, ectopic expression of full-length Bcl-2 significantly attenuated the capacity of paclitaxel (10 nM) to induce release of cytochrome c and the inhibitor of apoptosis antagonist Smac/DIABLO34 35into the cytosolic S-100 fraction, and the loop-deleted protein was even more effective in this regard. However, when cells were exposed to bryostatin 1, which exerted minimal effects by itself, paclitaxel-induced redistribution of cytochrome c and Smac/DIABLO in cells ectopically expressing full-length or loop-deleted Bcl-2 was restored to levels equivalent to those observed in empty-vector controls. Similar results were obtained when cells were exposed to 5 nM paclitaxel (data not shown). These findings suggest that bryostatin 1 acts by interfering with the ability of Bcl-2 to attenuate paclitaxel-mediated release of proapoptotic mitochondrial proteins into the cytosol and that this phenomenon does not depend upon Bcl-2 phosphorylation.
Cotreatment with bryostatin 1 dramatically overcomes protection of Bcl-2 against paclitaxel-induced mitochondrial dysfunction.
U937/neo, U937/Bcl-2, and U937/ΔBcl-2 cells were treated with paclitaxel (10 nM) with or without bryostatin 1 (10 nM) for 24 hours. Cytosolic expression of cytochrome c and Smac/DIABLO was monitored by Western analysis as described under “Materials and methods.” Blots were reprobed for actin to ensure equal loading and transfer of protein (data not shown). Two additional studies yielded equivalent results.
Cotreatment with bryostatin 1 dramatically overcomes protection of Bcl-2 against paclitaxel-induced mitochondrial dysfunction.
U937/neo, U937/Bcl-2, and U937/ΔBcl-2 cells were treated with paclitaxel (10 nM) with or without bryostatin 1 (10 nM) for 24 hours. Cytosolic expression of cytochrome c and Smac/DIABLO was monitored by Western analysis as described under “Materials and methods.” Blots were reprobed for actin to ensure equal loading and transfer of protein (data not shown). Two additional studies yielded equivalent results.
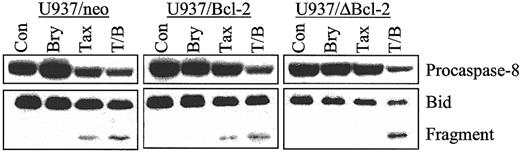
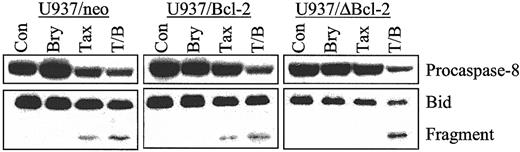
Bryostatin 1 enhances caspase-8 activation and Bid cleavage in parental and Bcl-2–overexpressing U937 cells
To assess the effects of bryostatin 1 on paclitaxel-mediated activation of the extrinsic apoptotic pathway, procaspase-8 degradation and Bid cleavage were monitored in empty-vector control cells as well as those ectopically expressing full-length and phosphorylation loop–deleted Bcl-2. As shown in Figure4, cotreatment with paclitaxel and bryostatin 1 induced equivalent degrees of activation of caspase-8 and Bid cleavage in U937/neo, U937/Bcl-2, and U937/ΔBcl-2 cells. Such findings indicate that ectopic expression of these antiapoptotic proteins is ineffective in blocking activation of the extrinsic apoptotic pathway by the bryostatin 1/paclitaxel regimen.
Cotreatment of bryostatin 1 and paclitaxel induces equivalent caspase-8 activation and Bid cleavage in parental and cells ectopically expressing Bcl-2 and ΔBcl-2.
U937/neo, U937/Bcl-2, and U937/ΔBcl-2 cells were treated with paclitaxel (10 nM) with or without bryostatin 1 (10 nM) for 24 hours. Cleavage of procaspase-8 and Bid was detected by Western analysis as described in “Materials and methods.” Blots were reprobed for actin to ensure equal loading and transfer of protein. Two additional experiments yielded identical results.
Cotreatment of bryostatin 1 and paclitaxel induces equivalent caspase-8 activation and Bid cleavage in parental and cells ectopically expressing Bcl-2 and ΔBcl-2.
U937/neo, U937/Bcl-2, and U937/ΔBcl-2 cells were treated with paclitaxel (10 nM) with or without bryostatin 1 (10 nM) for 24 hours. Cleavage of procaspase-8 and Bid was detected by Western analysis as described in “Materials and methods.” Blots were reprobed for actin to ensure equal loading and transfer of protein. Two additional experiments yielded identical results.
Caspase-8 activation is central to the potentiation of paclitaxel lethality by bryostatin 1
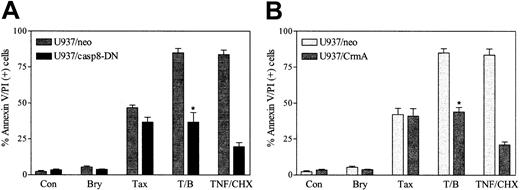
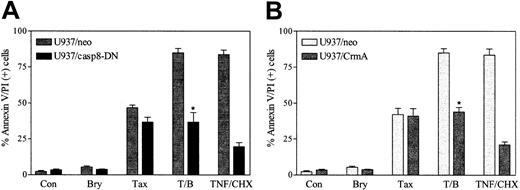
To define further the functional role of the extrinsic cell death pathway in bryostatin 1/paclitaxel lethality, parallel studies were conducted in cells ectopically expressing dominant-negative caspase-8 (U937/casp8-DN). As shown in Figure5A, ectopic expression of U937/casp8-DN minimally attenuated apoptosis in cells exposed to paclitaxel alone (5 nM), suggesting that the extrinsic pathway plays only a minor role in the lethal effects of this agent. However, in marked contrast, ectopic expression of DN caspase-8 completely abrogated the ability of bryostatin 1 to potentiate paclitaxel-induced cell death. Similar results were obtained when cells were exposed to 10 nM paclitaxel (data not shown). Thus, these data indicate that caspase-8 plays a central role in potentiation of paclitaxel lethality by bryostatin 1 but not in cell death induced by paclitaxel alone.
