Abstract
Relapsed or refractory multiple myeloma has a poor outlook. Some patients respond to thalidomide; however, criteria for predicting response have not been conclusively identified. We initiated a prospective multicenter phase 2 trial in patients with relapsed/refractory myeloma using thalidomide up to the maximum dose, 800 mg/d. Interferon-α-2B (1.5-3.0 × 106 U, subcutaneously, 3 times per week) was added at week 12 if disease was responsive or stable. Patients intolerant of interferon continued thalidomide alone. Thalidomide with or without interferon was continued until disease progression. Objectives were to determine toxicity, response rate (RR), progression-free survival (PFS), and overall survival (OS) and to elucidate relevant prognostic factors. We enrolled 75 patients, with median age 64 years (range, 36-83 years). Median individual maximum-tolerated dose of thalidomide was 600 mg/d; 41% reached 800 mg/d. Overall RR was 28%, and 55% stable disease (SD). The only predictor for response was age 65 years or younger (38% versus 17%; P = .043). At 18 months median follow-up, the actuarial median PFS and OS were 5.5 and 14.6 months, respectively. Multivariate analysis for OS demonstrated age exceeding 65 years (median, 9.2 months versus longer than 26 months; P = .011), raised serum lactate dehydrogenase (P = .002), and raised serum creatinine (P = .007) predicted inferior outcomes. Nineteen patients received interferon. Ten discontinued owing to toxicity. Four of 12 patients who received interferon for longer than 4 weeks were converted from SD to partial response. Our findings confirm substantial activity of thalidomide in relapsed/refractory myeloma. Interferon may improve response in selected patients, but is often not tolerated. The inferior outcome demonstrated in those with the identified prognostic factors is important in planning management for such patients. (Blood. 2003;102:69-77)
Introduction
Multiple myeloma is predominantly a disease of the elderly, with a median age at diagnosis of 65 years.1 Despite advances in the use of high-dose chemotherapy with autologous stem cell rescue and allogeneic transplantation,2 such strategies are suitable only for selected patients. Older patients and those with relapsed or refractory disease have a poor outlook, and disease control in this setting remains a therapeutic challenge.
Within the last decade, thalidomide has been reported to show activity in myeloma. A number of investigators have reported results of phase 2 trials and retrospective series.3-13 Similar response rates have been reported although a variety of response criteria have been used to evaluate efficacy. The largest of these studies is a single-center phase 2 trial with results reported by Barlogie et al5 for 169 patients. In this trial, 30% of patients achieved a 50% reduction in myeloma protein, and 14% achieved a complete or near-complete remission. However, no prospective multicenter confirmatory study with an analysis of baseline prognostic factors has been reported.
In 1999, we initiated a prospective multicenter phase 2 trial in patients with relapsed or refractory multiple myeloma using escalating doses of thalidomide with the addition of interferon—α-2B (IFN) once disease stabilization was achieved. Both thalidomide and interferon have established activity in myeloma, and there was potential for a synergistic antiangiogenic effect with the combination.14,15 The objectives of the trial were to determine the toxicity of thalidomide alone and in combination with interferon; to determine the response rate and progression-free and overall survival; and to identify prognostic factors for response, progression-free survival (PFS), and overall survival (OS).
Patients and methods
Patients
Eligible patients were those with relapsed or resistant multiple myeloma following prior systemic combination chemotherapy (failure of dexamethasone monotherapy was not acceptable) who required further systemic therapy. Patients considered to have resistant disease were those whose disease was refractory to front-line induction therapy that included at least 2 cycles of a regimen containing an alkylating agent or an anthracycline, or who had an initial transient response but experienced a relapse during front-line induction therapy. Minimum age for enrollment was 18 years, with no upper age limit. Further eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2 and written informed consent. This consent included agreement by both male and female patients to take precautions to prevent conception during the treatment program. Exclusion criteria were the following: females who were pregnant or lactating, concurrent serious medical or psychiatric illness that would preclude treatment administration or patient compliance with the protocol, and pre-existing peripheral neuropathy exceeding grade 1 (National Cancer Institute criteria, version 2.0, 1998). The Ethics Committee of each participating institution gave approval for the study.
