Mutations at natural resistance-associated macrophage protein 1(Nramp1) impair phagocyte function and cause susceptibility to infections while mutations at Nramp2 (divalent metal transporter 1 [DMT1]) affect iron homeostasis and cause severe microcytic anemia. Structure-function relationships in the Nramp superfamily were studied by mutagenesis, followed by functional characterization in yeast and in mammalian cells. These studies identify 3 negatively charged and highly conserved residues in transmembrane domains (TM) 1, 4, and 7 as essential for cation transport by Nramp2/DMT1. The introduction of a charged residue (Gly185Arg) in TM4 found in the naturally occurring microcytic anemiamk (mouse) and Belgrade (rat) mutants is shown to cause a partial or complete loss of function in mammalian and yeast cells, respectively. A pair of mutation-sensitive and highly conserved histidines (His267, His272) was identified in TM6. Surprisingly, inactive His267 and His272 mutants could be rescued by lowering the pH of the transport assay. This indicates that His267/His272 are not directly involved in metal binding but, rather, play an important role in pH regulation of metal transport by Nramp proteins.
Introduction
Natural resistance-associated macrophage protein 2(Nramp2) (divalent metal transporter 1 [DMT1], divalent cation transporter 1 [DCT1]) is essential for nutritional iron (Fe2+) uptake by the duodenum brush border1-3 and for iron transport across the endosomal membrane in peripheral tissues.3-5 Nramp2 is an integral membrane protein composed of 12 predicted transmembrane (TM) domains.6 The Nramp2 gene produces 2 mRNAs by alternative splicing of the terminal 3′ exon that show different 3′ untranslated regions containing (isoform I, +IRE) or not (isoform II, −IRE) an iron response element (IRE) as well as distinct C-terminal protein sequences.7,8 In normal tissues, Nramp2 protein (isoform I) is expressed at the brush border of the proximal portion of the duodenum, where it is regulated by dietary iron deprivation.9 Nramp2 (isoform II) is also present in the Tf receptor–positive recycling endosome compartment of erythroid precursors that can be recruited in vivo by treatment with phenylhydrazine or erythropoietin.10 Recently, Nramp2 mRNA expression was detected in the kidney (isoforms I and II),11 and Nramp2 protein expression (isoform I) was detected at the brush border of the kidney proximal tubule.12 Direct transport studies in Xenopus laevis oocytes suggest that Nramp2 is a pH-dependent divalent metal transporter with broad substrate specificity including Fe2+, Mn2+, Co2+, Cd2+, Cu2+, Ni2+, Pb2+, and possibly Zn2+ and may function by a H+ cotransport mechanism.1 Parallel studies with metal-sensitive dyes have shown similar transport properties for Nramp2 expressed at the plasma membrane.13,14 An independently arising natural mutation in TM4 of Nramp2 (Gly185Arg) is responsible for microcytic anemia of the mk mouse2 and theBelgrade rat,3 both associated with a severe defect in intestinal iron absorption and impaired use by erythroid cells.15 Transport experiments in vitro in transfected HEK293 cells, as well as subcellular localization studies in vivo in target tissues from mk/mk mice, have shown that theNramp2Gly185Arg mutation causes a severe loss of function characterized by reduced activity and possibly impaired maturation/targeting.16,17 Together, these studies suggest a dual role for Nramp2 as the Tf-independent iron acquisition system of the duodenum and as the transporter of reduced Tf-iron across the endosomal membrane.18 19
The Nramp2 homolog, Nramp1,6,20 is expressed in the lysosomal compartment of macrophages and neutrophils21 and is recruited to the membrane of pathogen-containing phagosomes formed in these cells,22,23where it may function as a Mn2+ efflux pump.24A naturally occurring mutation in predicted TM4 of Nramp1 (Gly169Asp) impairs maturation and membrane targeting of the protein and causes susceptibility to infection with unrelated intracellular pathogens.20,25Nramp1 and Nramp2define a large superfamily of membrane transporters highly conserved from bacteria to humans.6,26,27 Demonstration of divalent cation transport by distant Nramp homologs from bacteria (MntH, Mramp), yeast (Smf1, Smf2, Smf3), fly (Mlv), and plant (AtNramp) has highlighted functional conservation in this family.6,28-30 In addition, expression of mouse Nramp2 protein in a double smf1/smf2 mutant can restore the ability of this mutant to grow at alkaline pH and on medium containing metal chelators.31,32 Likewise, expression of humanNRAMP1 in the fly mutant malvolio corrects the taste discrimination defect of this mutant,30 in a manner similar to that produced by increasing dietary Fe2+ or Mn2+.33
In the present study, we have used multiple sequence alignments to identify highly conserved residues in the Nramp superfamily. We have studied the functional role of conserved charged TM domain residues in substrate selectivity and pH regulation of Nramp2.
Materials and methods
Materials
Calcein acetoxymethylester (calcein-am; 500 μM stock solution prepared in dimethyl sulfoxide [DMSO]) was obtained from Molecular Probes (Eugene, OR). Stock Fe2+ (2 mM) aqueous solutions of ferrous ammonium sulfate (FAS; Sigma, Oakville, ON, Canada) were always prepared fresh in degassed, deionized water. CoCl2 (Sigma) was prepared as a stock solution (2 mM) in water. The membrane-permeable iron chelator salicylaldehyde isocotinoyl hydrazone (SIH; 25 mM stock solution) was prepared in DMSO, and the membrane-impermeable iron chelator HES-DFO (6 desferroxamine Mr 50 000 starch molecule; 38 mM stock) was prepared in water and stored at −20°C. SIH and HES-DFO were generous gifts from Dr P. Ponka (McGill University, Lady Davis Institute, Montreal, QC, Canada).
Plasmids and constructs
A full-length mouse Nramp2 (DMT1) cDNA isoform II lacking the IRE (GenBank accession no. L33415) was modified by the in-frame addition of 2 antigenic cMyc epitope tags at the carboxy terminus of the protein (N2-2Myc).32 Mutations at specific amino acid positions were created by site-directed mutagenesis using a recombinant polymerase chain reaction (PCR) protocol34 and using oligonucleotide primers listed in Table 1. The mutants were cloned into the mammalian expression vector pCB6. For complementation studies in yeast, mutants were introduced in the yeast expression vector pVT.
