Abstract
We and others have recently defined that Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1/CD31) functions as a negative regulator of platelet-collagen interactions involving the glycoprotein VI/Fc receptor gamma chain (GPVI/FcR-γ chain) signaling pathway.1,2 In this study, we hypothesized that PECAM-1 may be physically and functionally associated with FcγRIIa on the platelet membrane. The functional relationship between PECAM-1 and FcγRIIa was assessed by determining the effect of anti-PECAM-1 monoclonal antibody Fab fragments on FcγRIIa-mediated platelet aggregation and heparin-induced thrombocytopenia (HITS)-mediated platelet aggregation. Preincubation of washed platelets with monoclonal antibody fragments of 2BD4 directed against PECAM-1 and IV.3 directed against FcγRIIa completely blocked FcγRIIa-mediated platelet aggregation and HITS-mediated platelet aggregation, whereas anti-CD151 antibody had no blocking effect. Coengagement of FcγRIIa and PECAM-1 resulted in negative regulation of FcγRIIa-mediated phospholipase Cγ2 activation, calcium mobilization, and phosphoinositide 3-kinase-dependent signaling pathways. In addition, the physical proximity of FcγRIIa and PECAM-1 was confirmed by using fluorescence resonance energy transfer and coimmunoprecipitation studies. These results indicate that PECAM-1 and FcγRIIa are colocalized on the platelet membrane and PECAM-1 down-regulates FcγRIIa-mediated platelet responses. (Blood. 2003;102:3637-3645)
Introduction
Platelets are central to the physiologic process of hemostasis and pathologic process of thrombosis. The mechanisms underlying the role of platelets in these processes are highly complex, integrated, and regulated, involving the interaction of multiple surface platelet receptors, including the glycoprotein Ib-IX-V (GPIb-IX-V) complex, αIIbβ3, and glycoprotein VI/Fc receptor gamma chain (GPVI/FcR-γ chain) complex with subendothelial proteins, and plasma ligands.3
The low-affinity immunoglobulin G (IgG) Fc receptor, FcγRIIa, and the collagen receptor GPVI/FcR-γ chain complex are 2 IgG-bearing immunoreceptor tyrosine-based activation motif (ITAM)-containing receptors expressed on the human platelet surface. These ITAM-containing receptors bear tandem ITAM motifs (D/ExYxxL/I-x7-YxxL/I) either within their cytoplasmic tails or within an associated protein and are the actual signal transducing element of the receptor. On receptor clustering, activation of receptor-associated tyrosine kinases of the Src family occurs, which phosphorylate the ITAM motif. The phosphorylated ITAM then serves as a docking site for Src-homology 2 (SH2) domain-containing adapter proteins and enzymes, including Syk protein-tyrosine kinase, thereby recruiting molecules to the membrane and to the proximity of their substrates, including phospholipase Cγ (PLCγ), the Ras activating enzyme, Sos and phosphoinositide 3-kinase (PI-3K).4-6 In contrast to the GPVI/FcR-γ chain complex, FcγRIIa is unique where the ligand binding domain and the intracellular ITAM are contained in the one polypeptide, whereas the GPVI/FcR-γ chain complex consists of GPVI noncovalently associating with the ITAM-containing FcR-γ chain.7,8 FcγRIIa is the sole Fc platelet receptor existing as a dimer with each molecule being a 40-kDa molecular weight (MW) type I membrane glycoprotein possessing 2 extracellular immunoglobulin domains that bind IgG (KD ∼10-6), a transmembrane domain, and an ITAM-containing intracellular domain. The extracellular domain contains a polymorphism at amino acid position 131 (Arg/His) and has been implicated in immune complex-related diseases such as systemic lupus erythematosus.9 In addition, in vitro and in vivo murine models have demonstrated that FcγRIIa is involved in heparin-induced thrombocytopenia (HITS).10 In vitro activation of platelet FcγRIIa via clustering with aggregated human IgG or activatory anti-FcγRIIa antibodies causes platelet activation, secretion, shape change, and aggregation through an ITAM signaling pathway.11 Such tyrosine kinase-dependent activity pathways are regulated by phosphatases in which activity is dependent on immunoreceptor tyrosine-based inhibition motif (ITIM) sequences. Platelets also express several ITIM-containing surface receptors, including PECAM-1, signal regulatory protein α (SIRPα), and protein zero related (PZR).12-14 PECAM-1 is a 130-kDa MW transmembrane protein member of the immunoglobulin-ITIM superfamily with an extracellular domain containing 6 Ig-domains, a transmembrane domain, and a dual ITIM-containing intracellular domain.15,16
It is now recognized that regulation of cell signaling involves a balance between activatory and inhibitory signals as observed in many immune cells.17 In particular, the negative regulation of ITAM-containing receptors by their inhibitory counterparts, ITIM-containing receptors, determines both the threshold and magnitude of activatory responses and corresponding cellular effects. For regulation of one receptor signaling pathway by another, a close physical proximity and coengagement between these receptors is required for induction of interactions between target substrates, adaptors, and effector molecules in their individual signaling pathways. Previous studies have defined physical associations of several platelet receptors, including GPIbα with FcγRIIa, GPIbα and the GPVI/FcR-γ chain complex, GPIbα and protein-disulfide isomerase, and CD151 with integrin αIIbβ3.18-20 More importantly, a functional relationship has been demonstrated between the ITAM-containing GPVI/FcR-γ chain complex that is negatively regulated by the ITIM-containing PECAM-1 receptor on both murine and human platelets to down-modulate platelet-collagen interactions.1,2 It is therefore conceivable that PECAM-1 may also negatively regulate FcγRIIa in human platelets, particularly because platelets lack the inhibitory coreceptor, FcγRIIB, that is known to negatively regulate FcγRIIA in other cell types. However, the fact that ITIM-containing receptors are known to have different affinities for different ITAM-containing receptors, and, unlike the GPVI/FcR-γ chain complex, the ITAM is associated with the ligand binding polypeptide of FcγRIIa, prompted us to investigate the possible existence of a physical and functional relationship between FcγRIIa and PECAM-1. The importance of FcγRIIa in both pathophysiologic conditions and healthy platelet function further necessitates these studies.