Treatment
Patients commenced treatment with thalidomide (Thalomid; Celgene, Warren, NJ) at a dose of 200 mg/d orally, with planned dose escalation each 14 days by a further 200 mg/d, up to a total dose of 800 mg/d. Dose escalation was ceased if patients developed intolerable side effects as determined by the investigator. The dose could be reduced if necessary so that patients continued on an individual maximum tolerated dose (iMTD). At week 12, patients with stable or responsive disease continued on their iMTD of thalidomide and began treatment with interferon—α-2B (Intron; Shering Plough, Sydney, New South Wales, Australia). To be eligible to receive IFN, patients were required to have acceptable blood parameters, including serum bilirubin level below 34 μM, alkaline phosphatase level no more than 3 times the upper limit of normal (ULN) for the institution, aspartate transaminase and/or alanine aminotransferase level no more than 3 times ULN, absolute neutrophil count (ANC) at least 1.5 × 109/L, and platelet count at least 75 × 109/L. Patients were ineligible to receive IFN if they had one of the following: a history of hypersensitivity to IFN, a psychiatric condition, autoimmune hepatitis, a history of other severe autoimmune disease, or pre-existing thyroid function abnormalities. Four patients with ANC between 1.0 and 1.5 × 109/L began treatment with IFN (eligibility infringements): they were retained in the analysis.
IFN was commenced at a dose of 3.0 × 106 U by subcutaneous injection 3 times per week. If patients developed an ANC below 1.0 × 109/L, or a platelet count below 50 × 109/L, IFN was withheld until recovery to an ANC at least 1.5 × 109/L and a platelet count at least 75 × 109/L. IFN was then recommenced at a dose of 1.5 × 106 U thrice weekly, with escalation up to a dose of 3.0 × 106 U attempted at the discretion of the investigator. IFN was ceased in patients unable to maintain ANC and platelet count above the required thresholds, or those who developed grade 3 neurotoxicity or grade 2 or 3 hypersensitivity. Patients who developed any other type of grade 3 toxicity were to have IFN withheld until the toxicity resolved to grade 1 level or lower. IFN could then be recommenced at a dose of 1.5 × 106 U thrice weekly but was ceased if grade 3 or greater toxicity recurred. IFN was permanently ceased if any type of grade 4 toxicity developed. Such patients continued on thalidomide alone. Thalidomide with or without IFN was continued until progressive disease or patient intolerance.
Evaluation of patients and criteria for response
At trial entry, baseline history and examination findings, including ECOG performance status, were recorded for patients. Baseline bone marrow aspirate and trephine with cytogenetics was performed as well as a skeletal survey and nerve conduction study. Fluorescent in situ hybridization (FISH) studies for otherwise occult chromosomal deletions or translocations were not routinely performed. A baseline full blood count and the following biochemistry data were recorded: serum creatinine level, serum calcium level, liver function tests, lactate dehydrogenase (LDH) level, C-reactive protein (CRP) level, CA-153 level,16 and β2-microglobulin (β2M) level. Baseline serum and 24-hour urine collection were obtained for protein electrophoresis, immunoelectrophoresis, and immunofixation. Full blood count and basic biochemistry were repeated every 2 weeks up to 24 weeks, and then every 4 weeks during treatment. Toxicity assessments and serum and urine protein electrophoresis were performed every 4 weeks. Bone marrow aspirate and biopsy were repeated every 3 months until disease progression. Nerve conduction studies were repeated every 3 months and skeletal surveys every 4 months. Patients who discontinued thalidomide with or without IFN because of toxicity were reassessed every 3 months, and the date of disease progression or death from any cause was recorded.
Adverse events, apart from laboratory tests, were categorized according to their relationship to study drugs (not related; possibly, probably, or definitely related) and were graded according to the National Cancer Institute (NCI) Common Toxicity Criteria, version 2.0, 1998.
Response criteria, based on those of the Chronic Leukemia and Myeloma Task Force,17 were as previously used in the Australian Leukaemia Study Group myeloma II study.18 A complete response (CR) was defined as disappearance of serum M protein and/or Bence Jones protein-uria (determined by immunofixation) on 2 determinations at least 4 weeks apart plus fewer than 5% plasma cells in the bone marrow in a patient with no signs or symptoms of disease. Partial response (PR) was defined by all of the following: reduction of serum M protein level to less than 50% of the pretreatment value on 2 determinations at least 4 weeks apart; a decrease of at least 50% in urinary light-chain excretion from a pretreatment value of more than 1.0 g per 24 hours, or a fall to less than 0.1 g per 24 hours if pretreatment value was between 0.5 and 1.0 g per 24 hours; a decrease in the size of measurable plasmacytoma(s) of at least 50% of the sum of the products of the cross-diameters of each measurable lesion; and a decrease in bone pain from moderate or severe to none or mild. Stable disease (SD) was defined as failure to meet response criteria for disease response or progression. An assessment of stable disease did not require confirmation on a second assessment. Progression of disease was defined as any of the following: an increase in serum M protein level to more than 50% above the previous nadir; an increase in urinary M protein level to more than 50% above the previous nadir and a light-chain excretion of at least 0.2 g per 24 hours; appearance of a new plasmacytoma or an increase in a pre-existing plasmacytoma by more than 50%; appearance of a new lytic bone lesion or a more than 50% increase in the size of any existing lesion.