Caspase-8 activation is critical for potentiation of paclitaxel lethality by bryostatin 1.
U937 cells ectopically expressing dominant-negative caspase-8 (U937/casp8-DN) and CrmA were treated with paclitaxel (5 nM) with or without bryostatin 1 (10 nM) or treated with TNF-α (1 ng/mL) plus CHX (1 μM) for 24 hours. Apoptotic cells were quantified by annexin V and PI positivity as described in “Materials and methods.” Results shown are representative of 3 experiments (means ± SE) employing several U937/casp8-DN and U937/CrmA clones. *Significant difference relative to empty-vector control cells (P < .01).
Caspase-8 activation is critical for potentiation of paclitaxel lethality by bryostatin 1.
U937 cells ectopically expressing dominant-negative caspase-8 (U937/casp8-DN) and CrmA were treated with paclitaxel (5 nM) with or without bryostatin 1 (10 nM) or treated with TNF-α (1 ng/mL) plus CHX (1 μM) for 24 hours. Apoptotic cells were quantified by annexin V and PI positivity as described in “Materials and methods.” Results shown are representative of 3 experiments (means ± SE) employing several U937/casp8-DN and U937/CrmA clones. *Significant difference relative to empty-vector control cells (P < .01).
To confirm further that potentiation of paclitaxel-induced apoptosis by bryostatin 1 was mediated through the death receptor pathway, cells ectopically expressing the serpin CrmA, a potent inhibitor of caspase-8,3 were exposed to paclitaxel (5 nM) with or without bryostatin 1 (10 nM) for 24 hours, after which apoptosis was monitored. As shown in Figure 5B, ectopic expression of CrmA did not modify the apoptotic response to paclitaxel alone but essentially abrogated potentiation of paclitaxel-induced cell death by bryostatin 1. Ectopic expression of CrmA, like DN caspase-8, also substantially blocked apoptosis triggered by the combination of TNF and cycloheximide (CHX). Taken together, these findings argue strongly that potentiation of paclitaxel-induced apoptosis by bryostatin 1 is mediated by the extrinsic cell death pathway.
Coadministration of paclitaxel and bryostatin 1 results in potentiation of TNF-α mRNA levels and prolonged protein accumulation
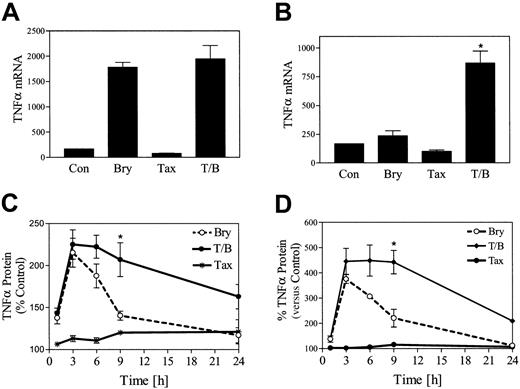
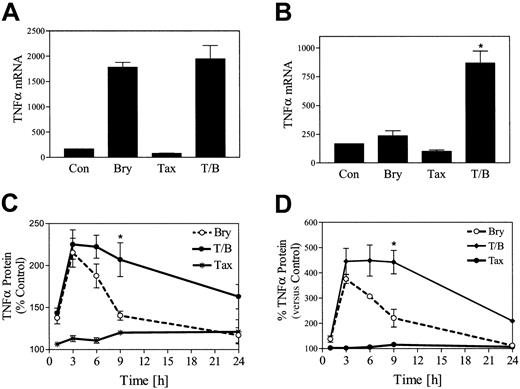
In view of evidence that bryostatin 1 induces a variety of cytokines, including TNF-α, in hematopoietic cells,36RT-PCR was employed to assess the impact of bryostatin 1 and paclitaxel exposure on TNF-α mRNA production in U937 cells. As shown in Figure6A, paclitaxel (5 nM) by itself exerted minimal effects on TNF-α mRNA levels, while bryostatin 1 by itself induced a 10-fold increase in TNF-α mRNA levels at 3 hours, which declined to baseline by 6 hours (Figure 6B). Cotreatment of cells with paclitaxel and bryostatin 1 did not increase TNF-α mRNA levels at 3 hours relative to cells exposed to bryostatin 1 alone. However, cells treated with both bryostatin 1 with paclitaxel continued to exhibit a 4-fold increase in TNF-α mRNA levels at 6 hours (Figure6B). Assessment of TNF-α protein levels by ELISA revealed equivalent increases in bryostatin 1– and bryostatin 1 plus paclitaxel–treated cells at 3 hours (Figure 6C). However, whereas TNF-α protein levels declined to baseline by 24 hours in cells exposed to bryostatin 1 alone, a persistent increase in protein levels was noted in bryostatin 1/paclitaxel–treated cells at 24 hours. Essentially identical results were noted in HL-60 cells (Figure 6D). These findings indicate that coexposure of U937 cells to the combination of paclitaxel and bryostatin 1 results in sustained increases in TNF-α mRNA and protein levels.
Coadministration of paclitaxel and bryostatin 1 results in potentiation of TNF-α mRNA levels and prolonged protein accumulation.
