Salzer et al, from the University of Vienna, have recently described that vesicles released from Ca++/Ca++ionophore-treated erythrocytes are enriched in lipids and proteins that are typically found within lipid microdomains of the parent cell's plasma membrane, the so-called “lipid rafts.”1 The microvesicles shed from Ca++-loaded erythrocytes, processed for the separation of lipid domains, appear to contain the conventional markers of lipid rafts, cholesterol and ganglioside GM1.1 The novelty of the data is in the large number of membrane-associated proteins, most of which were previously undetected, found by Salzer et al in the human erythrocytes: stomatin, flotillin-1, flotillin-2, synexin, and sorcin. These proteins appear to be associated with lipid rafts, and, most important, some of them are enriched in the vesicles, relative to the parent cell's membrane. They share this property with the family of exofacial proteins inserted in the membrane via a glycosyl phosphatidylinositol (GPI) anchor. In erythrocytes, the most famous member of this family is acetylcholinesterase (AChE), whose enrichment in Ca++-dependent vesicles was known well before the structure of the GPI anchor was elucidated.2 3
If one takes the amount of membrane phospholipids as a measure of membrane surface extension (as is common and correct practice) and then normalizes the amount of AChE, as enzymatic activity, over membrane phospholipids, a 3- to 4-fold enrichment of AChE in microvesicles, with respect to erythrocytes, is observed. This figure, supported by several independent reports in the past,3 was easily confirmed in our lab. For reasons that were not explicitly stated in their paper, and that therefore remain obscure to the reader, Salzer et al chose to normalize AChE activity to hemoglobin content of vesicles and cells, and were able to calculate a different figure. Thus, the amount of AChE in vesicles, when referred to hemoglobin, is “roughly 80 times” the amount in the parent cells.1 This figure may seem confusing for a reader more accustomed to the old notion of a 3- to 4-fold enrichment. However, we must say that this result is correct. A 185-nm spherical vesicle2 has a volume of approximately 0.0033 μm3, and a surface area of approximately 0.108 μm2. The surface-to-volume ratio (S/V) is therefore approximately 33 μm−1. The measured S/V of erythrocytes4 (total population of cells) is approximately 1.59 μm−1. Thus, the S/V in vesicles is roughly 20 times the S/V in erythrocytes. A 3- to 4-fold enrichment in AChE in vesicles, calculated by normalization of AChE activity over membrane surface extension (phospholipids), becomes a 60- to 80-fold enrichment when normalizing to cell volume (hemoglobin): a good fit. However, it was probably not necessary to introduce a new procedure for quantifying AChE enrichment in vesicles, since it does not constitute an original finding. However, Salzer et al go one step further and claim that band 3 protein and glycophorins are strongly decreased in vesicles compared with cells. The way they demonstrate this is clearly incorrect: sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels were loaded with equivalent amounts of erythrocyte membranes and vesicle membranes by taking AChE as the normalizing parameter. Then, band 3 was stained (glycophorins were not, however) and shown to be decreased in vesicles.1(Fig 2) But since AChE is enriched in the membrane of vesicles with respect to cells, this method will always detect a decrease of anything that would otherwise keep constant in the membrane of vesicles. Therefore, we would prefer to adhere to the well-documented notion that band 3 is equally represented, per unit surface area, in vesicles and cells,3 as we have found the same result by direct measurements of properly loaded gels in our lab. Salzer et al conclude that, “interestingly, only trace amounts of the flotillins are found in the vesicles [italics added].”1 Maybe flotillins are indeed present in more than trace amounts in vesicles. It is only a matter of watching more carefully.
Segregation of lipid raft proteins during calcium-induced vesiculation of erythrocytes
The erythrocyte membrane contains lipid rafts,1-1-1-3with stomatin, the flotillins, and glycosylphosphatidyl-inositol (GPI)–linked proteins as the major integral proteins, whereas band 3 protein and the glycophorins are largely absent. As microvesicles released from erythrocytes after Ca++ treatment are specifically enriched in the GPI-linked enzyme acetylcholinesterase (AChE),1-4 it is likely that lipid rafts are involved in the vesiculation process. We showed that microvesicles in fact contain lipid rafts; however, the relative amounts of the raft proteins differ between microvesicles and the erythrocyte membrane.1-5 These results suggest a calcium-induced segregation of different types of lipid rafts, with stomatin-specific rafts enriched in microvesicles and flotillins depleted.