Statistical methods
Prognostic factors prospectively stipulated for analyses were age at commencement of treatment (65 or younger versus older than 65 years); β2M level (3 mg/L or lower, versus more than 3 mg/L but below 6 mg/L, versus at least 6 mg/L); LDH level (within normal range, above normal); hemoglobin level (at least 110 g/L versus below 110 g/L); CRP level (below 6 mg/L, versus between 6 and 10 mg/L, versus more than 10 mg/L); serum creatinine level (130 μM or lower versus more than 130 μM [0.13 mM or lower versus more than 0.13 mM]); serum calcium level (2.6 mM or lower versus more than 2.6 mM); plasma cells in bone marrow (50% or fewer versus more than 50%); response to last prior chemotherapy (no versus yes); chromosome 13 loss (no versus yes); and CA-153 (within normal range versus above normal).
Response rates were calculated as percentages of all patients, and 95% exact confidence intervals (CIs) were estimated by means of the probabilities of the binomial distribution.19 Time to response was estimated by Kaplan-Meier analysis. The 2-sided Fisher exact test or the Cochran-Armitage test for trend was used to compare the response rates between subgroups and patient characteristics between age groups.19
All patients who commenced treatment were included in the analyses of PFS and OS. All patients were followed to a close-out date of at least January 2, 2002, with the vital status of each patient taken to be the vital status on the close-out date. PFS time was measured from the date of commencing protocol treatment to the date of first progression or death from any cause without prior progression. OS was measured from the date of commencing protocol treatment to the date of death from any cause. The Kaplan-Meier method was used to estimate OS and PFS, with censoring of survival times at the close-out date.20 The Brookmeyer-Crowley method was used to estimate 95% confidence intervals for median survival times. The 95% confidence intervals for the percentages surviving at particular times were calculated by means of the logit transformation. Differences or trends between groups were tested by means of the Mantel-Cox log-rank test.20,21 Multifactor analyses of prognostic factors for PFS and OS were carried out by means of Cox proportional hazards regression, with P based on the likelihood ratio test.21 Factors included in the models were those with log rank P < .1 in unifactor analyses. Patients with unknown values of any prognostic factor in the model were excluded from the multifactor analyses. Chromosome 13 deletion was not included in multifactor analyses because it was unknown for a large number of patients.
Because some patients were still on therapy at the close-out date, the Kaplan-Meier method was used to estimate median total doses and duration of thalidomide treatment. The Wilcoxon rank sum test was used to compare the iMTDs between age groups. The worst grades of adverse events, which were categorized as probably or definitely related to study drugs, have been reported as toxicities.
Results
Patient characteristics
Seventy-five patients were accrued from 7 Australian centers. The median age was 64 years (range, 36-83 years), with 36 patients (48%) older than 65 years. Table 1 summarizes the baseline characteristics of the patients and their prior treatments by age group. Patient characteristics were fairly similar in the 2 age groups with 2 exceptions; only one patient older than 65 years had received a prior autograft (as opposed to 49% aged 65 years or younger), and there was some difference in distribution of number of prior chemotherapy regimens. However, the number of patients who received 3 or fewer regimens (56% versus 62%) or more than 3 regimens (43% versus 39%) were similar in both groups. The median duration of follow-up from commencement of treatment to the close-out date was 18 months (range, 6-26 months). Fourteen patients (19%) remained on treatment at the close-out date.