U937 cells were treated with paclitaxel (5 nM) with or without bryostatin 1 (10 nM) for 3 hours (A) and 6 hours (B). Total RNA was isolated, and TNF-α mRNA expression was determined by RT-PCR as described in “Materials and methods.” U937 cells (C) and HL-60 cells (D) were treated with paclitaxel (10 nM) with or without bryostatin 1 (10 nM). The amount of TNF-α protein in the media was monitored at various time intervals (0 to 24 hours) by ELISA assay as described in “Materials and methods.” TNF-α protein is expressed as fold increase, reflected by changes in optical density, relative to untreated control cells. Results are representative of 3 separate experiments (means ± SE). *Significantly greater than the sum of the effects of each agent administered alone (P < .01).
Coadministration of paclitaxel and bryostatin 1 results in potentiation of TNF-α mRNA levels and prolonged protein accumulation.
U937 cells were treated with paclitaxel (5 nM) with or without bryostatin 1 (10 nM) for 3 hours (A) and 6 hours (B). Total RNA was isolated, and TNF-α mRNA expression was determined by RT-PCR as described in “Materials and methods.” U937 cells (C) and HL-60 cells (D) were treated with paclitaxel (10 nM) with or without bryostatin 1 (10 nM). The amount of TNF-α protein in the media was monitored at various time intervals (0 to 24 hours) by ELISA assay as described in “Materials and methods.” TNF-α protein is expressed as fold increase, reflected by changes in optical density, relative to untreated control cells. Results are representative of 3 separate experiments (means ± SE). *Significantly greater than the sum of the effects of each agent administered alone (P < .01).
Recombinant human TNF-α potentiates paclitaxel-induced apoptosis
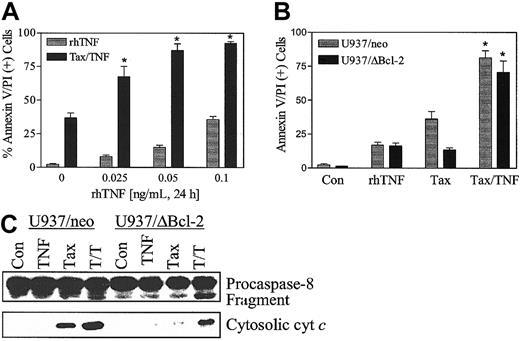
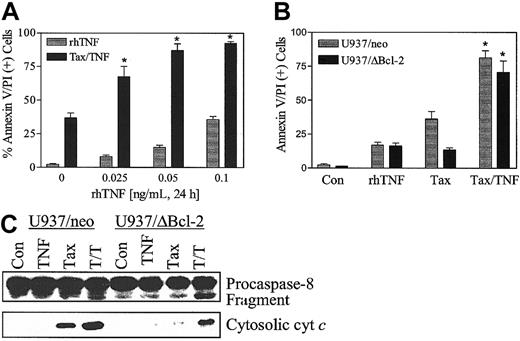
To define further the effects of TNF-α on paclitaxel-induced lethality, U937 cells were exposed to paclitaxel (5 nM) in combination with recombinant human TNF-α (rhTNF-α) (0.025 to 1.0 ng/mL) for 24 hours. As shown in Figure 7A, paclitaxel-induced cell death was markedly enhanced by rhTNF-α. Coadministration of paclitaxel with rhTNF-α also circumvented the blockade of paclitaxel-mediated apoptosis conferred by ectopic expression of Bcl-2 protein (Figure 7B), analogous to results obtained with bryostatin 1. Consistent with these findings, coadministration of rhTNF-α restored the ability of paclitaxel to promote procaspase-8 degradation and cytochrome c release in U937/ΔBcl-2 cells (Figure 7C). Equivalent results were obtained when cells were exposed to 10 nM paclitaxel (data not shown). These findings indicate that the capacity of bryostatin 1 to overcome paclitaxel resistance in leukemia cells ectopically expressing the phosphorylation loop–deleted Bcl-2 protein is closely mimicked by rhTNF-α.
Recombinant human TNF-α (rhTNF-α) potentiates paclitaxel-induced apoptosis.
(A) U937 cells were treated with paclitaxel (5 nM) with or without rhTNF (0 to 0.1 ng/mL) for 24 hours. (B) U937/neo (░) and U937/ΔBcl-2 (▪) were treated with paclitaxel (5 nM) with or without rhTNF (0.025 ng/mL) for 24 hours. Apoptotic cells were quantified by annexin V and PI positivity as described in “Materials and methods.” Data are shown in panels A and B as the means ± SE. *Significant difference compared with paclitaxel alone or TNF-α alone treatment in the corresponding cell lines (P < .025). (C) Cells were exposed to paclitaxel with or without rhTNF as in panel B, after which the degradation of native procaspase-8 and expression of cytosolic cytochrome c were monitored by Western analysis as described in “Materials and methods.” Blots were reprobed for actin to ensure equal protein loading and transfer. Two additional experiments yielded similar results.
Recombinant human TNF-α (rhTNF-α) potentiates paclitaxel-induced apoptosis.
(A) U937 cells were treated with paclitaxel (5 nM) with or without rhTNF (0 to 0.1 ng/mL) for 24 hours. (B) U937/neo (░) and U937/ΔBcl-2 (▪) were treated with paclitaxel (5 nM) with or without rhTNF (0.025 ng/mL) for 24 hours. Apoptotic cells were quantified by annexin V and PI positivity as described in “Materials and methods.” Data are shown in panels A and B as the means ± SE. *Significant difference compared with paclitaxel alone or TNF-α alone treatment in the corresponding cell lines (P < .025). (C) Cells were exposed to paclitaxel with or without rhTNF as in panel B, after which the degradation of native procaspase-8 and expression of cytosolic cytochrome c were monitored by Western analysis as described in “Materials and methods.” Blots were reprobed for actin to ensure equal protein loading and transfer. Two additional experiments yielded similar results.