In light of evidence indicating the potential existence of a physical and functional relationship between platelet FcγRIIa and PECAM-1, we hypothesize that PECAM-1 negatively regulates FcγRIIa-mediated platelet responses through attenuation of signaling via the recruitment of protein-tyrosine phosphatases (PTPs) such as SHP-2 (Src homology 2-containing protein-tyrosine phosphatase 2) subsequent to the phosphorylation of the dual PECAM-1 ITIMs, resulting in dephosphorylation of important signaling effector and adaptor molecules involved in the FcγRIIa ITAM-mediated signaling pathway. To address this hypothesis using in vitro studies, we investigated the physical proximity and possible colocalization of FcγRIIa and PECAM-1 on the surface of human platelets using distinct complementary biochemical techniques, flow cytometry, fluorescence resonance energy transfer (FRET), coimmunoprecipitation, and Western blot studies. We next examined the functional effect of PECAM-1 on FcγRIIa-mediated platelet aggregation induced by aggregated IgG, activatory anti-FcγRIIa antibody cross-linking, and heparin-induced thrombocytopenia antibody. Finally, we delineated the molecular signaling mechanisms that govern the above functional relationship by using immunoprecipitation/Western blot analysis to profile important protein-tyrosine phosphorylated signaling molecules in FcγRIIa and PECAM-1 signaling pathways.
These studies show for the first time that FcγRIIa and PECAM-1 are physically and functionally associated on the surface of human platelets. More importantly, they provide a greater insight into the function and regulation at the signaling level of the physiologically and pathophysiologically important sole platelet Fc receptor, FcγRIIa, and potential novel therapeutic targets.
Materials and methods
Aggregated human IgG
Human IgG (10 mg; Sigma Chemical, St Louis, MO) was aggregated as described previously.18
Monoclonal antibodies
The following monoclonal antibodies (mAbs) were used in this study: PECAM-1.1 (IgG2a), PECAM-1.2 (IgG1), PECAM-1.3 (IgG1) were kindly provided by Dr Peter Newman (Blood Research Institute, Milwaukee, WI)21 ; anti-PECAM-1 mAbs 2BD4 (IgG1) and B2B1 (IgG1), and anti-CD151 mAbs 11B1.G4 (IgG2a) and 14A2 (IgG1)22,23 ; IV.3 (IgG1) was kindly provided by Prof Clark Anderson (Ohio State University, Columbus)24,25 ; VM16d mAb was kindly provided by Dr Michael Berndt (Monash University, Clayton, Victoria, Australia).26
Preparation and biotinylation of monoclonal antibody Fab fragments
Fab fragments were prepared for the following mAbs: PECAM-1.1, PECAM-1.2, PECAM-1.3, 2BD4, B2B1, 11B1, and IV.3 with the use of a standard protocol using Ficin as previously described.27 Biotinylation of IV.3, PECAM-1.1, 2BD4, B2B1, and 11B1 Fab fragments was performed according to manufacturers' instructions.
Preparation of human platelets
Platelet-rich plasma (PRP) and washed platelets were prepared from freshly obtained human blood as previously described.16
Binding of aggregated human IgG to washed platelets
The binding of aggregated human IgG and IV.3 to human platelets was assessed as previously described.18 Acquisition flow cytometry analysis was performed with an Epics XL-MCL (Beckman-Coulter, Fullerton, CA) using a platelet-specific fluorescein isothiocyanate (FITC) protocol with forward scatter (FSC), side scatter (SSC), and fluorescent parameters in log scale.
Aggregated human IgG-mediated platelet aggregation
Aggregated IgG induction of platelet aggregation mediated through FcγRIIa was analyzed using a Packs-4 Platelet Aggregation Chromogenic Kinetic System (Helena Laboratories, Beaumont, TX). For aggregated IgG-mediated platelet aggregation, 225 μg/mL aggregated IgG was added to 450 μL washed platelets (3 × 108/mL) in Ringer-citrate-dextrose (RCD) pH 7.4. In blocking experiments, 10 μg/mL final concentration of nonbiotinylated anti-FcγRIIa (IV.3 Fab), biotinylated anti-PECAM-1 (PECAM-1.1, 2BD4, and B2B1 Fab), and biotinylated anti-CD151 (11B1 Fab) was added to the washed platelets with stirring for 5 minutes, prior to the addition of aggregated IgG to induce platelet aggregation.
IV.3-mediated platelet aggregation
This assay system involves the simultaneous coengagement of FcγRIIa and PECAM-1 using optimal concentrations of biotinylated anti-FcγRIIa (IV.3) and biotinylated anti-PECAM-1 (2BD4 Fab) with cross-linking induced by the addition of Neutravidin (Pierce, Rockford, IL). Specificity control of Neutravidin to induce FcγRIIa-mediated aggregation through cross-linking of biotinylated IV.3 mAb was applied using biotinylated IV.3 mAb without the addition of Neutravidin. Anti-CD151 (biotinylated 11B1 Fab) was used as a negative control for inhibition of platelet aggregation. Cross-linking of receptors was induced by 10 μg/mL Neutravidin, resulting in either platelet aggregation or inhibition.
HITS-mediated platelet aggregometry
The assay system used was essentially the same as that in FcγRIIa-mediated platelet aggregation studies using aggregated human IgG. However, HITS-positive donor sera along with 1 U/mL heparin instead of aggregated IgG were used to induce platelet aggregation. A volume of 450 μL of 3 × 106/mL PRP with 20 μL HITS-positive donor sera and 1 U/mL heparin were used in these experiments.
Fluorescence resonance energy transfer (FRET)
IV.3, PECAM-1.1, PECAM-1.2, and VM16d mAb F(ab′)2 were labeled with Alexa Fluor 546 by using an Alexa Fluor 546 Protein Labeling Kit (Molecular Probes, Eugene, OR) and fluorescein 5-isothiocyanate isomer 1 (FITC) (Sigma Chemical) according to manufacturer's instructions. Washed platelets (100 μL of 3 × 108/mL) in RCD, pH 6.5, containing 0.2% (vol/vol) bovine serum albumin (BSA) were incubated with the respective donor and acceptor fluorochrome-labeled F(ab)′2 fragments directed to the receptors at 10 μg/mL for 20 minutes. Samples were then centrifuged for 1500g for 5 minutes at room temperature, then washed with 200 μL RCD, pH 6.5, containing 0.2% (wt/vol) BSA and resuspended in 0.5 mL RCD, pH 7.4, for FRET detection by using an LS-50B luminescence spectrometer (Perkin Elmer, Bucks, England).
Calcium mobilization
Washed platelets (4 mL of 3 × 108 cells/mL) in RCD, pH 6.5, were loaded with 3 μM fluorescent calcium indicator, Fura-2/am (Molecular Probes) as previously described.28 To induce calcium mobilization, 5 μg/mL biotinylated IV.3 IgG was added and allowed 5 minutes for binding prior to the addition of 10 μg/mL Neutravidin to induce clustering of FcγRIIA. Biotinylated anti-PECAM-1 Fab and a negative control biotinylated anti-CD151 Fab at 5 μg/mL were used for coengagement of PECAM-1 and CD151 as required.