Yeast transformation and smf1/smf 2complementation assays
The Saccharomyces cerevisiae smf1/smf 2 double mutant is a mutant in which the SMF1 and SMF2 genes have been insertionally inactivated (MATa ura3-52leu2-3 −112 gal2SMF1::LEU2,SMF2::LEU2).32This mutant cannot grow on alkaline medium or on medium containing metal chelators.31,32 Yeast cells were transformed with pVT/Nramp2 constructs,35 andura+ transformants were grown as mass populations, and crude membrane fractions36 were prepared for Nramp2 protein expression by immunoblotting using the mouse anti-cMyc monoclonal antibody 9E10 (Babco, Berkeley, CA).9To test for possible complementation of the growth defects of thesmf1/smf2 mutant by Nramp2 variants, duplicate aliquots (1 mL) of ura+ transformants were resuspended in either YPD medium or in alkaline YPD medium (pH 7.9, OD595 = 0.02) in 96-well plates (100 μL per well). Growth was measured after 24 hours of incubation at 30°C using an enzyme-linked immunosorbent assay (ELISA) microplate reader (Bio-Rad model 450). Sensitivity to alkaline pH was measured as relative growth of each transformant (expressed as a percentage) in alkaline YPD 7.9 compared with growth of the same transformant in normal YPD medium (pH 5.5 to 6.0). For complementation studies on alkaline agar, cells grown to saturation in normal YPD were diluted to OD595 = 0.2, 0.02, 0.002, 0.0002, and 0.000 02 in YPD 7.9, and 20 μL of each dilution was spotted on alkaline YPD agar plates and grown for 36 to 48 hours at 30°C before photography.
Cell culture and transfection
LR-73 Chinese hamster ovary (CHO) cells were routinely grown in α-minimum essential medium (α-MEM) supplemented with 10% fetal bovine serum, 50 U/mL penicillin, and 50 μg/mL streptomycin (Invitrogen, Burlington, ON, Canada). All pCB6 (neo) plasmid constructs were transfected into cells as calcium phosphate coprecipitates, according to a procedure we have previously described.37 Clones of stable transfectants were selected in medium containing Geneticin (G418, 770 μg/mL; Invitrogen) and were picked after 8 to 13 days of selection.
Crude membrane preparation from CHO transfectants
Cell pellets were resuspended in 250 μL TNE buffer (100 mM NaCl; 10 mM Tris [tris(hydroxymethyl)aminomethane]–Cl, pH 7.0; 10 mM EDTA [ethylenediaminetetraacetic acid]) containing protease inhibitors (1 mM phenylmethylsulfonyl fluoride [PMSF], 1 μM pepstatin, 0.3 μM aprotinin, 1 μM leupeptin). Cells were homogenized by 20 passages through a 25-gauge, 5/8-inch needle, followed by centrifugation (4°C, 2000g, 10 minutes) to eliminate nuclei and unbroken cells. Membranes were then pelleted from the supernatant by ultracentrifugation (75 000 rpm, TLA-100 rotor [Beckman, Mississauga, ON, Canada], 4°C) and were resuspended in TNE containing 30% glycerol and protease inhibitors. Recombinant Nramp2 protein variants were detected using the mouse monoclonal anti–c-Myc antibody 9E10 (1:1000; Babco) as previously described.13
Calcein loading of the cells and divalent metal transport assay
CHO Nramp2 transfectants were loaded with the metal-sensitive fluorescent dye calcein-am, as we have previously described.13 Briefly, CHO transfectants (1 × 106 cells) were incubated with 0.250 μM calcein-am for 10 minutes at 37°C in 1 mL loading medium (α-minimum essential medium, 1 mg/mL bovine serum albumin [BSA], 20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethane sulfonic acid], pH 7.4). The cells were washed twice in and resuspended in 500 μL transport buffer (150 mM NaCl, 20 mM MES [2-[N-morpholino]ethanesulfonic acid monohydrate], pH 5.0 to 6.5). The cell suspension was transferred to a stirred thermostated (37°C) semimicrocuvette, and fluorescence was recorded using an LS-50B fluorescence spectrometer (PerkinElmer, Woodbridge, ON, Canada; excitation, 488 nm; emission, 517 nm; excitation and emission bandpass, 5 nm; response time, 6 seconds; data interval, 0.5 seconds). Divalent metals (20 μM final concentration of Fe2+ or Co2+) were added to the cell suspension after allowing fluorescence to stabilize for 60 seconds. For assays using iron, a combination of membrane-permeable (SIH) and membrane-impermeable (HES-DFO) iron chelators were used at various time points (Figure 5A) to distinguish intracellular from cell-associated quenchable fluorescence: After fluorescence was allowed to stabilize for 60 seconds, 20 μM Fe2+ was added to the cell suspension (Figure 5A, arrow 1). After 240 seconds, the membrane-impermeable iron chelator HES-DFO was added (Figure 5A, arrow 2) to release metal-induced quenching of extracellular cell-associated complexed calcein. At 300 seconds, a membrane-permeable iron chelator SIH was added (Figure 5A, arrow 3) to release metal-induced quenching of intracellular calcein fluorescence. Initial rates were calculated from quenching curves, and the size of the intracellular labile iron pool was extracted from data obtained with the 2 metal chelators.
Cell surface protein biotinylation
CHO Nramp2 transfectants were washed thoroughly with cold phosphate-buffered saline (PBS) (supplemented with 1 mM MgCl2, 0.1 mM CaCl2) and then with cold borate buffer (10 mM boric acid, 154 mM NaCl, 7.2 mM KCl, 1.8 mM CaCl2, pH 9.0). Cells were labeled for 15 minutes on ice with 0.5 mg/mL Sulfo-NHS-SS-Biotin (sulfosuccinimidyl-2-(biotinamido) ethyl-1,3-dithiopropionate) (Pierce, Rockford, IL) in cold borate buffer. After washing 3 times with cold quenching buffer (PBS, 200 mM glycine), cells were scraped, collected, and resuspended in 1 mL lysis buffer (1% Triton X-100, 150 mM NaCl, 2 mM EDTA, 10 mM Tris-Cl [pH 7.4], 30% glycerol) plus protease inhibitors. Lysates were incubated on ice for 20 minutes and pelleted (10 minutes, 10 000g, 4°C). Supernatants were collected, and protein levels were quantified by Bradford assay. A total of 500 μg total protein lysate was incubated overnight at 4°C with 50 μL immobilized streptavidin beads (Pierce) in a final volume of 500 μL (with lysis buffer and protease inhibitors). Streptavidin beads were washed 3 times with lysis buffer and then once with PBS. Labeled cell surface proteins were eluted with 50 μL 1 × sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer and separated by SDS-PAGE.