TNF soluble receptor blocks bryostatin 1–mediated potentiation of paclitaxel-induced apoptosis
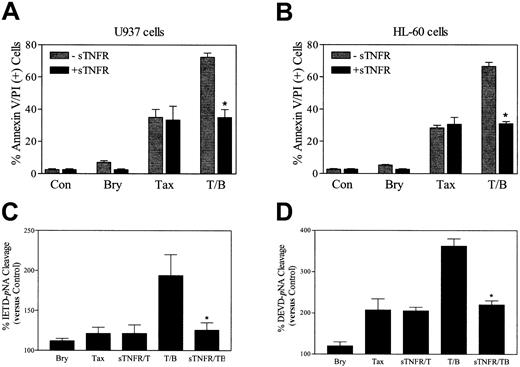
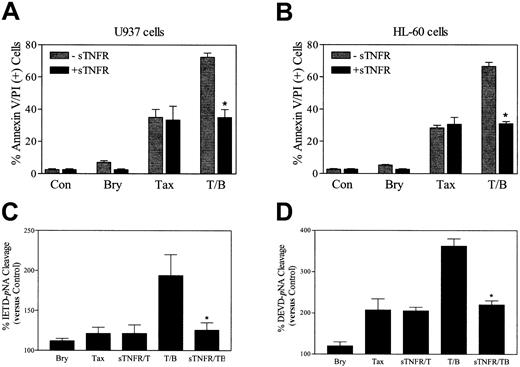
To investigate the functional significance of induction of TNF-α in bryostatin 1–related potentiation of paclitaxel-induced cell death, U937 cells were exposed to TNF soluble receptor (sTNFR, 100 ng/mL) 30 minutes prior to the addition of paclitaxel and bryostatin 1. As shown in Figure 8A, sTNFR abrogated the potentiation of paclitaxel-induced cell death by bryostatin 1, analogous to results obtained when exogenous TNF-α was added (data not shown). The sTNFR also blocked potentiation of paclitaxel lethality by bryostatin 1 in HL-60 cells (Figure 8B). Consistent with these findings, sTNFR blocked the increase in caspase-8 and caspase-3 activation in U937 cells exposed to the combination of paclitaxel and bryostatin 1 but exerted minimal effects in cells exposed to paclitaxel alone (Figure 8C-D). Together, these findings support the notion that induction of TNF-α by bryostatin 1 plays a major role in potentiating the lethal actions of paclitaxel in U937 and HL-60 cells.
TNF soluble receptor (sTNFR) abrogates bryostatin 1–mediated potentiation of paclitaxel-induced apoptosis.
U937 cells (A) and HL-60 (B) cells were exposed to paclitaxel (5 nM) with or without bryostatin 1 (10 nM) for 24 hours in the presence (▪) and absence (░)of sTNFR (100 ng/mL). Apoptosis was determined by annexin V and PI positivity as described in “Materials and methods.” Values are representative of 3 separate experiments (means ± SE). U937 cells were exposed to the treatments described for panels A and B for 24 hours, after which caspase-8 (C) and caspase-3 (D) activities, reflected by IETD-pNA or DEVD-pNA cleavage, respectively, were determined as described in “Materials and methods.” Values represent percentages relative to untreated controls and correspond to the 3 separate experiments performed in triplicate (means ± SE). *Significant difference from cells exposed to paclitaxel/bryostatin 1 in the absence of sTNFR (P < .01).
TNF soluble receptor (sTNFR) abrogates bryostatin 1–mediated potentiation of paclitaxel-induced apoptosis.
U937 cells (A) and HL-60 (B) cells were exposed to paclitaxel (5 nM) with or without bryostatin 1 (10 nM) for 24 hours in the presence (▪) and absence (░)of sTNFR (100 ng/mL). Apoptosis was determined by annexin V and PI positivity as described in “Materials and methods.” Values are representative of 3 separate experiments (means ± SE). U937 cells were exposed to the treatments described for panels A and B for 24 hours, after which caspase-8 (C) and caspase-3 (D) activities, reflected by IETD-pNA or DEVD-pNA cleavage, respectively, were determined as described in “Materials and methods.” Values represent percentages relative to untreated controls and correspond to the 3 separate experiments performed in triplicate (means ± SE). *Significant difference from cells exposed to paclitaxel/bryostatin 1 in the absence of sTNFR (P < .01).
Initial PKC activation is required for potentiation of paclitaxel-induced TNF-α induction, caspase-3 activation, and cell death by bryostatin 1
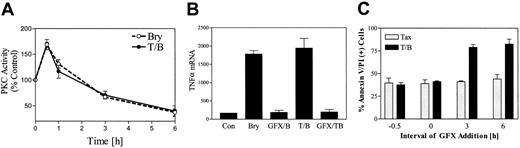
To examine the possible relationship between initial PKC activation and potentiation of paclitaxel lethality by bryostatin 1, a time course analysis of PKC activity in U937 cells exposed to 10 nM bryostatin 1 with or without paclitaxel (5 nM) was performed (Figure 9A). Thirty minutes following bryostatin 1 treatment, an increase in total PKC activity was noted. PKC activity declined rapidly after 1 hour, and at 6 hours only 40% of basal activity was detected. Parallel results were obtained when membrane-associated PKC activity was monitored (data not shown). This biphasic effect was qualitatively similar to that which we have previously reported in HL-60 cells exposed to bryostatin 1.33 PKC activity was unaltered by treatment with paclitaxel alone (data not shown); moreover, PKC activity in bryostatin 1/paclitaxel–treated cells was similar to that observed in cells exposed to bryostatin 1 alone.
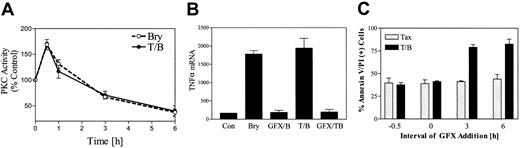
Initial PKC activation is required for potentiation of paclitaxel-induced TNF-α induction and apoptosis by bryostatin 1.