IV.3 cross-linking with and without PECAM-1 coengagement and immunoprecipitation/Western blot studies
A volume of 0.5 mL washed human platelets (3 × 108/mL) in RCD, pH 7.4, was stirred at 200g in an aggregometer cuvette at 37°C for 1 minute in a Payton dual channel aggregometer module (Paton Scientific, Victor Harbour, South Australia). Biotinylated IV.3 IgG (10 μg/mL) was then added and allowed to bind to the platelets for 5 minutes before cross-linking by the addition of 10 μg/mL Neutravidin. For PECAM-1 coengagement, 10 μg/mL biotinylated anti-PECAM-1, 2BD4, or PECAM-1.1 Fab fragments were added after the addition of biotinylated IV.3 IgG. Samples were cross-linked with 10 μg/mL Neutravidin for 0, 15 seconds, 30 seconds, 1 minute, 2 minutes, 5 minutes, and up to 15 minutes before stopping the reaction with the addition of 0.5 mL Triton lysis buffer. Specific details of immunoprecipitation procedures, Western blotting, and stripping are as previously described.16
Chemical cross-linking of cell surface antigens and digitonin lysis of platelet preparations
Chemical cross-linking of platelet cell surface antigens and digitonin lysis conditions are essentially as previously described.18 Following lysis, samples were centrifuged at 11 000g for 10 minutes at 4°C, and digitonin-soluble supernatants were removed for preclearing and immunoprecipitation studies.
Statistical analysis of data
Acquisition flow cytometry analysis data were analyzed statistically with Excel (Microsoft, Redmond, WA) for statistical significance using Student t test (P < .05 as significant), standard deviation, and error.
Results
Effect of mAbs on aggregated human IgG and IV.3 binding to platelets
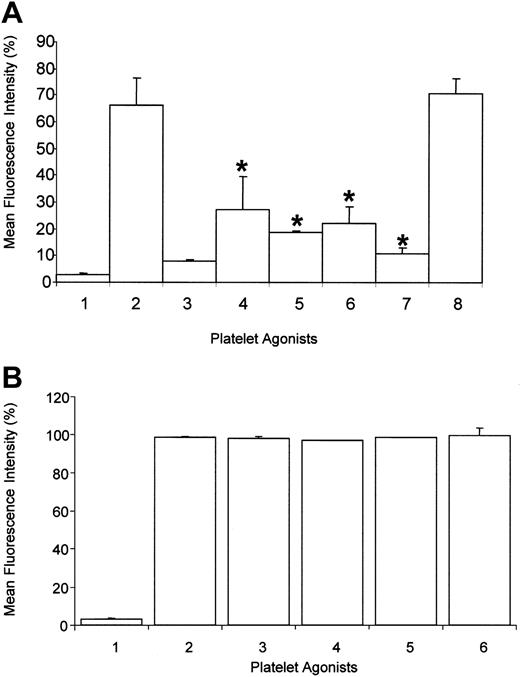
To establish a possible physical proximity relationship between FcγRIIa and PECAM-1, aggregated IgG binding to FcγRIIa studies were conducted in the presence and absence of anti-PECAM-1 Fab fragments. These studies have revealed significant reduction (P < .05) of binding of aggregated IgG (75 μg/mL) to FcγRIIa with treatment of platelets with 4 different anti-PECAM-1 Fab fragments (PECAM-1.1 and PECAM-1.3, B2B1 and 2BD4) (Figure 1A). Specifically, a 2.5-fold reduction in aggregated IgG binding was observed following pretreatment with anti-PECAM-1 Fab fragments, whereas a 6-fold decrease in aggregated IgG binding to FcγRIIa was observed with FcγRIIa-specific blocking mAb, IV.3 Fab (10 μg/mL). Conversely, pretreatment with an antibody recognizing a platelet tetraspanin family member, CD151, had no observable effect in terms of inhibition of aggregated IgG binding. CD151 has a similar copy number to PECAM-1 on platelets on the basis of 11B1 binding.23
Inhibition of aggregated IgG binding to human platelets by anti-PECAM-1 antibodies. (A) Binding of aggregated human IgG to washed platelets was measured by flow cytometry. Platelets were (1) untreated or (2) incubated with aggregated human IgG. Platelets were also incubated with aggregated human IgG after pretreatment with 10 μg/mL Fab fragments of (3) IV.3 mAb; (4) PECAM-1.1 mAb; (5) PECAM-1.3 mAb; (6) B2B1 mAb; (7) 2BD4 mAb; or negative control (8) CD151 mAb. * denotes statistical significance (P < .05) (n = 3). (B) Binding of biotinylated IV.3 to washed platelets in the presence of anti-PECAM-1 Fab fragments was measured by flow cytometry. Platelets were (1) untreated or (2) incubated with biotinylated IV.3 Fab (10 μg/mL). Platelets were also incubated with biotinylated IV.3 Fab (10 μg/mL) after pretreatment with increasing doses of anti-PECAM-1 2BD4 Fab fragments (3) 5 μg/mL, (4) 10 μg/mL, (5) 20 μg/mL, (6) 40 μg/mL, and IV.3 binding detected with streptavidin-PE and measured with flow cytometry. Results are representative of 3 experiments. Error bars represent mean ± SEM.
Inhibition of aggregated IgG binding to human platelets by anti-PECAM-1 antibodies. (A) Binding of aggregated human IgG to washed platelets was measured by flow cytometry. Platelets were (1) untreated or (2) incubated with aggregated human IgG. Platelets were also incubated with aggregated human IgG after pretreatment with 10 μg/mL Fab fragments of (3) IV.3 mAb; (4) PECAM-1.1 mAb; (5) PECAM-1.3 mAb; (6) B2B1 mAb; (7) 2BD4 mAb; or negative control (8) CD151 mAb. * denotes statistical significance (P < .05) (n = 3). (B) Binding of biotinylated IV.3 to washed platelets in the presence of anti-PECAM-1 Fab fragments was measured by flow cytometry. Platelets were (1) untreated or (2) incubated with biotinylated IV.3 Fab (10 μg/mL). Platelets were also incubated with biotinylated IV.3 Fab (10 μg/mL) after pretreatment with increasing doses of anti-PECAM-1 2BD4 Fab fragments (3) 5 μg/mL, (4) 10 μg/mL, (5) 20 μg/mL, (6) 40 μg/mL, and IV.3 binding detected with streptavidin-PE and measured with flow cytometry. Results are representative of 3 experiments. Error bars represent mean ± SEM.
To exclude the possibility that anti-PECAM-1 Fab fragments interfere with FcγRIIa ligand binding, we performed a dose-response of nonbiotinylated anti-PECAM-1 Fab fragments (0-40 μg/mL) in the presence of biotinylated IV.3 Fab fragments and detected IV.3 binding to platelets with streptavidin-phycoerythrin (PE) and flow cytometry. As shown in Figure 1B, the anti-PECAM-1 Fab fragments had no effect on the alteration of the IV.3 binding to FcγRIIa on the platelet membrane. Overall, these results suggest the existence of either a close physical or functional proximity between FcγRIIa and PECAM-1, whereas no such relationship can be observed between FcγRIIa and CD151.