Results
Mutagenesis strategy
Alignment of 28 eukaryotic and prokaryotic Nramp sequences (supplement on Blood website; see the Supplemental Document link at the top of the online article) identify a common and conserved hydrophobic core of 10 TM domains (about 30% identity between bacteria and humans)6 that contain 4 absolutely invariant (Asp86, Glu154, Glu299, Arg416) and 5 highly conserved (Arg119, Arg146, Asp161, Asp192, Glu225) charged residues (6 negative, 3 positive) in TM domains (Figure 1, dark blue residues). Another unique feature of the Nramp family is the presence of 2 invariant histidine residues (His267, His272) in the predicted TM domain 6 (Figure 1, red residues). In mice, naturally occurring mutations at adjacent residues in TM4 of Nramp1 andNramp2 cause susceptibility to infection and microcytic anemia, respectively, and the loss-of-function phenotype of the microcytic anemia Nramp2 variant (Gly185Arg) was studied. Mutant cDNAs were expressed in yeast S cerevisiae and tested for their ability to complement 2 phenotypes of an smf1/smf2mutant: (a) impaired growth on metal chelators31 and (b) impaired growth at alkaline pH.32 Mutants showing partial or complete loss of function were subsequently expressed in CHO cells and tested for Fe2+ and Co2+ transport at the plasma membrane.13
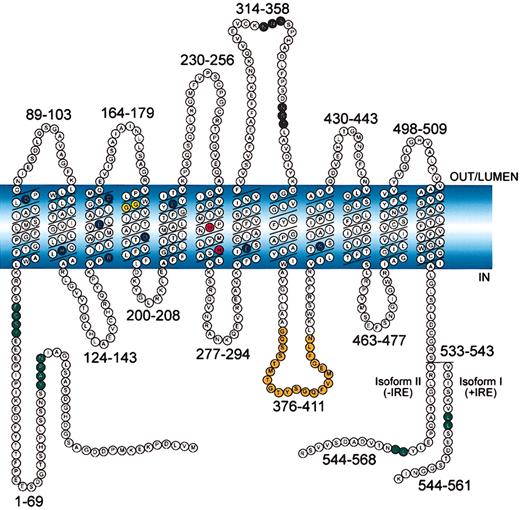
Schematic representation of mouse Nramp2 (DMT1) isoform II (−IRE) and isoform I (+IRE).
The 12 transmembrane domains are predicted from hydropathy profiling, calculations of hydrophobic moment, and other computer-assisted analyses6 and from direct epitope mapping studies.13 Individual predicted intracellular and extracellular segments are identified, and their position within the primary sequence is shown. Amino acid residues defining sequence landmarks and signature motifs are depicted in different colors, including negatively and positively charged residues within predicted TM domains (dark blue), conserved histidine residues in TM6 (red), glycine residues in TM4 altered in anemicmk/Belgrade mutants (Gly185Arg), and mutated (inNramp1) in mice susceptible to infections (Gly184Asp) (yellow). Also identified are Asn-linked glycosylation signals in the TM7-TM8 extracytoplasmic loop (black), predicted membrane targeting/sorting motifs (tyrosine-based and dileucine) (green), and consensus transport signature common to Nramp orthologs and present in the cytoplasmic face of membrane anchors of bacterial periplasmic permeases (orange). The 2 different C-termini of the protein generated by alternative mRNA splicing containing or not an iron-response element (isoform I, +IRE; isoform II, −IRE) in the 3′ untranslated region are identified, with corresponding numbering. Finally, the polarity of the protein and membrane domains with respect to the membrane (light blue) is indicated (in, out, lumen).
Schematic representation of mouse Nramp2 (DMT1) isoform II (−IRE) and isoform I (+IRE).
The 12 transmembrane domains are predicted from hydropathy profiling, calculations of hydrophobic moment, and other computer-assisted analyses6 and from direct epitope mapping studies.13 Individual predicted intracellular and extracellular segments are identified, and their position within the primary sequence is shown. Amino acid residues defining sequence landmarks and signature motifs are depicted in different colors, including negatively and positively charged residues within predicted TM domains (dark blue), conserved histidine residues in TM6 (red), glycine residues in TM4 altered in anemicmk/Belgrade mutants (Gly185Arg), and mutated (inNramp1) in mice susceptible to infections (Gly184Asp) (yellow). Also identified are Asn-linked glycosylation signals in the TM7-TM8 extracytoplasmic loop (black), predicted membrane targeting/sorting motifs (tyrosine-based and dileucine) (green), and consensus transport signature common to Nramp orthologs and present in the cytoplasmic face of membrane anchors of bacterial periplasmic permeases (orange). The 2 different C-termini of the protein generated by alternative mRNA splicing containing or not an iron-response element (isoform I, +IRE; isoform II, −IRE) in the 3′ untranslated region are identified, with corresponding numbering. Finally, the polarity of the protein and membrane domains with respect to the membrane (light blue) is indicated (in, out, lumen).
Conserved charged residues in membrane domains
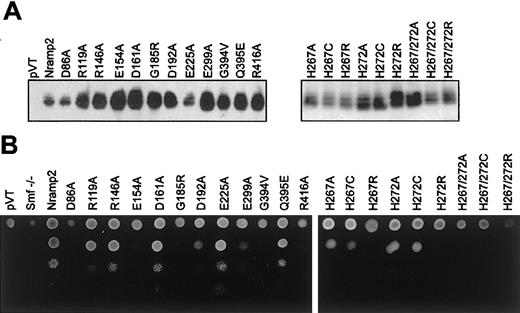
Immunoblotting indicated that all mutants could be stably expressed as 60- to 65-kDa immunoreactive proteins in yeast membrane fractions (Figure 2A, left panel). These results suggest that none of the mutations had a major effect on protein expression or stability in yeast. The ability of each mutant to complement the null smf1/smf2 yeast mutant was tested in parallel by plating serial dilutions of each transformant on YPD agar, pH 7.9 (Figure 2B, left panel), and using a growth inhibition assay in liquid YPD (pH 7.9). Routinely, yeast wild-type (WT)Nramp2 transformants showed a 9-fold stimulation for growth on alkaline medium over negative controls (pVT, smf−/−;Figure 3, left panel). TwoNramp2 mutants previously shown to either abrogate (Gln394Val) or to have no effect (Gln395Glu) on Nramp2 function in yeast32 were used as additional controls (Figure 3, left panel).
Nramp2 protein expression in
smf1/smf2 mutant yeast and functional complementation of growth at alkaline pH. (A) Crude membrane fractions fromsmf1/smf2 yeast cells (pVT) expressing either wild-type (Nramp2) or individual mutant variants of Nramp2 (indicated on top) were separated by SDS-polyacrylamide gel electrophoresis. Immunoblotting was performed using an affinity-purified rabbit antimouse polyclonal anti-Nramp2 antibody. Apparent electrophoretic mobility of the immunoreactive species is consistent with a molecular mass of 60 to 65 kDa. (B) Functional complementation of the growth defect of the smf1/smf2 mutant was tested on solid YPD agar adjusted at alkaline pH (pH 7.9). Serial 10-fold dilutions of cultures corresponding to individual Nramp2 mutants (identified) were spotted (from top to bottom) on YPD agar plates (pH 7.9), followed by incubation for 48 hours at 30°C and photography.