The letter of Minetti and Ciana refers to a paragraph in “Results” in our study,1-5 in which we discuss the relative amounts of certain membrane proteins in the erythrocyte membrane compared with whole microvesicles and nanovesicles (and not to vesicle membranes as stated by the authors). There are 2 misunderstandings in the comments of Minetti and Ciana. First, we did not “normalize AChE activity to hemoglobin content.” We mentioned the enrichment of AChE relative to hemoglobin in microvesicles (80 times) solely to explain the low abundance of cytosolic proteins in the vesicles (silver stain5(Fig 2)). Second, as stated in “Results,” we used the raft marker AChE for normalization to compare the relative amounts of other raft proteins, particularly stomatin and the flotillins, and contrasted these findings to nonraft proteins. The term “relative” in the criticized statement (“the relative amounts of the major integral membrane proteins band 3 and glycophorins are diminished [italics added]”1-5) should be understood as relative to AChE and not relative to phospholipid/cell surface. However, in contrast to the unpublished results of Minetti and Ciana (the Butikofer et al1-3 reference cited by the authors does not contain original data on the band 3 distribution), Hagelberg and Allan reported that “band 3 and glycophorins are depleted from microvesicles”1-6 using phospholipid content for normalization. They found that only 40% of the band 3 protein is present in the microvesicles when compared with erythrocyte membranes.
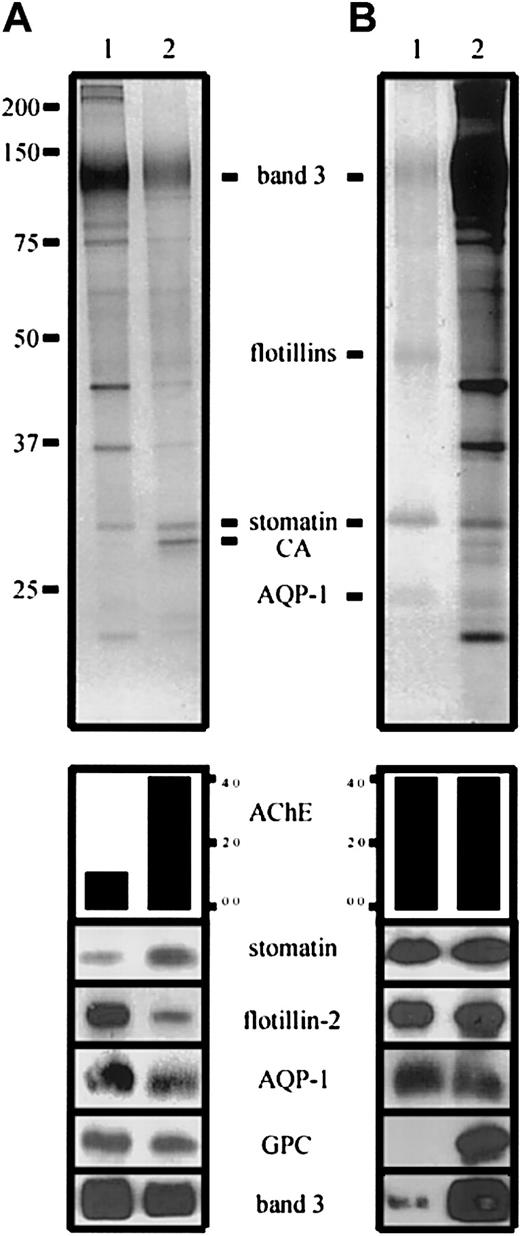
To address the question of membrane protein dynamics during microvesiculation in more detail, we compared erythrocyte membranes and increasing amounts of microvesicles by quantitative immunoblotting, thereby assessing the protein distribution relative to AChE (data not shown). As an example, we show the relative abundance of the respective proteins at a 4-fold AChE activity in the microvesicle sample (Figure1-1A). Whereas similar fractions of the total erythrocyte AChE, stomatin, flotillin-2, and aquaporin-1 content are found in lipid rafts (Figure1-1B), the relative amounts of these proteins in microvesicles are quite different, thereby demonstrating the segregation of erythrocyte raft proteins during vesiculation. A small fraction of band 3 protein is also found in the lipid rafts; however, the significance of this finding remains to be evaluated. Taking the well-supported factor of 3 for the AChE enrichment in microvesicles relative to phospholipid,1-4,1-6,1-7 we calculated the enrichment/depletion of the following proteins in microvesicles relative to membrane surface: stomatin (1.7), flotillin-2 (0.2), aquaporin-1 (0.4), glycophorin C (0.5), and band 3 (0.4; in agreement with Hagelberg and Allan1-6).
Distribution of erythrocyte membrane proteins in microvesicles and lipid rafts.