Analysis of receptor colocalization by FRET
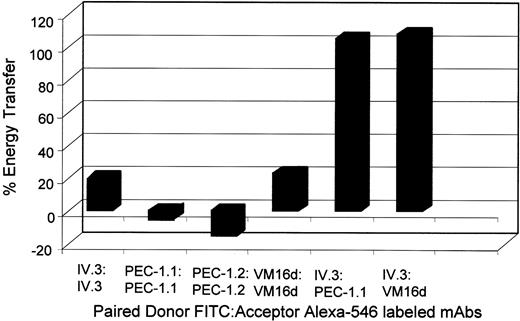
On the basis of the ability of anti-PECAM-1 mAbs to inhibit the binding of aggregated human IgG to washed human platelets, we hypothesized that PECAM-1 and FcγRIIa were located in close physical proximity on the platelet membrane. To test the proximity we used FRET between antibodies bound to FcγRIIa and PECAM-1 and coimmunoprecipitation studies. By using FITC-conjugated anti-FcγRIIa IV.3 F(ab′)2 and Alexa Fluor 546-conjugated anti-PECAM-1.1 as the donor and acceptor fluorochromes, respectively, significant (100%) FRET was observed relative to background controls (Figure 2). FITC-conjugated anti-FcγRIIA IV.3 F(ab′)2 and Alexa Fluor 546-conjugated anti-GPIbα VM16d was also positive (100%) for FRET as shown previously (Figure 2).18 Relatively lower FRET (6%-20%) was observed with donor and acceptor fluorochromes labeling of the same receptor for all 3 receptors (Figure 2), indicating the proximity of FcγRIIa to PECAM-1 and to GPIbα is much greater than that between identical receptors.
Fluorescence resonance energy transfer showing close physical proximity of FcγRIIa with platelet glycoprotein receptors, PECAM-1, and GPIbα Fluorescence resonance energy transfer efficiency values between platelet membrane epitopes using monoclonal antibody F(ab′)2 fragments to glycoprotein Ibα (VM16d), PECAM-1 (PEC-1.1, PEC-1.2), and FcγRIIa (IV.3). Energy transfer was measured between FITC-labeled (donor excitation) and Alexa-546-labeled (acceptor emission) epitopes using a luminescence spectrometer. Results are representative of 4 experiments.
Fluorescence resonance energy transfer showing close physical proximity of FcγRIIa with platelet glycoprotein receptors, PECAM-1, and GPIbα Fluorescence resonance energy transfer efficiency values between platelet membrane epitopes using monoclonal antibody F(ab′)2 fragments to glycoprotein Ibα (VM16d), PECAM-1 (PEC-1.1, PEC-1.2), and FcγRIIa (IV.3). Energy transfer was measured between FITC-labeled (donor excitation) and Alexa-546-labeled (acceptor emission) epitopes using a luminescence spectrometer. Results are representative of 4 experiments.
Physical association between FcγRIIa and PECAM-1
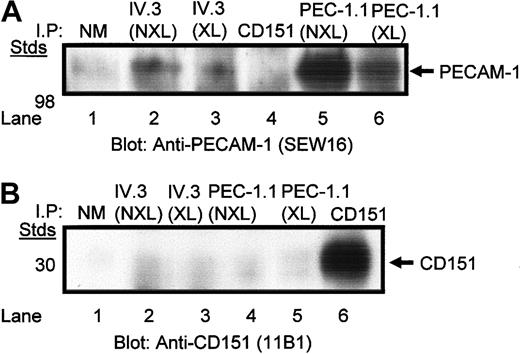
Immunoprecipitation with anti-PECAM-1.1 and anti-FcγRIIa IV.3 IgG mAbs from chemically cross-linked and non-cross-linked digitonin-lysed platelets and blotting for PECAM-1 reveals the presence of PECAM-1 bands (Figure 3A). In contrast, platelet lysates immunoprecipitated with healthy mouse IgG1 and anti-CD151, 11B1.G4 IgG mAb, displayed no PECAM-1 bands. Conversely, when similarly treated platelet lysates were blotted for CD151 with 11B1.G4 IgG mAb, only anti-CD151, 11B1.G4 immunoprecipitates displayed the presence of CD151 (Figure 3B). In addition, cross-linking decreased the yield of monomeric PECAM-1 but induced the formation of dimeric and oligomeric forms of PECAM-1 (results not shown) in both digitonin-lysed platelets immunoprecipitated with anti-PECAM-1 and anti-FcγRIIa mAb, as previously described.29 Taken as a whole, these results indicate that FcγRIIa is constitutively associated with PECAM-1, whereas CD151 is not physically associated with either receptor, on the platelet membrane.
Coimmunoprecipitation of PECAM-1 and FcγRIIa from digitonin-lysed platelets. (A) Washed platelets were treated with and without chemical cross-linking prior to digitonin lysis and immunoprecipitation using antibodies against the polypeptides: normal mouse IgG1, FcγRIIa (IV.3), PECAM-1 (PEC-1.1), and CD151 (11B1). Immunoblot shown reveals presence of PECAM-1 antigen. (B) Same as panel A. The only exception is that the immunoblot is probed for the presence of CD151 under nonreduced conditions. NM indicates normal mouse IgG; NXL, nonchemically crosslinked; and XL, chemically crosslinked.
Coimmunoprecipitation of PECAM-1 and FcγRIIa from digitonin-lysed platelets. (A) Washed platelets were treated with and without chemical cross-linking prior to digitonin lysis and immunoprecipitation using antibodies against the polypeptides: normal mouse IgG1, FcγRIIa (IV.3), PECAM-1 (PEC-1.1), and CD151 (11B1). Immunoblot shown reveals presence of PECAM-1 antigen. (B) Same as panel A. The only exception is that the immunoblot is probed for the presence of CD151 under nonreduced conditions. NM indicates normal mouse IgG; NXL, nonchemically crosslinked; and XL, chemically crosslinked.
Effect of mAbs on aggregated IgG-mediated platelet aggregation
Whereas aggregated human IgG induced rapid and robust platelet aggregation, there was complete inhibition of aggregated IgG-induced platelet aggregation following pretreatment of washed platelets with anti-PECAM-1 IgG mAb, PECAM-1.1 (Figure 4). Similar results were obtained with anti-PECAM-1 mAbs, 2BD4 and B2B1 (results not shown). Similarly, the anti-FcγRIIa, IV.3 IgG mAb (10 μg/mL) displayed the same inhibitory outcome (Figure 4A). Conversely, as predicted from initial inhibition of aggregated IgG binding studies, anti-CD151, 11B1.G4 mAb IgG, does not inhibit platelet aggregation (Figure 4A).