Nramp2 protein expression in
smf1/smf2 mutant yeast and functional complementation of growth at alkaline pH. (A) Crude membrane fractions fromsmf1/smf2 yeast cells (pVT) expressing either wild-type (Nramp2) or individual mutant variants of Nramp2 (indicated on top) were separated by SDS-polyacrylamide gel electrophoresis. Immunoblotting was performed using an affinity-purified rabbit antimouse polyclonal anti-Nramp2 antibody. Apparent electrophoretic mobility of the immunoreactive species is consistent with a molecular mass of 60 to 65 kDa. (B) Functional complementation of the growth defect of the smf1/smf2 mutant was tested on solid YPD agar adjusted at alkaline pH (pH 7.9). Serial 10-fold dilutions of cultures corresponding to individual Nramp2 mutants (identified) were spotted (from top to bottom) on YPD agar plates (pH 7.9), followed by incubation for 48 hours at 30°C and photography.
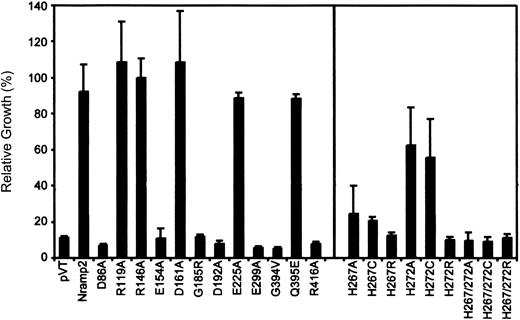
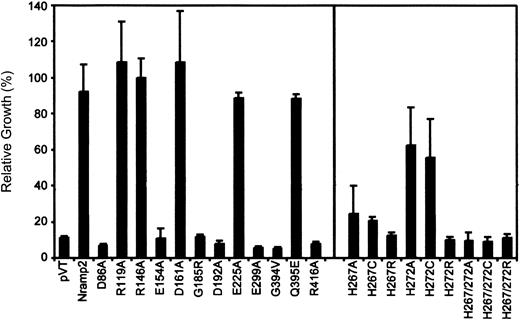
Quantitation of complementation of the
smf1/smf2 yeast mutant by Nramp2 variants. Individual Nramp2 mutants (identified) together with positive (Nramp2) and negative (pVT) controls were grown to saturation and diluted to OD595 = 0.02 in either YPD or YPD pH 7.9. Cells were seeded in 96-well plates, and growth was measured after 24 hours' incubation at 30°C by measuring OD595. Results represent relative growth (expressed as a percentage) of individual mutant grown at pH 7.9 versus normal YPD. Error bars represent standard deviations of the means.
Quantitation of complementation of the
smf1/smf2 yeast mutant by Nramp2 variants. Individual Nramp2 mutants (identified) together with positive (Nramp2) and negative (pVT) controls were grown to saturation and diluted to OD595 = 0.02 in either YPD or YPD pH 7.9. Cells were seeded in 96-well plates, and growth was measured after 24 hours' incubation at 30°C by measuring OD595. Results represent relative growth (expressed as a percentage) of individual mutant grown at pH 7.9 versus normal YPD. Error bars represent standard deviations of the means.
In both assays, mutants Arg119Ala, Arg146Ala, Asp161Ala, and Glu225Ala showed full complementation of the smf1/smf2 growth defect, while mutants Asp86Ala, Glu154Ala, Asp192Ala, Glu299Ala, and Arg416Ala failed to do so. In addition, testing these 11 mutants for their ability to complement susceptibility of smf1/smf2 mutant to metal chelator (growth in EGTA [ethylenebis (oxyethylenenitrilo)–tetraacetic acid]–containing liquid medium) showed relative activities similar to that seen for growth at alkaline pH (data not shown). These results identify 5 of the 9 charged residues in TM domains as essential for Nramp2 function and smf1/smf2complementation in yeast.
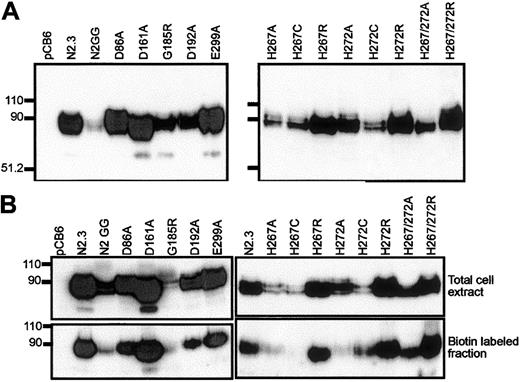
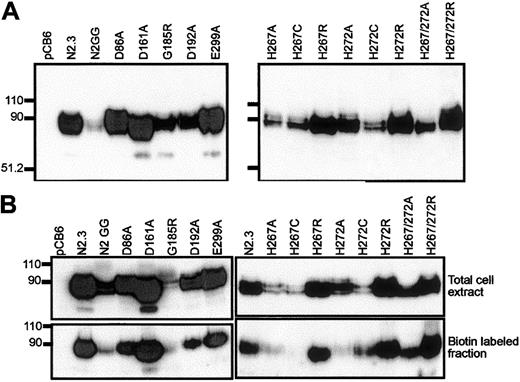
The Asp86Ala, Glu154Ala, Asp161Ala, Asp192Ala, Glu299Ala, and Arg416Ala mutants were analyzed for metal transport after transfection in CHO cells (Figure 4A, left panel). Transfectants were screened by immunoblotting for expression of the corresponding Nramp2 variants at the cell membrane. Membranes from cells transfected with the pCB6 empty vector were used as negative controls, while 2 previously characterized transfectants13expressing either low (N2GG) or high (N2.3) levels of Nramp2 protein were used as positive controls. CHO cell clones stably expressing mutants Glu154Ala and Arg416Ala could not be obtained in 4 independent transfections (141 clones screened) for Glu154Ala and 3 independent transfections (75 clones screened) for Arg416Ala, suggesting possible effect of these mutations on protein folding/processing and/or toxicity for the cells. Stable CHO transfectants expressing Asp86Ala, Asp192Ala, and Glu299Ala could be readily isolated. In these clones the level of expression varied but was in the range of levels seen in positive controls expressing WT (N2.3, N2GG) or Asp161Ala proteins (Figure 4A, left panel). In all cases, variability in the level of expression of individual mutants (including those that could not be expressed) can be attributed to a combination of the site of integration of the vector in the host genome and the effect of the introduced mutation on cell growth and/or survival. In addition, biotin labeling experiments in intact cells identified cell surface reactivity of all expressed mutants (Asp86Ala, Asp161Ala, Asp192Ala, Glu299Ala), with levels approximately proportionate to total expression levels detected in membrane fractions by immunoblotting (Figure 4B, left panel). These results indicate that mutations at Asp86, Asp161, Asp192, and Glu299 do not have a major effect on Nramp2 protein maturation or membrane targeting.