Treatment received
Fifty-six patients received thalidomide alone, and 19 received IFN in addition. The median total dose of thalidomide received was 58 g, with a median duration of thalidomide treatment of 22 weeks. The median iMTD was 600 mg/d (range, 200-1000 mg/d), with 41% of patients receiving at least 800 mg/d. For patients 65 years old or younger, the average iMTD was 638 mg/d (median dose, 800 mg/d). For patients older than 65 years, the average iMTD of thalidomide was 492 mg/d (median, 400 mg/d) (P = .006), with 75% receiving an iMTD of 400 mg/d or greater (Figure 1). By the close-out date, 61 patients had discontinued thalidomide, 39 patients (52%) because of progressive disease and 16 (21%) because of toxicity or patient refusal (Table 2). Of the 19 patients who received thalidomide and interferon, 12 patients received concurrent treatment for more than 4 weeks, and 68% of these patients were able to receive a maximum dose of 3.0 × 106 U 3 times per week. Of the patients who received interferon, 13 were aged 65 years or younger and 6 were older than 65 years. The majority of patients were ineligible to commence interferon because of progressive disease prior to week 12 (24 patients), cessation of thalidomide prior to week 12 due to toxicity (9 patients), low ANC (11 patients), and depression (2 patients). Of the 10 patients that were eligible to receive interferon but did not, reasons included investigator concern about existing significant toxicity from thalidomide, prior intolerance of interferon (3 patients), and active graft-versus-host disease (1 patient).
Maximum doses of thalidomide according to age group. Solid bars represent patients aged 65 years or younger, and cross-hatched bars represent patients older than 65 years. Note the minor protocol violations in that 2 patients received more than 800 mg/d.
Maximum doses of thalidomide according to age group. Solid bars represent patients aged 65 years or younger, and cross-hatched bars represent patients older than 65 years. Note the minor protocol violations in that 2 patients received more than 800 mg/d.
Efficacy
The overall response rate to the protocol treatment based on intention-to-treat analysis was 28% (CI, 18%-40%), with 1 complete response and 20 partial responses. Forty-one patients (55%) achieved stable disease as their best response, and 11 patients (15%) progressed, died early, or received fewer than 28 days of thalidomide owing to toxicity. The remaining 2 could not be assessed for response: 1 because initial serum monoclonal protein was not detectable and the urinary Bence-Jones protein was not assessed after treatment, and 1 because concurrent plasma exchange was given during thalidomide treatment. The median time to first response was 12.4 weeks (range, 4-114 weeks), with all but one response occurring within 9 months of commencing treatment. Fifteen of the 39 patients who were 65 years old or younger at the commencement of treatment (38%) responded to treatment compared with 6 of 36 (17%) of the older patients (P = .043). Disease stabilization was achieved in 64% (23 of 36) of patients older than 65 years and 46% of those aged 65 years or younger (18 of 39).