Inhibition of aggregated IgG FcγRIIA-mediated platelet aggregation by anti-PECAM-1 intact and Fab fragments. (A) Addition of aggregated IgG to washed platelets results in a lag phase of 2 minutes followed by platelet aggregation. Pretreatment of platelets with anti-CD151 mAb 11B1 (10 μg/mL) did not block aggregated IgG-mediated platelet aggregation. Pretreatment of platelets with either anti-FcγRIIA mAb IV.3 (10 μg/mL) or anti-PECAM-1.1 mAb (10 μg/mL) completely blocked aggregated IgG-mediated platelet aggregation. (B) Platelet aggregation of washed platelets induced by subthreshold concentration of aggregated IgG. Pretreatment of washed platelets with biotinylated anti-CD151 mAb 11B1 Fab (10 μg/mL) for 5 minutes prior to Neutravidin cross-linking did not block aggregated IgG-mediated platelet aggregation. Pretreatment of washed platelets with either FcγRIIA mAb IV.3 Fab (10 μg/mL) or biotinylated anti-PECAM-1 mAb 2BD4 Fab (10 μg/mL) for 5 minutes prior to 10 μg/mL Neutravidin cross-linking completely blocked aggregated IgG-mediated platelet aggregation.
Inhibition of aggregated IgG FcγRIIA-mediated platelet aggregation by anti-PECAM-1 intact and Fab fragments. (A) Addition of aggregated IgG to washed platelets results in a lag phase of 2 minutes followed by platelet aggregation. Pretreatment of platelets with anti-CD151 mAb 11B1 (10 μg/mL) did not block aggregated IgG-mediated platelet aggregation. Pretreatment of platelets with either anti-FcγRIIA mAb IV.3 (10 μg/mL) or anti-PECAM-1.1 mAb (10 μg/mL) completely blocked aggregated IgG-mediated platelet aggregation. (B) Platelet aggregation of washed platelets induced by subthreshold concentration of aggregated IgG. Pretreatment of washed platelets with biotinylated anti-CD151 mAb 11B1 Fab (10 μg/mL) for 5 minutes prior to Neutravidin cross-linking did not block aggregated IgG-mediated platelet aggregation. Pretreatment of washed platelets with either FcγRIIA mAb IV.3 Fab (10 μg/mL) or biotinylated anti-PECAM-1 mAb 2BD4 Fab (10 μg/mL) for 5 minutes prior to 10 μg/mL Neutravidin cross-linking completely blocked aggregated IgG-mediated platelet aggregation.
To exclude any Fc-mediated effects, Fab fragments were used in subsequent platelet experiments. Pretreatment with biotinylated anti-PECAM-1 Fab (2BD4) (10 μg/mL) and cross-linking of PECAM-1 with Neutravidin (10 μg/mL) results in inhibition of aggregated IgG-induced platelet aggregation (Figure 4B). Nonbiotinylated anti-FcγRIIA F(ab) (IV.3) exhibits potent inhibition of platelet aggregation, whereas no inhibition of platelet aggregation is observed with biotinylated anti-CD151 Fab (11B1.G4) (10 μg/mL) (Figure 4B). This inhibitory effect mediated by anti-PECAM Fab fragments on FcγRIIa-mediated platelet aggregation required cross-linking of PECAM-1.
Effect of mAbs on FcγRIIa-mediated platelet aggregation
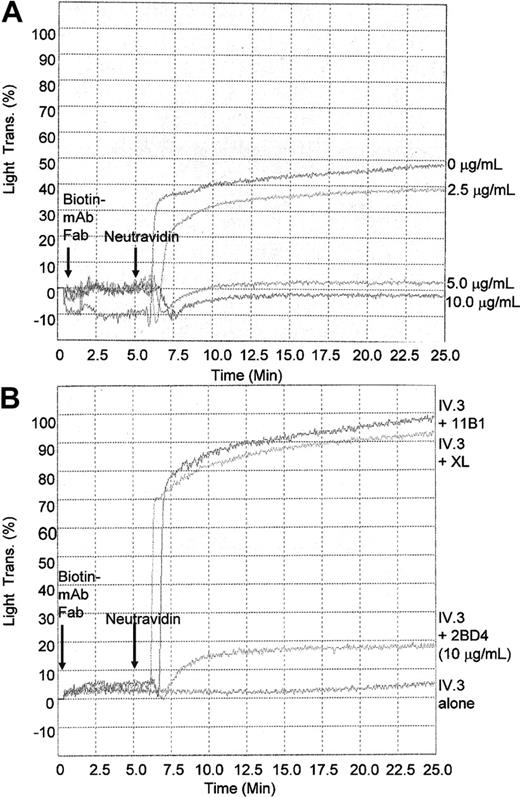
FcγRIIa-mediated platelet aggregation was performed using biotinylated IV.3 mAb (10 μg/mL) and cross-linking with Neutravidin (10 μg/mL). The addition of biotinylated anti-PECAM-1 Fab (2BD4) prior to cross-linking with Neutravidin allows the simultaneous coengagement of PECAM-1. Coengagement of PECAM-1 induced by addition of biotinylated anti-PECAM-1 Fab (2BD4) with biotinylated IV.3 mAb followed by cross-linking with Neutravidin resulted in a dose-dependent inhibition of platelet aggregation with biotinylated 2BD4 anti-PECAM-1 Fab fragments (Figure 5A). Conversely, when biotinylated anti-CD151 Fab (11B1.G4) was used to simultaneously cross-link CD151 and FcγRIIa, no inhibition of platelet aggregation was observed (Figure 5B). Similarly, in the absence of Neutravidin, no platelet aggregation response was observed, signifying the specificity of biotinylated anti-PECAM-1 Fab to inhibit FcγRIIa-mediated platelet aggregation (Figure 5B).
Inhibition of FcγRIIa-mediated platelet aggregation by anti-PECAM-1 Fab fragments. (A) Washed platelets (3 × 108 platelets/mL) were pretreated with varying concentrations of biotinylated Fab fragments of anti-PECAM-1 (2BD4) (0-10 μg/mL as indicated) and anti-FcγRIIa IV.3 mAb (10 μg/mL) for 5 minutes. Following pretreatment, where indicated cross-linking (XL) was initiated by addition of 10 μg/mL Neutravidin to induce FcγRIIa-mediated platelet aggregation. (B) Washed platelets (3 × 108 platelets/mL) were pretreated with 10 μg/mL biotinylated Fab antibody fragments of mAbs directed to CD151 (11B1), PECAM-1 2BD4, or FcγRIIa (IV.3) for 5 minutes. Following pretreatment, cross-linking was initiated in appropriate cuvettes by addition of 10 μg/mL Neutravidin to induce FcγRIIa-mediated platelet aggregation.