Expression of Nramp2 variants in stably transfected CHO cells.
(Left panels) Mutants at conserved charged residues in TM domains; (right panels) multiple mutants at the conserved histidines in TM6. Crude membrane fractions (A) or total cell lysates (B) were prepared from various transfected cell clones (identified) and separated by SDS-polyacrylamide gel electrophoresis. Immunoblotting was performed using a mouse monoclonal anti-cMyc antibody directed against a corresponding epitope tag inserted in-frame in the Nramp2constructs. Controls include cells transfected with the plasmid alone (pCB6) and cells expressing low (N2GG) or high amounts (N2.3) of wild-type Nramp2.13 Molecular mass markers are identified in kilodaltons to the left of the immunoblots. (B) Prior to electrophoresis, intact cells were labeled with biotin and disrupted with lysis buffer (upper panels). Labeled cell surface proteins were then isolated with strepavidin beads (lower panels).
Expression of Nramp2 variants in stably transfected CHO cells.
(Left panels) Mutants at conserved charged residues in TM domains; (right panels) multiple mutants at the conserved histidines in TM6. Crude membrane fractions (A) or total cell lysates (B) were prepared from various transfected cell clones (identified) and separated by SDS-polyacrylamide gel electrophoresis. Immunoblotting was performed using a mouse monoclonal anti-cMyc antibody directed against a corresponding epitope tag inserted in-frame in the Nramp2constructs. Controls include cells transfected with the plasmid alone (pCB6) and cells expressing low (N2GG) or high amounts (N2.3) of wild-type Nramp2.13 Molecular mass markers are identified in kilodaltons to the left of the immunoblots. (B) Prior to electrophoresis, intact cells were labeled with biotin and disrupted with lysis buffer (upper panels). Labeled cell surface proteins were then isolated with strepavidin beads (lower panels).
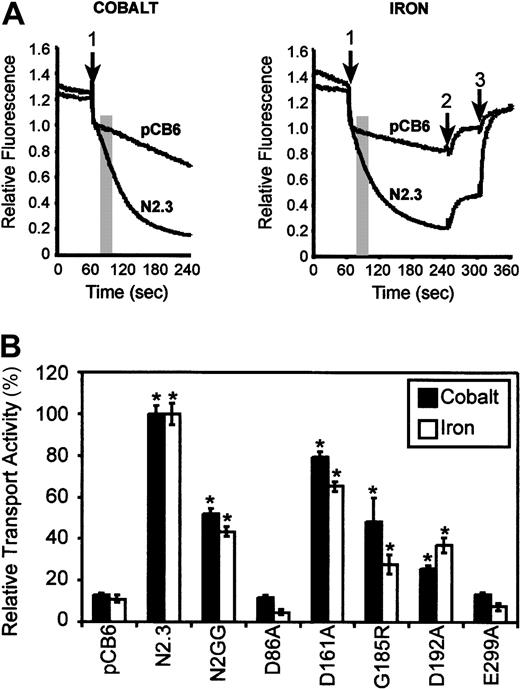
Transport properties of the mutants were investigated in intact cells using a calcein-quenching assay.13 Metal transport by Nramp2 using the calcein-quenching assay and transport of isotopic55Fe have been shown to be comparable in the identical control Nramp2 CHO transfectants13 as well as some Nramp2 mutant variants (data not shown). For the calcein-quenching assay, CHO transfectants were loaded with the metal-sensitive fluorescent dye calcein, and the effect of externally added divalent cations Fe2+ or Co2+ on the rate of quenching of fluorescence was monitored (at optimal pH 6.0), and the slope of the initial quenching curve was calculated (initial rate13). Results shown in Figure 5A show a typical set of fluorescence quenching traces for positive and negative controls. Transport activities of mutants are shown in Figure 5B and are expressed as a relative transport activity (%). Mutants were classified as having either low (less than 33% of WT), intermediate (between 34% and 67%), or WT activity (above 67%). Expression of WT Nramp2 in clones N2.3 and N2GG caused a 10-fold and 5-fold stimulation of calcein quenching and hence Co2+ and Fe2+transport, respectively, compared with controls. Likewise, cells expressing mutant Asp161Ala showed transport rates similar to WT (Figure 5B). On the other hand, mutant Asp192Ala in TM4 showed intermediate transport activity when compared with WT for both Fe2+ and Co2+. Finally, mutants Asp86Ala and Glu299Ala were completely inactive for the 2 substrates tested. These results identify residues Asp86 and Glu299 in corresponding TM1 and TM7 as playing key roles in metal transport by Nramp2.
Relative Fe2+ and Co2+ transport activity of Nramp2 mutations affecting conserved charged residues in TM domains.
(A) Control CHO cells (pCB6) as well as CHO transfectants expressing wild-type Nramp2 (N2.3) were loaded with the metal-sensitive fluorescent dye calcein (introduced as 0.250 μM calcein-am) during 10 minutes at 37°C. Cells were washed, resuspended in 500 μL transport buffer (pH 6.0), and fluorescence recorded with an LS-50B fluorescence spectrometer. When fluorescence stabilized, divalent metals (20 μM final concentration of Fe2+ or Co2+) were added to the cell suspension (arrow 1), and fluorescence was continuously monitored for an additional 3 minutes. For iron transport studies (right panel), the membrane-impermeable iron chelator HES-DFO (200 μM) was added at 4 minutes (arrow 2) to reveal cell-associated extracellular Fe-calcein complexes. At 5 minutes, the membrane-permeant iron chelator SIH (250 μM) was added (arrow 3) and revealed intracellular Fe-calcein complexes (see “Materials and methods”). The transport activity of Nramp2 was measured as an initial rate (slope) during the early portion of the calcein-quenching curve and is shown as a shaded area (slope interval). (B) Relative transport activities of wild-type (N2.3, N2GG) and mutant Nramp2 variants (identified) were calculated from the initial slope of calcein-quenching curves as shown in panel A and are expressed as a relative transport activity with transport in the positive control N2.3 set at 100%. Error bars represent standard errors on the means of 3 or more independent experiments. *Transport activities significantly different (ie, more than 2 standard deviations) from those detected in the negative control, vector-transfected (pCB6) cells.