(A) U937 cells were treated with bryostatin 1 (10 nM) alone (○) or in combination with paclitaxel (5 nM, ●). Total cellular PKC activity was assessed as described in “Materials and methods.” (B) U937 cells were exposed to paclitaxel/bryostatin 1 in the presence or absence of GFX (1 μM) for 3 hours. Total RNA was isolated, and TNF-α mRNA levels were determined by RT-PCR. (C) U937 cells were treated with GFX (1 μM) before (−30 or 0 minutes) or after (3 or 6 hours) administration of paclitaxel (5 nM) and bryostatin 1 (10 nM). Cell death was assessed after 24 hours by annexin and PI staining as described in “Materials and methods.” Results for panels A and C correspond to 3 separate experiments (means ± SE).
Initial PKC activation is required for potentiation of paclitaxel-induced TNF-α induction and apoptosis by bryostatin 1.
(A) U937 cells were treated with bryostatin 1 (10 nM) alone (○) or in combination with paclitaxel (5 nM, ●). Total cellular PKC activity was assessed as described in “Materials and methods.” (B) U937 cells were exposed to paclitaxel/bryostatin 1 in the presence or absence of GFX (1 μM) for 3 hours. Total RNA was isolated, and TNF-α mRNA levels were determined by RT-PCR. (C) U937 cells were treated with GFX (1 μM) before (−30 or 0 minutes) or after (3 or 6 hours) administration of paclitaxel (5 nM) and bryostatin 1 (10 nM). Cell death was assessed after 24 hours by annexin and PI staining as described in “Materials and methods.” Results for panels A and C correspond to 3 separate experiments (means ± SE).
To determine the relationship between PKC activation and TNF-α production, U937 cells were exposed to the selective PKC inhibitor bisindolylmaleimide I (GF 109203X; GFX; 1 μM) 30 minutes prior to treatment with paclitaxel with or without bryostatin 1, after which TNF-α mRNA was monitored by RT-PCR. As shown in Figure 9B, pretreatment with GFX completely blocked bryostatin 1–mediated induction of TNF-α mRNA in U937 cells, suggesting the up-regulation of TNF is induced by bryostatin 1–mediated PKC activation.
To evaluate further the possibility that PKC activation might be involved in bryostatin1-related potentiation of paclitaxel-induced cell death, U937 cells were pretreated with GFX (1 μM, 0.5 hours) prior to the addition of paclitaxel/bryostatin1. As shown in Figure 9C, pretreatment of cells with GFX essentially abrogated potentiation of paclitaxel actions by bryostatin 1. To determine whether the duration of PKC activation by bryostatin 1 represents an important determinant of potentiation of paclitaxel-mediated apoptosis, GFX was added at varying exposure intervals before or after treatment of cells with paclitaxel/bryostatin 1. Potentiation of paclitaxel actions by bryostatin 1 was essentially abrogated when GFX was added at time 0 or 30 minutes prior to bryostatin1/paclitaxel. In contrast, addition of GFX at later time points (eg, 3 or 6 hours after bryostatin 1/paclitaxel administration) was associated with the persistence of the ability of bryostatin 1 to potentiate paclitaxel-induced apoptosis. In separate studies, pretreatment of cells with 10 nM bryostatin 1 for 6 hours, an interval when PKC activity was significantly down-regulated (Figure 9A), failed to potentiate paclitaxel-induced apoptosis (data not shown). Collectively, these findings suggest that initial PKC activation, rather than PKC down-regulation or inhibition, is essential for promotion of paclitaxel lethality by bryostatin 1.
Bryostatin 1 increases paclitaxel-induced apoptosis in cells that have undergone mitotic arrest
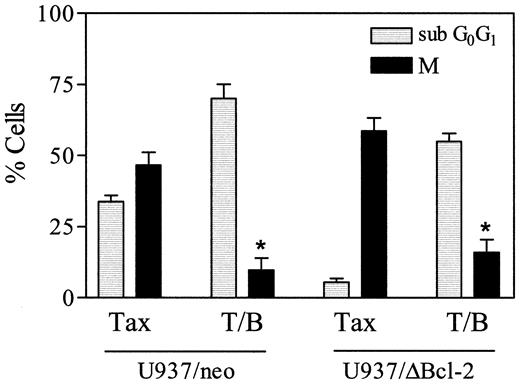
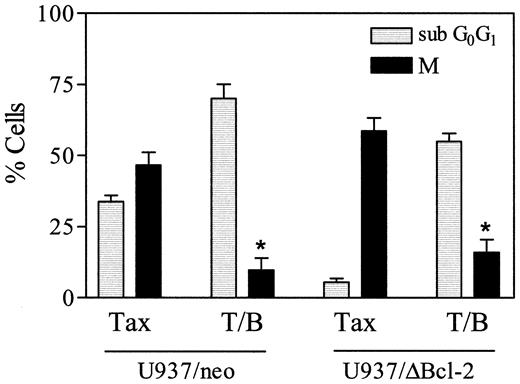
As shown in Figure 10, exposure of U937/neo cells to 10 nM paclitaxel in combination with bryostatin 1 for 18 hours resulted in a clear increase in the subdiploid (apoptotic) population and a reciprocal decline in the MPM-2+ (mitotic) fraction compared with cells exposed to paclitaxel alone (P < .02 in each case). In U937/ΔBcl-2 cells exposed to paclitaxel alone, the subdiploid population was minimal, whereas more than 50% of cells were MPM-2+, consistent with the notion that cells protected from apoptosis underwent extensive mitotic arrest. When paclitaxel-resistant U937/ΔBcl-2 cells were treated with the combination of paclitaxel and bryostatin 1, there was a very substantial increase in the subdiploid fraction and a large decline in the MPM-2+ population. Taken together, these findings suggest that paclitaxel-treated U937 cells that have undergone mitotic arrest are particularly susceptible to potentiation of apoptosis by bryostatin 1.