Inhibition of FcγRIIa-mediated platelet aggregation by anti-PECAM-1 Fab fragments. (A) Washed platelets (3 × 108 platelets/mL) were pretreated with varying concentrations of biotinylated Fab fragments of anti-PECAM-1 (2BD4) (0-10 μg/mL as indicated) and anti-FcγRIIa IV.3 mAb (10 μg/mL) for 5 minutes. Following pretreatment, where indicated cross-linking (XL) was initiated by addition of 10 μg/mL Neutravidin to induce FcγRIIa-mediated platelet aggregation. (B) Washed platelets (3 × 108 platelets/mL) were pretreated with 10 μg/mL biotinylated Fab antibody fragments of mAbs directed to CD151 (11B1), PECAM-1 2BD4, or FcγRIIa (IV.3) for 5 minutes. Following pretreatment, cross-linking was initiated in appropriate cuvettes by addition of 10 μg/mL Neutravidin to induce FcγRIIa-mediated platelet aggregation.
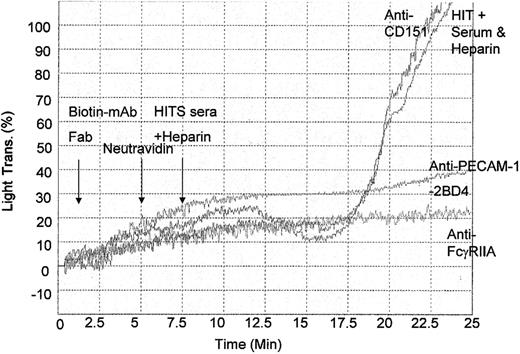
Effect of mAbs on HITS-mediated platelet aggregation
In humans, the sole Fc receptor for IgG, FcγRIIa, in platelets plays an important role in the pathophysiology of drug-induced thrombocytopenia, including HITS. On the basis of these features, we hypothesized that PECAM-1 may negatively regulate HITS-mediated platelet aggregation via its down-regulation of FcγRIIa-mediated signaling events. To test this possibility, we examined the effect of anti-PECAM-1 Fab fragments in HITS-mediated platelet aggregation. As shown in Figure 6, 3 × 108 washed platelets/mL pretreated with 10 μg/mL biotinylated anti-PECAM-1 Fab fragments (2BD4) and cross-linking with 10 μg/mL Neutravidin resulted in 5-fold inhibition of platelet aggregation induced by 20 μL HITS-positive donor sera. Similar results were observed with anti-PECAM-1 Fab fragments, PECAM-1.1 and B2B1 (results not shown). In addition, pretreatment with nonbiotinylated anti-FcγRIIa Fab fragments (IV.3) (10 μg/mL) also inhibited HITS-mediated platelet aggregation (Figure 6). In contrast, no inhibitory effect was observed with biotinylated anti-CD151 Fab fragments (11B1.G4) (10 μg/mL). Taken together, these studies strongly suggest the negative inhibitory effect of PECAM-1 activation on FcγRIIa-mediated platelet aggregation induced by HITS antibodies.
Inhibition of FcγRIIa-mediated HITS platelet aggregation by cross-linked anti-PECAM-1 Fab fragments. Platelet-rich plasma (3 × 106 platelets/mL) was pretreated with various biotinylated Fab antibody fragments directed to CD151 (11B1) (10 μg/mL); nonbiotinylated anti-FcγRIIa (IV.3) (10 μg/mL), and anti-PECAM-1 (2BD4) (10 μg/mL) for 5 minutes prior to cross-linking with 10 μg/mL Neutravidin and stimulation with HITS-positive donor sera and 1 U/mL heparin to induce HITS-mediated platelet aggregation.
Inhibition of FcγRIIa-mediated HITS platelet aggregation by cross-linked anti-PECAM-1 Fab fragments. Platelet-rich plasma (3 × 106 platelets/mL) was pretreated with various biotinylated Fab antibody fragments directed to CD151 (11B1) (10 μg/mL); nonbiotinylated anti-FcγRIIa (IV.3) (10 μg/mL), and anti-PECAM-1 (2BD4) (10 μg/mL) for 5 minutes prior to cross-linking with 10 μg/mL Neutravidin and stimulation with HITS-positive donor sera and 1 U/mL heparin to induce HITS-mediated platelet aggregation.
Coengagement of FcγRIIa and PECAM-1 leads to negative regulation of phospholipase Cγ2 and PI 3-kinase-dependent signaling pathways
On clustering, FcγRIIa is tyrosine phosphorylated and recruits Syk protein-tyrosine kinase (PTK) via its intrinsic ITAMs The recruitment of Syk PTK allows tyrosine phosphorylation of PLCγ2 and recruitment of phosphatidylinositol 3 (PI 3) kinase, whose activation results in downstream rapid accumulation of phosphatidylinositol-3, -4, -5 phosphate [PtdIns(3,4,5)P3] and Akt phosphorylation. Activation of the PLCγ2 signaling pathway results in calcium mobilization to promote platelet secretion and engagement of integrin αIIbβ3 to initiate platelet aggregation. On the basis of our studies with PECAM-1, we hypothesize that coengagement of PECAM-1 and its recruitment and activation of protein-tyrosine phosphatases such as SHP-2 delivers a negative feedback signal to down-modulate FcγRIIa-mediated signaling events. To test this hypothesis, we examined a number of phosphorylation profiles of key signaling molecules (antiphosphotyrosine content, tyrosine phosphorylation of PLCγ2, phosphoSer/Thr Akt) and calcium mobilization associated with FcγRIIa signaling by using immunoprecipitation, Western blot, and calcium mobilization studies.
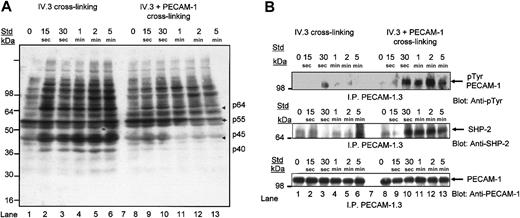
The antiphosphotyrosine blot of total tyrosine phosphorylated platelet proteins following IV.3 cross-linking of FcγRIIA alone or with PECAM-1 coengagement revealed induction of several tyrosine phosphorylated platelet proteins following clustering of FcγRIIA alone (Figure 7A, lanes 1-6). In contrast, coengagement of PECAM-1 with FcγRIIA cross-linking induced a significant reduction in tyrosine phosphorylation over time of at least 4 identifiable tyrosine phosphorylated platelet proteins as indicated by arrows with approximate molecular weights of p64, p55, p45, and p40 (Figure 7A, lanes 8-13). After 1 minute of coengagement with PECAM-1, a significant decrease in tyrosine phosphorylation of these 4 platelet proteins was seen, as observed in lanes 12 and 13. Three of the 4 platelet proteins displayed inducible protein-tyrosine phosphorylation subsequent to clustering of FcγRIIa after 15 seconds with IV.3 cross-linking as indicated in lanes 2 to 6 (Figure 7A). Overall, these results indicate a reduction in tyrosine phosphorylation of platelet proteins on PECAM-1 coengagement consistent with its inhibitory activity.