Relative Fe2+ and Co2+ transport activity of Nramp2 mutations affecting conserved charged residues in TM domains.
(A) Control CHO cells (pCB6) as well as CHO transfectants expressing wild-type Nramp2 (N2.3) were loaded with the metal-sensitive fluorescent dye calcein (introduced as 0.250 μM calcein-am) during 10 minutes at 37°C. Cells were washed, resuspended in 500 μL transport buffer (pH 6.0), and fluorescence recorded with an LS-50B fluorescence spectrometer. When fluorescence stabilized, divalent metals (20 μM final concentration of Fe2+ or Co2+) were added to the cell suspension (arrow 1), and fluorescence was continuously monitored for an additional 3 minutes. For iron transport studies (right panel), the membrane-impermeable iron chelator HES-DFO (200 μM) was added at 4 minutes (arrow 2) to reveal cell-associated extracellular Fe-calcein complexes. At 5 minutes, the membrane-permeant iron chelator SIH (250 μM) was added (arrow 3) and revealed intracellular Fe-calcein complexes (see “Materials and methods”). The transport activity of Nramp2 was measured as an initial rate (slope) during the early portion of the calcein-quenching curve and is shown as a shaded area (slope interval). (B) Relative transport activities of wild-type (N2.3, N2GG) and mutant Nramp2 variants (identified) were calculated from the initial slope of calcein-quenching curves as shown in panel A and are expressed as a relative transport activity with transport in the positive control N2.3 set at 100%. Error bars represent standard errors on the means of 3 or more independent experiments. *Transport activities significantly different (ie, more than 2 standard deviations) from those detected in the negative control, vector-transfected (pCB6) cells.
Gly185Arg mutation of mk mice
A naturally occurring mutation at Gly185 in TM4 (Gly185Arg) is associated with severe iron deficiency and microcytic anemia of themk mouse2 and of the Belgraderat.3 When expressed in the yeast smf1/smf2mutant, the Gly185Arg variant of Nramp2 can be expressed in the membrane fraction of these cells at levels similar to those seen for either the WT protein and for the other mutants (Figure 2A). Complementation studies indicated that Gly185Arg could not restore growth in alkaline medium (Figure 3) or in medium containing metal chelators (data not shown). These results suggest that in yeast cells, Gly185Arg is either transport incompetent or is active but mistargeted to an inappropriate transport site. CHO transfectants expressing robust levels of Gly185Arg mutant could be readily isolated (Figure 4). Interestingly, transport experiments in these cells (Figure 5B) indicated that the Gly185Arg mutant retains significant and intermediate transport activity, with 50% (Co2+) and 30% activity (Fe2+) of the WT protein. These results in CHO cells clearly suggest that the Gly185Arg mutation only attenuates but does not eliminate transport function of Nramp2 protein in mammalian cells.
Conserved histidine pair in TM6
Histidine pairs can form metal binding sites in soluble38 or in membrane proteins.39,40 The highly conserved histidine pair found in TM6 (His267, His272) of the Nramp superfamily was initially studied in Nramp2 by mutagenesis to the small neutral residue alanine in mutants His267Ala and His272Ala and in the double mutant His267Ala/His272Ala. Metal binding by His residues involves donating imidazole nitrogen lone-pair electrons to the unfilled orbitals of the metal, and cysteines can substitute for His in this process.38 41-43 Thus, mutants His267Cys, His272Cys, and His267Cys/His272Cys were created. The imidazole proton of His has a pKa of 6.6 and can mediate pH-dependent effects in proteins. Thus, a last set of mutants was created in which His was replaced by the positively charged Arg (pKa about 12), which may functionally mimic the protonated His, albeit with a much larger bulk. Mutants were transformed in yeast cells, and immunoblotting analyses show that all 9 single and double mutants could be stably expressed in the membrane fraction of the smf1/smf2 mutant (Figure 2A, right panel). Complementation studies for growth at alkaline pH (Figure3) and in the presence of metal chelators (data not shown) indicate that His267 is highly mutation sensitive, with replacements to Ala, Cys, and Arg causing severe or complete loss of function. His272 was less mutation sensitive than His267, with His272Ala and His272Cys retaining near WT activity, and only substitution to the bulkier Arg (His272Arg) abrogated complementation. Finally, the 3 double mutants (His267Ala/His272Ala, His267Cys/His272Cys, His267Arg/His272Arg) were completely inactive in yeast.
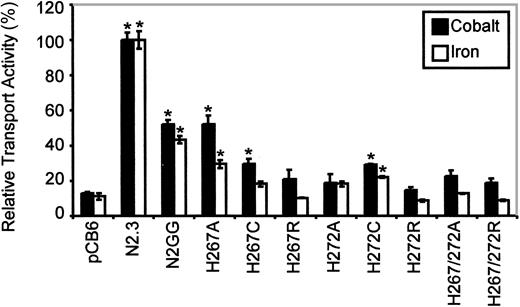
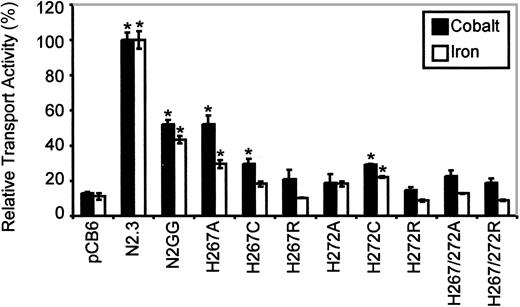
Immunoblotting analysis (Figure 4A, right panel) revealed that all mutants could be stably expressed after transfection in CHO cells, with the exception of the double His267/His272Cys mutant (3 independent transfections, 119 clones screened). The other 8 Nramp2mutants were expressed at varying levels but generally fell between those seen in the positive controls expressing low (N2GG) and high (N2.3) amounts of Nramp2. Biotin labeling experiments in intact cells identified cell surface reactivity of all expressed mutants, with levels approximately proportionate to total expression levels detected in membrane fractions by immunoblotting (Figure 4B, right panel). However, reduced trafficking of protein to the plasma membrane may have occurred in His267Cys and His272Ala. Metal transport activity of the mutants was tested in the calcein-quenching assay for Fe2+and Co2+ at pH 6.0. Mutants bearing either single mutations to Arg at either position (His267Arg, His272Arg) or double mutations (His267A/His272Ala, His267Arg/His272Arg) were completely inactive for transport (Figure 6). Likewise, mutant His272Ala was also inactive in these conditions, and only low transport activity for Co2+ was detected for His267Cys (Figure 6). Reduced trafficking to the plasma membrane (Figure 4B) may have contributed to reduced activity of His267Cys and His272Ala. Only His267Ala and His272Cys retained modest but significant transport activity toward both metal cations analyzed in the assay, with His267Ala possibly having a higher selectivity for cobalt. These results indicate that His267 and His272 play an important role in Nramp2 metal transport, both in yeast and mammalian cells.