Bryostatin 1 increases paclitaxel-induced apoptosis in cells that have undergone mitotic arrest.
U937/neo (░) and U937/ΔBcl-2 (▪) cells were treated with paclitaxel (10 nM) and bryostatin 1 (10 nM) for 18 hours, after which cells were labeled with the MPM-2 antibody (M) and analyzed by flow cytometry to determine the percentage of cells in mitosis as described under “Materials and methods.” Values represent the means for 3 separate experiments (± SE). *Significant difference compared with treatment with paclitaxel alone in corresponding cells (P < .02).
Bryostatin 1 increases paclitaxel-induced apoptosis in cells that have undergone mitotic arrest.
U937/neo (░) and U937/ΔBcl-2 (▪) cells were treated with paclitaxel (10 nM) and bryostatin 1 (10 nM) for 18 hours, after which cells were labeled with the MPM-2 antibody (M) and analyzed by flow cytometry to determine the percentage of cells in mitosis as described under “Materials and methods.” Values represent the means for 3 separate experiments (± SE). *Significant difference compared with treatment with paclitaxel alone in corresponding cells (P < .02).
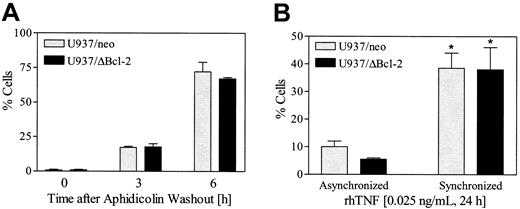
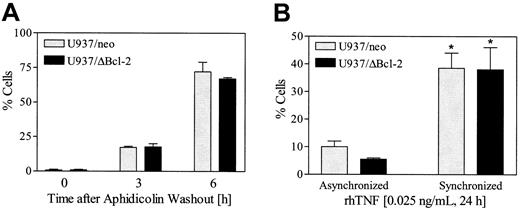
Cells synchronized in G2M display enhanced susceptibility to TNF-α– induced lethality
In view of evidence that cells in the G2M phase of the cell cycle exhibit increased vulnerability to intracellular death signals,37 an attempt was made to determine if cells arrested in G2M by paclitaxel might be more susceptible to TNF-α–induced lethality. To this end, U937/neo and U937ΔBcl-2 cells were synchronized with aphidicolin (100 nM, 24 hours), washed free of drug, and after 6 hours exposed to 0.025 ng/mL rhTNF for 24 hours. As shown in Figure 11A, about 75% of cells were in G2M at the time of TNF-α administration. The results shown in Figure 11B indicate that empty-vector and ΔBcl-2 cells synchronized in G2M display a marked increase in apoptosis following TNF-α exposure compared with their unsynchronized counterparts (P < .01). Together, these findings raise the possibility that induction of TNF-α by bryostatin 1 in cells arrested in G2M by paclitaxel contributes to the marked potentiation of apoptosis accompanying combined drug exposure.
Cells synchronized in G2M display enhanced susceptibility to TNF-α–induced lethality.
(A) U937/neo and U937/ΔBcl-2 cells were exposed to aphidicolin (100 ng/mL) for 24 hours, washed 3 times, and then resuspended in fresh medium. Cell cycle analysis was performed 3 and 6 hours following the washout. (B) At 6 hours after aphidicolin washout, synchronized and untreated (asynchronized) cells were exposed to TNF-α (0.025 ng/mL) for 24 hours. Apoptosis was determined by annexin V/PI positivity as described in “Materials and methods.” Values represent the means for 3 separate experiments (± SE). *Significant difference relative to asynchronous cells (P < .01).
Cells synchronized in G2M display enhanced susceptibility to TNF-α–induced lethality.
(A) U937/neo and U937/ΔBcl-2 cells were exposed to aphidicolin (100 ng/mL) for 24 hours, washed 3 times, and then resuspended in fresh medium. Cell cycle analysis was performed 3 and 6 hours following the washout. (B) At 6 hours after aphidicolin washout, synchronized and untreated (asynchronized) cells were exposed to TNF-α (0.025 ng/mL) for 24 hours. Apoptosis was determined by annexin V/PI positivity as described in “Materials and methods.” Values represent the means for 3 separate experiments (± SE). *Significant difference relative to asynchronous cells (P < .01).
Discussion
While previous studies have demonstrated that bryostatin 1 potentiates the lethal actions of paclitaxel in both hematopoietic29 as well as nonhematopoietic cells,30 the mechanism underlying this phenomenon remains unclear. One possibility is that bryostatin 1 promotes those events responsible for paclitaxel-mediated mitochondrial injury and apoptosis. In this regard, the lethal actions of paclitaxel have been linked to phosphorylation of Bcl-2 and interference with the capacity of this protein to block mitochondrial injury and cell death.38Consistent with this model, bryostatin 1–mediated circumvention of paclitaxel resistance in leukemic cells ectopically expressing Bcl-2 has been shown to be associated with Bcl-2 phosphorylation.23 An alternative possibility is that chronic exposure to bryostatin 1, which induces PKC down-regulation,20,39 generically sensitizes cells to a variety of noxious stimuli, including cytotoxic drugs.25,40 The observations that PKC inhibitors promote drug-induced apoptosis33 and that activation of PKC opposes hematopoietic cell death,41,42 including that induced by paclitaxel,43 support this notion.