Coengagement of PECAM-1 with IV.3 cross-linking leads to a reduction in tyrosine phosphorylation of platelet proteins, induction of tyrosine phosphorylation of PECAM-1, and recruitment of SHP-2 protein-tyrosine phosphatase. (A) Washed platelets were stimulated by FcγRIIa cross-linking with and without coengagement of PECAM-1 over 5 minutes at 37°C with stirring. Immunoblotting of 100 μg platelet whole proteins with horseradish peroxidase (HRP)-conjugated antiphosphotyrosine antibody, RC20, and enhanced chemiluminescence (ECL) development. Arrows indicate proteins with reduced antiphosphotyrosine content. (B, top panel) Washed platelets were stimulated by FcγRIIa cross-linking with and without coengagement of PECAM-1 over a time course of 0 to 5 minutes at 37°C with stirring. Immunoprecipitation of PECAM-1 with monoclonal anti-PECAM-1 antibody (PECAM-1.3) followed by immunoblotting with HRP-conjugated antiphosphotyrosine antibody (RC20) and ECL development. (B, middle panel) Blot was stripped and reprobed for SHP-2 antigen with polyclonal anti-SHP-2 antibody. (B, bottom panel) Blot was stripped and reprobed for PECAM-1 antigen content with polyclonal anti-PECAM-1 (SEW16) antibody.
Coengagement of PECAM-1 with IV.3 cross-linking leads to a reduction in tyrosine phosphorylation of platelet proteins, induction of tyrosine phosphorylation of PECAM-1, and recruitment of SHP-2 protein-tyrosine phosphatase. (A) Washed platelets were stimulated by FcγRIIa cross-linking with and without coengagement of PECAM-1 over 5 minutes at 37°C with stirring. Immunoblotting of 100 μg platelet whole proteins with horseradish peroxidase (HRP)-conjugated antiphosphotyrosine antibody, RC20, and enhanced chemiluminescence (ECL) development. Arrows indicate proteins with reduced antiphosphotyrosine content. (B, top panel) Washed platelets were stimulated by FcγRIIa cross-linking with and without coengagement of PECAM-1 over a time course of 0 to 5 minutes at 37°C with stirring. Immunoprecipitation of PECAM-1 with monoclonal anti-PECAM-1 antibody (PECAM-1.3) followed by immunoblotting with HRP-conjugated antiphosphotyrosine antibody (RC20) and ECL development. (B, middle panel) Blot was stripped and reprobed for SHP-2 antigen with polyclonal anti-SHP-2 antibody. (B, bottom panel) Blot was stripped and reprobed for PECAM-1 antigen content with polyclonal anti-PECAM-1 (SEW16) antibody.
Protein-tyrosine phosphorylation is an early event in FcγRIIa-mediated signal transduction. To test if PECAM-1 cross-linking attenuated FcγRIIa-mediated signal transduction, we performed a time course (0-5 minutes) of IV.3 ± PECAM-1 cross-linking, lysed the platelet suspensions, immunoprecipitated PECAM-1, and detected tyrosine phosphorylated PECAM-1 by immunoblotting with an antiphosphotyrosine antibody, RC20. As shown in Figure 7B (top panel), weak inducible tyrosine phosphorylation of PECAM-1 was observed following IV.3 cross-linking over time (lanes 1-6) and strongly inducible after 15 seconds following IV.3 ± PECAM-1 (lanes 8-13). Cross-linking of PECAM-1 alone with mAbs results in weak inducible tyrosine phosphorylation of PECAM-1 as previously described.28 In addition, as shown in Figure 7B (middle panel), inducible recruitment of SHP-2 protein-tyrosine phosphatase parallels the induction of tyrosine phosphorylation of PECAM-1 observed over time. The anti-PECAM-1 blot confirmed the proper PECAM-1 antigen loading in PECAM-1 immunoprecipitates (Figure 7B, bottom panel). These studies verify that induction of the tyrosine phosphorylation of PECAM-1 ITIMs leads to the recruitment of SHP-2 following clustering of FcγRIIa and PECAM-1 in platelets.
As recruitment of Syk by FcγRIIa leads to recruitment of PLCγ2 and subsequent downstream activation of signaling events, we tested the tyrosine phosphorylation status of PLCγ2 following clustering of FcγRIIA and coengagement of FcγRIIa and PECAM-1. As shown in Figure 8A (top panel), following clustering of FcγRIIa, there is an inducible tyrosine phosphorylation of PLCγ2 evident at 30 seconds and peaking at 1 minute. Following coengagement of FcγRIIa and PECAM-1, there was almost complete inhibition of the tyrosine phosphorylation of PLCγ2 (lanes 9-13). In contrast, coengagement of FcγRIIa and CD151 showed no difference in the level of tyrosine phosphorylation of PLCγ2 (Figure 8B, top panel). PLCγ2 antigen loading is consistent in all lanes (Figure 8A-B, bottom panels). To exclude the possibility that competition between biotinylated anti-IV.3 and biotinylated anti-PECAM-1 Fab fragments for a limiting amount of Neutravidin accounts for the lower level of tyrosine phosphorylated PLC-γ2 over time, we have performed a dose response of Neutravidin (0-100 μg/mL) at a 1 minute timepoint. As shown in Figure 8C, increasing doses of Neutravidin resulted in equivalent levels of tyrosine phosphorylation of PLC-γ2 following IV.3 cross-linking alone. Following cross-linking of IV.3 and PECAM-1, the reduced tyrosine phosphorylation of PLC-γ2 is not affected by super-saturating concentrations of Neutravidin. These studies have shown that coengagement of PECAM-1 with FcγRIIa leads to dephosphorylation of tyrosine phosphorylated PLCγ2, an inhibitory signaling event characteristic of PECAM-1-mediated signaling involving SHP-2 recruitment and activation.
Coengagement of PECAM-1 with FcγRIIa cross-linking with IV.3 results in dephosphorylation of PLCγ2. (A, top panel) Washed platelets were stimulated by FcγRIIa cross-linking with and without coengagement of PECAM-1 over 5 minutes at 37°C with stirring. Immunoprecipitation of PLCγ2 with polyclonal anti-PLCγ2 antibody followed by immunoblotting with HRP-conjugated antiphosphotyrosine antibody (RC20) and ECL development. (A, bottom panel) Blot was stripped and reprobed for PLCγ2 antigen content with PLCγ2 antibody. (B, top panel) Same experimental conditions as in panel A, with the exception that coengagement of CD151 was performed. (B, bottom panel) Blot was stripped and reprobed for PLCγ2 antigen content with PLCγ2 antibody. (C, top panel) Similar experimental conditions as in panel A, with the exception that a single 1-minute timepoint and a dose response with Neutravidin (0-100 μg/mL) were performed. (C, bottom panel) Blot was stripped and reprobed for PLCγ2 antigen content with PLCγ2 antibody.