Relative Fe2+ and Co2+ transport activity of Nramp2 mutations affecting conserved histidine residues in TM6.
Transport studies were conducted at pH 6.5. Results are presented for different mutants at His267 and His272, as described in the legend to Figure 5. Error bars represent standard errors on the means of 3 or more independent experiments.
Relative Fe2+ and Co2+ transport activity of Nramp2 mutations affecting conserved histidine residues in TM6.
Transport studies were conducted at pH 6.5. Results are presented for different mutants at His267 and His272, as described in the legend to Figure 5. Error bars represent standard errors on the means of 3 or more independent experiments.
Possible change in ion selectivity for the His267Ala mutant was investigated further through radioisotopic 55Fe transport measurements. Competition experiments with cold iron and cobalt failed to reveal a significant preference for cobalt in His267Ala compared with the WT Nramp2 (data not shown). Therefore, the change in substrate selectivity in His267Ala suggested by the calcein-quenching assay, while statistically significant, could not be validated by more direct measurements with radioisotopic 55Fe.
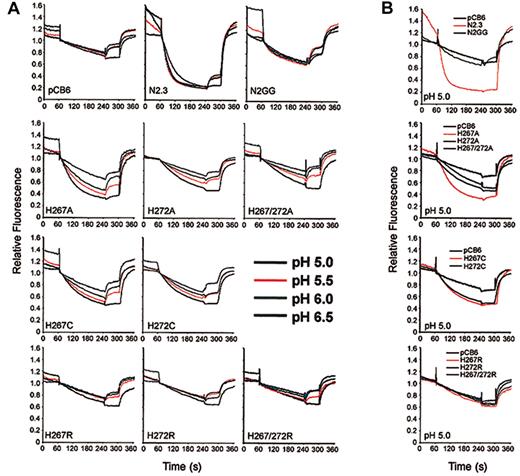
Possible pH effects on transport activity of the mutants were investigated (Figure 7). Fe2+(Figure 7A, top row) and Co2+ (data not shown) transport by Nramp2 in clones N2.3 and N2GG approached maximum at pH of about 6.0, and decreasing the pH to 5.0 had little effect on transport. Likewise, background uptake of metal in negative controls was not affected by lowering pH. However, the low level of transport activity detected at pH 6.0 in mutants His267Ala, His267Cys, and His272Cys (Figure 6) could be significantly enhanced by progressively lowering pH (Figure 7). This increase occurred gradually over background, pH-insensitive levels detected in control pCB6 cells. Surprisingly, mutants 272Ala and 267Ala/272Ala, which showed complete loss of function at pH 6.0 (Figure6), could be rescued by lowering the pH. Interestingly, however, lowering the pH of the extracellular medium had no effect on Co2+ (not shown) or Fe2+ transport (Figure 7B) of any of the histidine-to-arginine mutants (His267Arg, His272Arg, His267/272Arg). The pH effect seen in the Cys/Ala mutants was specific for the conserved His pair of TM6 and was not observed in any of the other inactivating mutations studied (eg, Asp86Ala, Glu299Ala, or Gly185Arg) (data not shown). Therefore, mutations at the 2 conserved histidines in TMD6 clearly affect pH sensitivity of Nramp2 transport and suggest that these residues may be involved in pH regulation of transport.
Effect of pH on transport activity of Nramp2 mutants at conserved histidine residues in TM6.
(A) Fe2+ transport in the various negative (pCB6) and positive controls (N2.3, N2GG) as well as in CHO clones expressingNramp2 mutants at the plasma membrane (His267Ala, His272Ala, His267/272Ala, His267Cys, His272Cys, His267Arg, His272Arg, His267/272Arg) was studied by the calcein-quenching assay as described in the legend to Figure 5A, with the following modifications. After loading with calcein-am at pH 7.4, cells were washed and resuspended in transport buffer at different pH (indicated). Fluorescence was allowed to stabilize for 1 minute, followed by addition of 20 μM Fe2+. (B) Summary of transport results obtained for the histidine mutants at pH 5.0.
Effect of pH on transport activity of Nramp2 mutants at conserved histidine residues in TM6.
(A) Fe2+ transport in the various negative (pCB6) and positive controls (N2.3, N2GG) as well as in CHO clones expressingNramp2 mutants at the plasma membrane (His267Ala, His272Ala, His267/272Ala, His267Cys, His272Cys, His267Arg, His272Arg, His267/272Arg) was studied by the calcein-quenching assay as described in the legend to Figure 5A, with the following modifications. After loading with calcein-am at pH 7.4, cells were washed and resuspended in transport buffer at different pH (indicated). Fluorescence was allowed to stabilize for 1 minute, followed by addition of 20 μM Fe2+. (B) Summary of transport results obtained for the histidine mutants at pH 5.0.
Discussion
We aimed to study the functional role of highly conserved charged residues in the TM domains of Nramp proteins on substrate transport and pH regulation. Such residues were mutated in the backbone of Nramp2, and corresponding mutants were tested in yeast and mammalian cells. With few exceptions, there was good general agreement betweensmf1/smf2 complementation results in yeast and transport data in mammalian cells. Interestingly, although all mutants could be expressed in the membrane fractions of yeast cells (Figure 2A), several could not be expressed in CHO cell membranes. These results suggest that such TM mutations (1) affected normal protein folding, maturation, and processing, possibly leading to protein instability and/or degradation, or (2) that overexpression of the corresponding mutant protein was somehow toxic for the cells. These results highlight possible differences in protein sorting, maturation, and targeting mechanisms in both cell systems but also stress the importance of maturation and membrane targeting for proper Nramp2 function.