(A) Erythrocyte membranes (lane 1) and microvesicles (lane 2) were prepared and AChE activity determined as described.1-5Aliquots were loaded on an 11% polyacrylamide gel, with a 4-fold AChE activity in the microvesicular sample, and analyzed by silver staining (upper panel) and Western blotting, as indicated and previously described1-5 (anti–AQP-1 was from Calbiochem, La Jolla, CA). AChE activity is given in arbitrary units. Note the differential distribution of stomatin and flotillin-2. (B) Erythrocyte lipid rafts were prepared by method B1-1 and AChE activity was determined. Aliquots with equal AChE activity of lipid rafts (lane 1) and erythrocyte membranes (lane 2) were loaded on an 11% polyacrylamide gel and analyzed by silver staining (upper panel) and Western blotting, as indicated. CA indicates carbonic anhydrase; GPC, glycophorin C; and AQP-1, aquaporin-1.
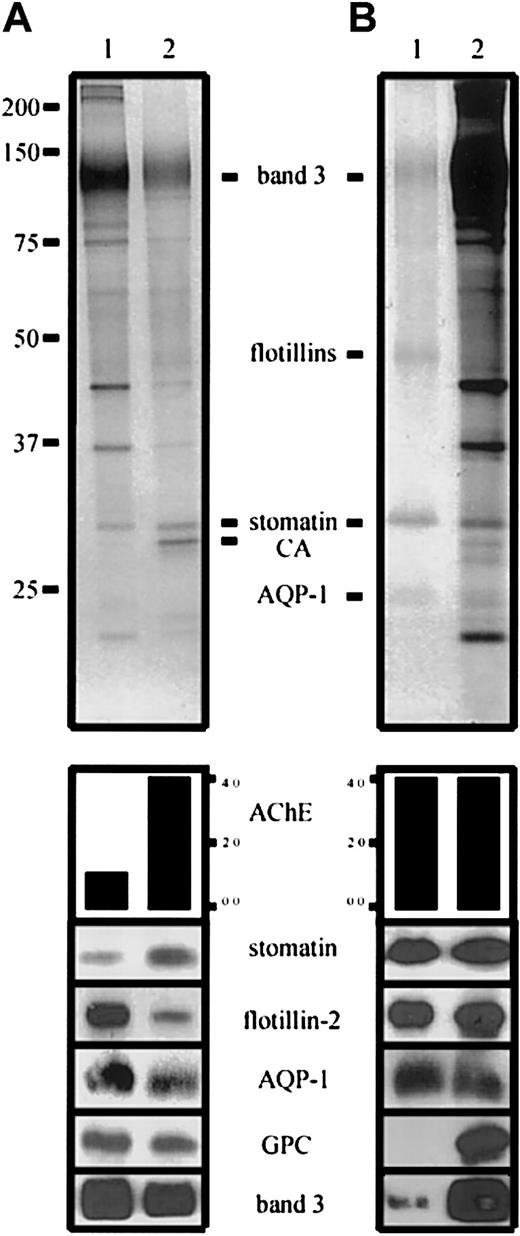
Distribution of erythrocyte membrane proteins in microvesicles and lipid rafts.
(A) Erythrocyte membranes (lane 1) and microvesicles (lane 2) were prepared and AChE activity determined as described.1-5Aliquots were loaded on an 11% polyacrylamide gel, with a 4-fold AChE activity in the microvesicular sample, and analyzed by silver staining (upper panel) and Western blotting, as indicated and previously described1-5 (anti–AQP-1 was from Calbiochem, La Jolla, CA). AChE activity is given in arbitrary units. Note the differential distribution of stomatin and flotillin-2. (B) Erythrocyte lipid rafts were prepared by method B1-1 and AChE activity was determined. Aliquots with equal AChE activity of lipid rafts (lane 1) and erythrocyte membranes (lane 2) were loaded on an 11% polyacrylamide gel and analyzed by silver staining (upper panel) and Western blotting, as indicated. CA indicates carbonic anhydrase; GPC, glycophorin C; and AQP-1, aquaporin-1.
In accordance with our previous study,1-5 these data clearly show the segregation of lipid raft proteins during calcium-induced vesiculation of erythrocytes. Whereas AChE and stomatin are enriched in the released microvesicles, the flotillins and aquaporin-1 are depleted. In line with the interpretation of Hagelberg and Allan,1-6 we assume a partial cytoskeletal association as the cause for the depletion of the respective membrane proteins in microvesicles. The mechanism of the enrichment of AChE and stomatin in microvesicles remains to be elucidated; however, the membrane aggregating and fusogenic properties of synexin, which is present in vesicular rafts,1-5 may play an important role in this process.
Acknowledgments
Supported by grant P15486 from the Austrian Science Fund.