However, results of the present studies argue against either of these mechanisms; instead, they strongly suggest that potentiation of paclitaxel-mediated lethality in human myeloid leukemia cells stems from bryostatin 1–mediated stimulation of TNF-α release and activation of the extrinsic apoptotic cascade. For example, while phosphorylation of Bcl-2 by PKC activators, including bryostatin 1 and PMA (phorbol-12-myristate-13-acetate), may exert divergent effects on apoptosis in various neoplastic and nonneoplastic cell types, there is considerable evidence that phosphorylation of Bcl-2 by taxanes contributes to the lethality of these agents.44,36The Δ32-80Bcl-2 mutant protein lacks the major Bcl-2 phosphorylation sites,37 and cells ectopically expressing this protein have been shown to exhibit marked resistance to growth factor deprivation45 as well as to diverse cytotoxic drugs, including paclitaxel.46 Although it is tempting to attribute the enhanced cytoprotective capacity of the Δ32-80Bcl-2 mutant protein to its resistance to phosphorylation,14 15 the possibility that loss of the N-terminal region results in conformational changes that prevent mitochondrial injury (ie, cytochrome c release) cannot be excluded. In any case, the ability of bryostatin 1 to potentiate paclitaxel-induced mitochondrial injury, caspase activation, and apoptosis in cells ectopically expressing the Δ32-80Bcl-2 mutant indicates that factors other than or in addition to Bcl-2 phosphorylation are involved in this phenomenon.
Analogously, the present results are also difficult to reconcile with the notion that potentiation of paclitaxel-mediated apoptosis by bryostatin 1 stems solely from interruption of the PKC cytoprotective pathway. While bryostatin 1 initially activates PKC,47 on chronic administration it down-regulates the enzyme48through a process that involves proteasomal degradation.49Thus, chronic exposure to bryostatin 1 may mimic the actions of pharmacologic PKC inhibitors (eg, staurosporine), many of which have been shown to be potent inducers of apoptosis.23 In this regard, the capacity of bryostatin 1 to promote apoptosis induced by the antimetabolite ara-C in human leukemia cells has also been correlated with PKC down-regulation.25 However, the observation that GFX, a highly specific PKC inhibitor,50blocked potentiation of paclitaxel lethality by bryostatin 1 argues strongly against the possibility that interruption of the PKC pathway is solely responsible for synergism between these agents. Moreover, preexposure of cells to bryostatin 1 for 6 hours failed to promote paclitaxel lethality despite down-regulating PKC activity (Figure 9A). Instead, these findings, as well as the observation that PKC activation is required for bryostatin 1–mediated potentiation of TNF-α release, are most compatible with the concept that initial PKC activation, rather than chronic down-regulation, is required for the proapoptotic actions of bryostatin 1 in this setting.
The notion that bryostatin 1 potentiates paclitaxel-mediated apoptosis by increasing TNF release is supported by several lines of evidence. First, coexposure of cells to paclitaxel and bryostatin 1 resulted in a marked increase in caspase-8 activation and Bid cleavage, events that are generally associated with activation of the extrinsic apoptotic pathway. Second, coadministration of these agents resulted in sustained elevations in TNF-α mRNA and protein release. Third, potentiation of paclitaxel-induced apoptosis by bryostatin 1 was blocked by interruption of the extrinsic pathway (eg, by ectopic expression of CrmA or DN caspase-8) or by administration of TNF soluble receptor. Finally, the ability of bryostatin 1 to enhance paclitaxel-induced cell death was closely mimicked by administration of rhTNF-α. The ability of the extrinsic apoptotic pathway to amplify apoptotic signals triggered by mitochondrial-directed agents (eg, cytotoxic drugs) has previously been described.7 However, in the latter study, extrinsic pathway activation occurred downstream of mitochondrial events—that is, as a consequence of caspase-8 cleavage by caspase-3, which was in turn activated by the caspase-9/cytochromec/Apaf-1 complex. In contrast, paclitaxel lethality was unperturbed by interventions that blocked the extrinsic pathway, indicating that cell death induced by this agent, at least when administered alone, proceeds largely through the intrinsic pathway. On the other hand, the ability of bryostatin 1 to potentiate this process by engaging the extrinsic cascade shares similarities with the capacity of agents such as Trail (TNF receptor apoptosis-inducing ligand) to enhance the lethality of cytotoxic drugs.51 In this regard, synergistic induction of apoptosis by paclitaxel and Trail has previously been described in prostate and lung cancer cells.52 Last, the ability of bryostatin 1 to promote paclitaxel lethality through activation of the receptor-related cascade is consistent with recent observation from our laboratory suggesting that a similar mechanism underlies synergistic interactions between bryostatin 1 and flavopiridol or ara-C (S.G., L. Cartee, Z.W., unpublished observations, August 2002).