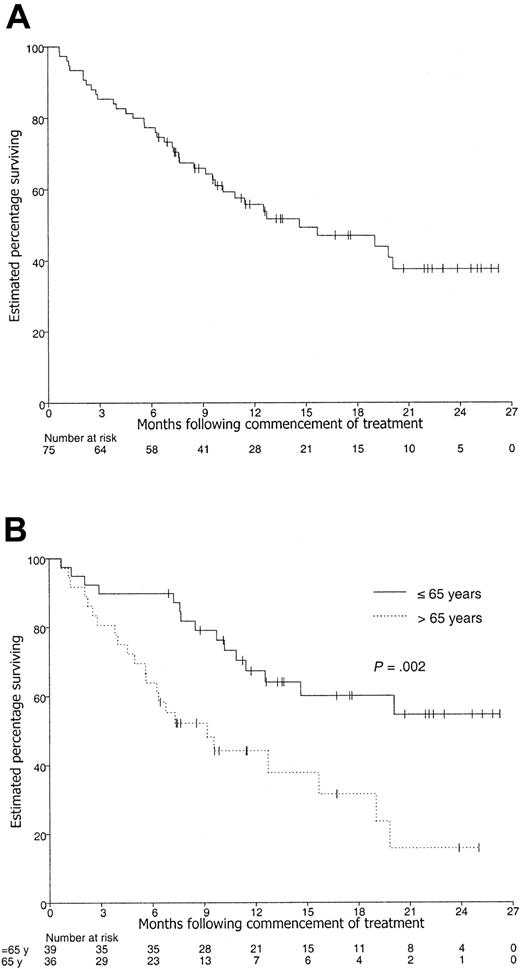
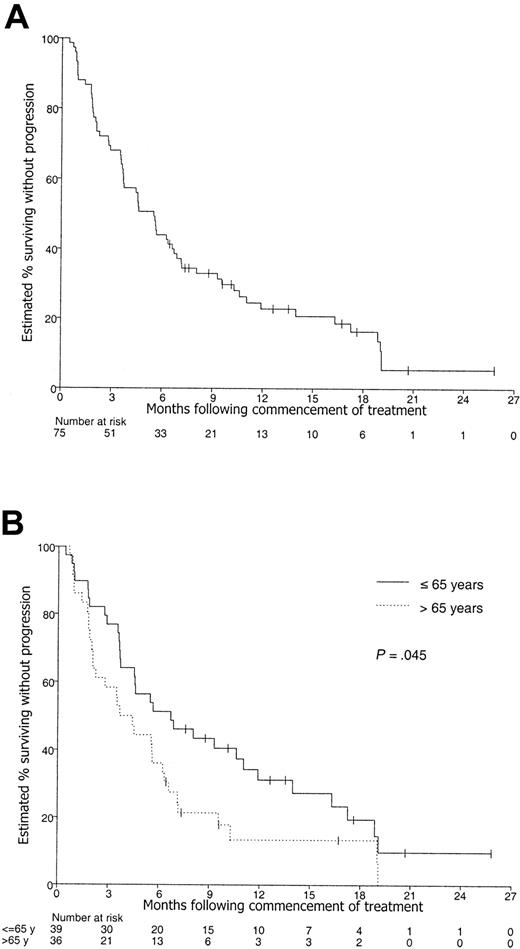
The actuarial median PFS and OS for all 75 patients was 5.5 months (CI, 3.6-6.8 months) and 14.6 months (CI, 9.7 to longer than 26.3 months), respectively (Figures 2, 3). The estimated 1-year PFS and OS were 23% (CI, 14%-34%) and 56% (CI, 44%-67%), respectively.
Kaplan-Meier curve of overall survival. Patients with censored OS times are shown by tick marks. (A) All 75 patients. (B) According to age group.
Kaplan-Meier curve of overall survival. Patients with censored OS times are shown by tick marks. (A) All 75 patients. (B) According to age group.
Kaplan-Meier curve of progression-free survival. Patients with censored PFS times are shown by tick marks. (A) All 75 patients. (B) According to age group.
Kaplan-Meier curve of progression-free survival. Patients with censored PFS times are shown by tick marks. (A) All 75 patients. (B) According to age group.
Of the 12 patients who received concurrent thalidomide and interferon for more than 4 weeks, 2 maintained SD, 5 maintained a PR, 4 were converted from SD to PR, and 1 progressed prior to commencing interferon. Three of the 4 patients who converted from SD to PR on interferon were 65 years old or younger.
Prognostic factors
An analysis of potential prognostic factors was performed for response: PFS (Table 3) and OS (Table 4). The only factor found to be predictive of higher likelihood of response by univariate analysis was age of 65 years or younger (P = .043, Fisher exact test).
Prognostic factors predictive for inferior PFS in unifactor analyses were age older than 65 years (P = .045), elevated β2M level (P = .015, log-rank trend test), and elevated LDH level (P = .003), with CRP level being of borderline significance (P = .060, log-rank trend test) (Table 3). Chromosome 13 loss was also highly significant for PFS in univariate analysis (P = .004), but this information was available for only 30 patients so it was not included in the multifactor analyses. Figure 3 shows the Kaplan-Meier curves according to age group, with PFS at 1 year estimated to be 31% of patients 65 years old or younger (CI, 18%-48%) and 13% of patients older than 65 years (CI, 5%-31%). On multifactor analysis, age older than 65 years, as well as elevated baseline levels of β2M, LDH, and CRP, was significant at P < .05 (Table 5).
Prognostic factors that significantly predicted inferior OS in unifactor Kaplan-Meier analyses were age older than 65 years (P = .002), elevated β2M level (P = .0008, trend), elevated LDH level (P = .0006), creatinine level exceeding 0.13 mM (P = .001), and chromosome 13 loss (P = .003) (Table 4). The estimated percentage surviving at 1 year was 67% of patients aged 65 years or younger (CI, 51%-81%) and 44% of patients older than 65 years (CI, 28%-62%) (Figure 2). On multifactor analysis, age group, LDH level, and creatinine level, but not β2M level, were significant at P < .05 (Table 5).