Gly185 is invariant in all Nramp orthologs from bacteria to humans. Strikingly, its mutation to Arg arose independently in 2 rodent models of iron deficiency, the microcytic anemia mousemk 2 and the anemic Belgrade (b)rat.3 The Gly185Arg mutation showed a complex phenotype. In yeast, Gly185Arg could be expressed in yeast membranes but could not complement the smf1/smf2 mutant, suggesting a loss of transport activity, in agreement with results from Su et al16 and from Worthington et al,44 who reported a 95% and 85% reduction in 55Fe2+transport activity of Gly185Arg in HEK293 cells and COS-7 cells, respectively. Gly185Arg could also be expressed in the membrane fraction of CHO cells (Figure 4), where it retained significant transport activity for both Co2+ (50% of WT) and Fe2+ (35% of WT). Similar transport activity of Gly185Arg has also been observed in stable transfectants in the LLC-PK1 porcine kidney cells (data not shown). This significant transport activity suggests that loss of transport function may not be the sole defect responsible for the mk phenotype in vivo. Rather, the mutation may affect membrane targeting in a cell-specific fashion, perhaps including targeting to a transport-incompetent compartment in yeast cells. Recent studies in vivo support such a tissue or cell-specific effect. Indeed, immunoblotting studies show robust expression of the Gly185Arg isoform in duodenum membrane fractions ofmk/mk mice, but immunohistochemistry studies revealed absence of protein targeting to the brush border, the site of active transport.17 Likewise, mk/mk mice show a strong reduction of Nramp2 (Gly185Arg) protein expression in the kidney,12 while mk/mk reticulocytes are completely devoid of Nramp2 expression.10 Parallel analysis of the disease susceptibility Gly169Asp mutation in TM4 ofNramp1 (reconstructed in Nramp2; data not shown) associated with susceptibility to infections also indicated absence ofsmf1/smf2 complementation in yeast. Also, we could not isolate CHO clones stably expressing this mutant, a situation similar to that seen in vivo in macrophages fromNramp1Gly169Asp mouse strains, where no mature protein is detected.45 Together, these results suggest an important role for this residue and TM segment for transport activity but also suggest that its integrity is required for proper maturation, folding, and/or targeting of the Nramp proteins.
Helical wheel projections in the conserved hydrophobic core of Nramp proteins reveal strong amphipathic character for several TM domains, including TM 3, 5, and 9.6 Sequence conservation expressed as a variability moment6 indicates periodicity with strong conservation of the polar face, with the apolar “lipid-accessible” face of the helix being heavily substituted. Several highly charged residues map to the polar side of TM helices. Such an arrangement is characteristic of families of ion transporters and ion channels.6 Mutations at the 9 conserved charges in TM domains had either no effect (Arg119, Arg146, Asp161, Glu225) or caused partial (Asp192) or complete loss of function (Asp86, Glu154, Glu299, Arg416). Asp86, Glu154, Glu299, and Arg416 are the most highly conserved, being invariant in multiple sequence alignments performed (supplemental document). It is striking that 3 of them have negatively charged side chains, raising the possibility that they may mediate interaction with the positively charged divalent cation substrates of Nramp transporters. Alternatively, such residues may be involved in hydrogen bonding, salt bridge formation (dipole), or other interactions in the formation of a water-filled pore or transport path.
The absolute conservation of the histidine pair His267/His272 in TM6 in eukaryotic and prokaryotic Nramp sequences suggests an important role. In addition, studies by us (data not shown) and others44show that Nramp2-mediated transport in transfected cells is sensitive to the action of the histidine-specific reagent diethyl pyrocarbonate (DEPC). Here, we show that both residues are mutation sensitive (in particular, His267), with independent substitutions at either or both residues causing loss of function. Strikingly, several poorly active (His267Ala, His267Cys, His272Cys) or completely inactive His mutants (His272Ala, His272Cys, His267Ala/His272Ala) at pH 6.0 could be rescued by lowering the pH of the transport assay (Figure 7). The observed pH effect was incremental, with maximal transport attained at or below pH 5.0. However, completely inactive histidine-to-arginine mutants (His267Arg, His272Arg, His267/272Arg) could not be rescued by lowering the pH of the transport assay. This may be a result of increased steric hindrance associated with replacement of histidine to bulky arginine. Thus, mutations at either His residues in TM6 shifted the pH required to achieve maximal transport to a more acidic value.
Several explanations can be put forward to account for the unique effect of pH on transport properties of the Nramp2 His mutants. First, His267 and His272 may be involved in direct binding of the metal substrate in a pH-dependent fashion. Metal binding by His pairs has been documented in soluble proteins such as transcription factors38 and has also been used in membrane proteins where they have been engineered to study proximity relationships between individual TM domains by electroparamagnetic resonance.46 Although His267 and His272 may indeed form part of a binding site for metals in the membrane portion of Nramp2, it appears unlikely. Indeed, an Nramp2 mutant lacking both histidines (His267Ala/His272Ala) is still transport-active at pH 5.0 (Figure 7B). A second possibility is that His267, His272, or both participate in H+ movement across the membrane in a H+ cotransport mechanism possibly by a proton relay system.1 Such a relay system has been described for the lactose permease of Escherichia coli and involves TM residues Arg302/His322/Glu325.47 Such a proton relay system may exist in Nramp2 and may involve conserved residues such as His267/His272 as well as other negatively and positively charged residues in TM domains. Partial or complete inactivation of this system would be predicted to have a major effect on the pH dependence of transport. A third explanation for the observed pH effect on transport properties of single or double His mutants is that His267/His272 may be implicated in pH regulation of the transporter through gain or loss of the imidazole proton (pKa 5.5 to 6.5). In this favored model, protonation of His267/His272 would be required to maintain the protein in a functional, transport-competent conformation. This effect could be either general and involve additional residues, resulting in a pH-dependent global conformational change from an inactive state (neutral pH) to an active state (acidic pH). In this model, loss of the key His residues would shift the pH for maximal transport to a more acidic value, requiring protonation of other groups or side chains to create the same overall conformational change. Alternatively, either or both His267/His272 could play a more specific role in creating a pH-dependent transport path in the transporter. For example, an interaction with adjacent and highly conserved negatively charged residues in other TM domains could be necessary to open an ion transport path. Loss of His267/His272 would require formation of compensatory interactions of conserved negatively charged residues with other protonated side chains and/or water molecules in the transport path. Although highly speculative, such a mechanism appears to account for the pH dependence of anion transport by the band 3 transporter.48 49
The authors are indebted to Drs S. Grinstein (University of Toronto) and H. R. Kaback (University of California, Los Angeles) for helpful discussions and suggestions during this work and to Dr P. Ponka (McGill University, Montreal) for the generous preparation and gift of iron chelators.
Supported by a research grant from the National Institute of Allergy and Infectious Diseases (RO1 AI35237-08) (P.G.), a studentship from the Canadian Insitutes of Health Research (S.L.), and a Distinguished Scientist salary award from the Canadian Institutes of Health Research (P.G.).
Submitted July 15, 2002; accepted December 19, 2002. Prepublished online as Blood First Edition Paper, January 9, 2003; DOI 10.1182/blood-2002-07-2108.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Philippe Gros, Professor, Department of Biochemistry, McGill University, 3655 Sir William Osler Promenade, Montreal, QC, Canada, H3G-1Y6; e-mail:gros@med.mcgill.ca.