Induction of TNF-α by bryostatin 1 and engagement of the extrinsic apoptotic pathway could also explain the ability of this agent to enhance paclitaxel lethality in leukemic cells ectopically expressing full-length Bcl-223 as well as the phosphorylation loop–deleted mutant (this study). Bcl-2 and related family members are believed to act by antagonizing the cytoprotective effects of proapoptotic proteins such as Bax and Bak, possibly by interfering with the formation of mitochondrial membrane channels that permit the egress of cytochrome c.53 Consistent with this model, the loop-deleted Bcl-2 protein, which is highly effective in antagonizing paclitaxel-mediated cytochrome crelease,34 also potently inhibits paclitaxel-induced apoptosis. However, in contrast to their ability to protect cells from agents that act through the intrinsic cascade, Bcl-2 and related proteins are generally ineffective in blocking apoptosis induced by stimuli that engage the receptor-related pathway. This may reflect the relative inability of Bcl-2 to prevent direct activation of caspase-8 and downstream effector caspases (eg, caspase-3) or to prevent mitochondrial injury induced by activated Bid (tBid).54Similar considerations would apply to the case of the loop-deleted Bcl-2 protein. Thus, activation of the extrinsic cascade by bryostatin 1 appears capable of initiating apoptotic events in cells otherwise protected from paclitaxel-mediated mitochondrial injury. The observation that cells arrested in G2M are more sensitive to the lethal actions of TNF-α has, to the best of our knowledge, not previously been reported. Induction of TNF-α by paclitaxel is well known, although this phenomenon appears to be separate from the lethal actions of this agent.55 In the present study, paclitaxel, administered at low concentrations, exerted little effect on TNF-α mRNA or protein levels, whereas bryostatin 1 was more effective in this regard, consistent with the results of earlier reports.56However, combined treatment with paclitaxel and bryostatin 1 resulted in a sustained increase in TNF-α levels, a phenomenon that might contribute to enhanced lethality. In view of findings suggesting that cells arrested in G2M are more susceptible to TNF-α–mediated apoptosis than their asynchronous counterparts (Figure 11), it is tempting to speculate that paclitaxel-mediated mitotic arrest of cells renders them more vulnerable to bryostatin 1–mediated potentiation of TNF-α release.
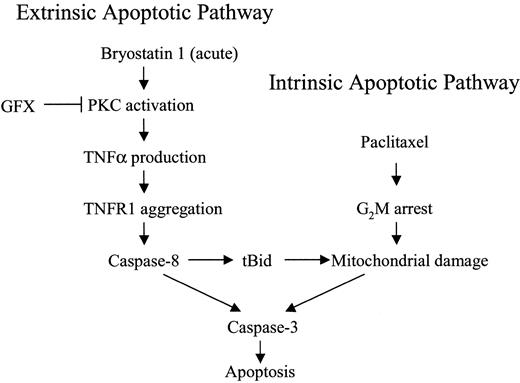
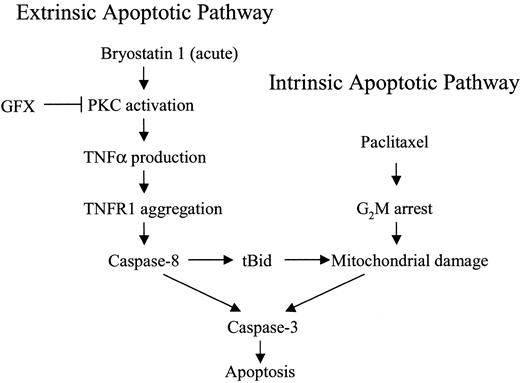
A model that might account for the marked potentiation of paclitaxel-induced apoptosis by bryostatin 1 is shown in Figure12. In this model, acute exposure to bryostatin 1 leads to the PKC-dependent induction of TNF-α, which by itself is insufficient to activate the extrinsic apoptotic pathway. However, coexposure to paclitaxel activates the intrinsic, mitochondrial pathway, the effects of which are amplified by engagement of the extrinsic apoptotic cascade.7 Last, for reasons that remain to be elucidated, cells arrested in G2M are more susceptible to the lethal effects of these events.
Model of bryostatin 1/paclitaxel interactions.
Acute exposure of leukemic cells to bryostatin 1 stimulates TNF-α production through a PKC-dependent mechanism and, in so doing, engages the extrinsic apoptotic pathway. However, activation of caspase-8 through this mechanism is insufficient, by itself, to trigger cell death. When leukemic cells are exposed to paclitaxel, mitochondrial dysfunction and cytochrome c release occur, resulting in a limited degree of caspase activation and cell death. The latter events are strikingly amplified by bryostatin 1–mediated activation of the extrinsic pathway. Finally, paclitaxel-mediated arrest of cells in G2M, where they are more sensitive to TNF-α–related lethality, contributes to the marked potentiation of cell death.
Model of bryostatin 1/paclitaxel interactions.
Acute exposure of leukemic cells to bryostatin 1 stimulates TNF-α production through a PKC-dependent mechanism and, in so doing, engages the extrinsic apoptotic pathway. However, activation of caspase-8 through this mechanism is insufficient, by itself, to trigger cell death. When leukemic cells are exposed to paclitaxel, mitochondrial dysfunction and cytochrome c release occur, resulting in a limited degree of caspase activation and cell death. The latter events are strikingly amplified by bryostatin 1–mediated activation of the extrinsic pathway. Finally, paclitaxel-mediated arrest of cells in G2M, where they are more sensitive to TNF-α–related lethality, contributes to the marked potentiation of cell death.
Although taxanes such as paclitaxel have been employed in the treatment of leukemia,57 activity to date has been limited. In this regard, intrinsic resistance of primary leukemia cells to paclitaxel-mediated lethality has been postulated.58However, the ability of agents such as bryostatin 1 to potentiate paclitaxel-induced apoptosis in human leukemia cells raises the possibility that combined treatment with these agents might exhibit increased activity in acute leukemia. The observation that bryostatin 1 potentiates paclitaxel-induced apoptosis in at least some primary AML specimens (data not shown) supports this notion. In this context, encouraging activity for a regimen combining paclitaxel and bryostatin 1 in patients with esophageal cancer has been reported.59Whether this phenomenon can be generalized to additional leukemia cell specimens and, if so, whether it stems from enhanced TNF-α release remains to be determined. Accordingly, studies designed to address these issues are currently under way.
Prepublished online as Blood First Edition Paper, January 9, 2003; DOI 10.1182/blood-2002-09-2739.
Supported by awards CA 63785 and CA 63753 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Steven Grant, Division of Hematology/Oncology, Virginia Commonwealth University/Medical College of Virginia, MCV Station Box 230, Richmond, VA 23298; e-mail:stgrant@hsc.vcu.edu.