Toxicity
During treatment with thalidomide alone, toxicity was generally manageable, with observed toxicities similar to those previously reported3-12 (Table 6). The most common toxicities included constipation, fatigue, and motor or sensory neuropathy. Two patients had febrile neutropenia; 3 patients had nonfatal thromboembolism; and 1 patient had grade 3 sinus bradycardia requiring insertion of a pacemaker. Eight patients developed a progressive rise in creatinine level while receiving thalidomide; in the majority there was clear evidence of progressive myeloma, and no cases were attributable to direct toxicity of thalidomide or interferon. Of the 16 patients (19%) who ceased thalidomide owing to toxicity or patient refusal, neurotoxicity was the primary reason in 8 and intolerable somnolence and lethargy in 5. Ten of 19 patients (53%) ceased interferon owing to toxicity, including fever (2 patients), muscle pain and anorexia (1), neutropenia (1), hyponatremia (1), anxiety (1), and depression (1) (Table 7). One patient ceased IFN following transient ischemic attacks, and 2 patients discontinued IFN because of complex partial seizures that resolved after discontinuation of IFN while thalidomide was continued. However, the overall rates of reported toxicities for concurrent thalidomide and interferon were similar to those for thalidomide alone.
Discussion
This trial confirms the activity of thalidomide in patients with relapsed or refractory multiple myeloma, many of whom were heavily pretreated. The patients had received a median of 3 prior chemotherapy regimens (range, 1-7 regimens), with 27% having had prior high-dose chemotherapy. The overall response rate (28%), while at the lower end of the range of previous reports (Table 8), should be considered in the context of a prospective, multicenter study with broad entry criteria, including patients with lower blood counts and more impaired renal function compared with other studies. Furthermore, we used an intention-to-treat analysis with very strict classical response criteria compared with those used by other investigators.16
An important finding of this trial was that patients of advanced age (older than 65 years) had an inferior outcome as measured by either RR, PFS, or OS. In terms of response, age was the only predictor (P = .043) among the prospectively determined prognostic factors tested. This finding is likely to have been masked in previous studies because of small study size and/or a study population that did not reflect the typical age distribution of myeloma patients (Table 8). Because we subjected our data to multiple comparisons, the criterion for statistical significance was more stringent (P < .0047), and it is possible that this may be a chance finding. In support of the validity of our results, we note that this reduced response rate appears to have translated into a significantly worse progression-free and overall survival for elderly patients, with a median survival time of only 9 months for those older than 65 years compared with more than 26 months for younger patients. Given the very low response rate to thalidomide among those older than 65 years in this study, there may well have been other contributing factors to the short median survival in this group. However, a substantial number of older patients did gain short-term benefit with thalidomide therapy. Two thirds of older patients achieved stabilization of disease before further progression (at a median of approximately 6 months), with about 13% remaining progression free at a year. Further support for the finding that advanced age is predictive of a poor outcome following thalidomide treatment appears in the recent publication from Yakoub-Agha et al,13 who describe a retrospective cohort of 83 patients treated with thalidomide for relapsed or refractory disease. In this study, age above 60 years was predictive of an inferior event-free and overall survival.
The reason for the reduced efficacy of thalidomide in older patients is likely to be multifactorial. In respect to prior therapy, older patients may indeed have been less heavily pretreated, with only one patient older than 65 years having undergone prior stem cell transplantation. Given that only 4 patients were converted from stable disease to a partial response with the addition of IFN, the responses observed were likely to be due to the single-agent activity of thalidomide, rather than to younger patients' being better able to tolerate IFN and thus achieving a higher response rate. Older patients and their treating physicians seemed less accepting of the side effects of thalidomide. Although there was no statistically significant difference in the rates of reported toxicities for older and younger patients (P > .2), patients older than 65 years were more likely than younger patients to cease thalidomide because of toxicity (19% versus 13%) or because of patient refusal (11% versus 0%). Hence, one possibility for the poorer outcome is the lower average daily dose that older patients received. A dose-response relationship has been recognized by Barlogie et al5 (in relatively young patients), with improved outcomes in patients who received more than 42 g thalidomide in the first 3 months of therapy, which translates to a daily dose exceeding 450 mg/d. However, arguing against this hypothesis is that all older patients received daily doses of at least 200 mg, with 75% receiving 400 mg or greater: doses that are generally considered effective in myeloma and are relatively high compared with those now being used in many centers.5,22-26
Another possible explanation for the age effect is alteration in thalidomide metabolism. Our study did not specifically assess plasma concentrations of thalidomide or its metabolites, but there is preliminary evidence indicating that clearance of thalidomide may be reduced in the elderly.27,28 It is not known whether thalidomide itself or one of its metabolites mediates the antimyeloma effect. Furthermore, the biology of malignant plasma cells and the bone marrow microenvironment may be different in older patients. Indeed, there are conflicting reports about the impact of patient age on prognosis in newly diagnosed multiple myeloma. Some studies have reported that patients with advanced age have an inferior survival compared with younger patients,29-33 whereas others have reported no effect once other prognostic variables such as C-reactive protein and β2M levels are considered.34-37 Advanced age does not appear to be predictive of inferior outcome following high-dose chemotherapy and autologous stem cell transplantation.38-41
In addition to age, we have confirmed other important independent prognostic factors for PFS (β2M, CRP, and LDH levels) and OS (LDH and creatinine levels), although elevated LDH and CRP levels were observed in a minority of patients. Unlike the study by Barlogie et al,5 we were not able to assess the presence of chromosome 13 deletions in more than half our patients, so we could not include this important factor in the multifactor analyses.
The real effect of adding IFN to thalidomide in patients with responsive or stable disease was not evaluable in this trial primarily because the majority of patients were not eligible to commence IFN at the week 12 time point. The main reasons for patients being ineligible were disease progression, cessation of thalidomide due to toxicity, or a low neutrophil count. Other reasons why interferon was not commenced included a patient history of previous intolerance of IFN and investigator concern that the addition of IFN would significantly worsen existing levels of thalidomide-induced fatigue and somnolence. Furthermore, over half of the patients subsequently discontinued IFN owing to toxicity. Nonetheless, the addition of IFN appeared to improve the response in 4 of the 19 patients, with conversion from stable disease to a partial response. We cannot exclude that this occurred because of a delayed response to thalidomide. However, one patient had a gradually declining paraprotein on the thalidomide/IFN combination that finally fulfilled the criteria for a partial response after nearly 2 years of treatment.
Recent concern has been raised about the adverse central neurological effects of the thalidomide/IFN combination. A recent trial combining thalidomide and interferon alpha-2b to treat patients with metastatic renal cell carcinoma was prematurely closed because 4 of the initial 13 patients developed significant neurological toxicity, including complex partial seizures and visual disturbance.42 Although that trial used doses of thalidomide (100 to 400 mg per day) similar to those in our trial, higher doses of IFN were used (9 × 106 U subcutaneously 3 times per week). An ECOG study combining thalidomide with lower doses of IFN in renal cancer is continuing. In our trial, one patient had transient ischemic attacks and 2 patients developed complex partial seizures. Rates of other neurological toxicities were otherwise similar to those of thalidomide alone. It may prove more feasible to combine IFN with the newer thalidomide derivatives, such as the immunomodulatory thalidomide derivatives (IMiDs), which are chemically linked but functionally distinct analogs of thalidomide that are generally better tolerated.43 Thalidomide is also being used in combination with corticosteroids, especially dexamethasone, with promising response rates, but as yet no analysis of a differential response by age has been reported.44,45
Our prospective trial confirms that thalidomide is an important addition to the therapeutic armamentarium for the treatment of myeloma. Single-agent activity of 38% in patients 65 years old or younger who have failed at least one standard therapy is certainly gratifying. However, for older patients, a response rate of only 17% is suboptimal, particularly given the median age of diagnosis of this disease. Nonetheless, two thirds of older patients achieve stabilization of disease, albeit for a short time. We believe this information enhances our ability to plan management for patients who have failed more standard therapy. Further studies examining the impact of thalidomide (or similar compounds) on the quality of life of elderly patients with myeloma are needed, and strategies to increase the response in elderly patients with relapsed or refractory disease, such as the combination of thalidomide with dexamethasone and/or chemotherapy, should continue to be investigated.
Prepublished online as Blood First Edition Paper, March 13, 2003; DOI 10.1182/blood-2002-09-2846.
Supported by Celgene (Warren, NJ), and Schering Plough, Australia. L.M. was supported by the Medical Oncology Group (Australia)/Novartis Clinical Research Fellowship.
J.B.Z., Chief Medical Officer at Celgene, has declared a financial interest in Celgene, whose product was used in this study.
This study was investigator driven.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We acknowledge the support of the Rotary Club of Camberwell, Australia, and Myeloma Victoria for assistance in patient education through the activities of the Myeloma Nurse.