Coengagement of PECAM-1 with FcγRIIa cross-linking with IV.3 results in dephosphorylation of PLCγ2. (A, top panel) Washed platelets were stimulated by FcγRIIa cross-linking with and without coengagement of PECAM-1 over 5 minutes at 37°C with stirring. Immunoprecipitation of PLCγ2 with polyclonal anti-PLCγ2 antibody followed by immunoblotting with HRP-conjugated antiphosphotyrosine antibody (RC20) and ECL development. (A, bottom panel) Blot was stripped and reprobed for PLCγ2 antigen content with PLCγ2 antibody. (B, top panel) Same experimental conditions as in panel A, with the exception that coengagement of CD151 was performed. (B, bottom panel) Blot was stripped and reprobed for PLCγ2 antigen content with PLCγ2 antibody. (C, top panel) Similar experimental conditions as in panel A, with the exception that a single 1-minute timepoint and a dose response with Neutravidin (0-100 μg/mL) were performed. (C, bottom panel) Blot was stripped and reprobed for PLCγ2 antigen content with PLCγ2 antibody.
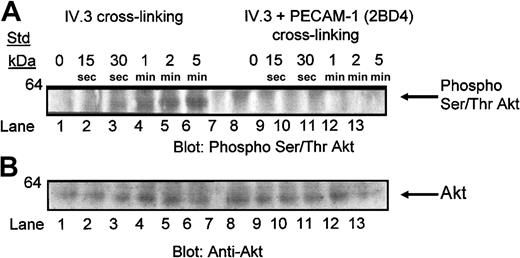
In addition, as recruitment of Syk by FcγRIIa also leads to recruitment of PI 3-kinase and subsequent downstream activation of signaling events, we tested the Ser/Thr phosphorylation status of Akt, a known downstream effector of PI 3-kinase-dependent signaling events following coengagement of FcγRIIa and PECAM-1. Following clustering of FcγRIIa, there is an inducible Ser/Thr phosphorylation of Akt evident by 30 seconds of stimulation (Figure 9A, lanes 3-6). In contrast, there was complete inhibition of Ser/Thr phosphorylation of Akt following coengagement of PECAM-1 with FcγRIIa and probing with antiphosphoSer/Thr Akt (lanes 9-13). Equal protein loading is observed in an Akt Western blot (Figure 9B). These results most likely suggest that recruited SHP-2 dephosphorylates Syk, an essential upstream effector of PI 3-kinase recruitment and activation by FcγRIIa, thereby initiating a block in PI 3-kinase-dependent signaling events.
Coengagement of PECAM-1 with FcγRIIa cross-linking with IV.3 results in dephosphorylation of serine/threonine kinase, Akt. (A) Washed platelets were stimulated by FcγRIIa cross-linking with and without coengagement of PECAM-1 over a time course of 0 to 5 minutes at 37°C with stirring. Platelet lysate (200 μg) was electrophoresed on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, followed by immunoblotting with a mixture of antiphosphoSer473 Akt and antiphosphoThr308 Akt (1/2000) followed by HRP-conjugated antirabbit (1/10 000) and ECL development. (B) Blot was stripped and reprobed for Akt antigen content with goat anti-Akt antibody (0.3 μg/mL) and HRP-conjugated antigoat (1/4000) and ECL development.
Coengagement of PECAM-1 with FcγRIIa cross-linking with IV.3 results in dephosphorylation of serine/threonine kinase, Akt. (A) Washed platelets were stimulated by FcγRIIa cross-linking with and without coengagement of PECAM-1 over a time course of 0 to 5 minutes at 37°C with stirring. Platelet lysate (200 μg) was electrophoresed on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, followed by immunoblotting with a mixture of antiphosphoSer473 Akt and antiphosphoThr308 Akt (1/2000) followed by HRP-conjugated antirabbit (1/10 000) and ECL development. (B) Blot was stripped and reprobed for Akt antigen content with goat anti-Akt antibody (0.3 μg/mL) and HRP-conjugated antigoat (1/4000) and ECL development.
As recruitment of Syk by FcγRIIa leads to tyrosine phosphorylation of PLCγ2, generation of secondary messengers inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) and subsequent calcium mobilization, we tested the effect on calcium mobilization following coengagement of FcγRIIa and PECAM-1. Following IV.3 cross-linking, there was release of calcium from intracellular stores. In contrast, coengagement of PECAM-1 with FcγRIIa resulted in inhibition of intracellular calcium release, whereas coengagement of CD151 with FcγRIIa cross-linking displayed no inhibitory effect (Figure 10). Inactivation of PLCγ2 leading to inhibition of Ca2+ mobilization is likely to be mediated on PECAM-1 inhibitory signaling involving down-regulation of phosphorylation of PLCγ2 by Syk, decrease in PIP3 (PI 3-kinase, phosphatidylinositol 3,4,5-trisphosphate) production as a result of down-regulation of PI 3-kinase activity.
Inhibition of FcγRIIa-mediated calcium mobilization mediated by anti-PECAM-1 Fab fragments. Calcium mobilization was measured after FcγRIIa cross-linking of Fura-2 labeled washed platelets in the presence and absence of coengagement of CD151 and PECAM-1. The Fura-2 ratio was determined using a luminescence spectrometer and recorded for 500 seconds.
Inhibition of FcγRIIa-mediated calcium mobilization mediated by anti-PECAM-1 Fab fragments. Calcium mobilization was measured after FcγRIIa cross-linking of Fura-2 labeled washed platelets in the presence and absence of coengagement of CD151 and PECAM-1. The Fura-2 ratio was determined using a luminescence spectrometer and recorded for 500 seconds.
Discussion
The concept that ITIM-bearing molecules have the capacity to negatively regulate cell activation induced by coaggregation with receptors bearing ITAMs has been demonstrated in a variety of immune cells. In human platelets, 2 different ITAM-associated receptor complexes are known. These receptors are the collagen GPVI receptor noncovalently coupled to a signal receptor ITAM-containing molecule, FcR-γ chain, and FcγRIIa containing an intrinsic ITAM in its intracellular domain. On the basis of this study, we have demonstrated that PECAM-1 and FcγRIIa are located in close physical proximity and are colocalized on the platelet membrane. This novel observation is supported by functional studies with PECAM-1-mediated signaling events inducing negative regulation of FcγRIIa-mediated signaling events and FcγRIIa-mediated platelet aggregation. From a clinical perspective, we found that PECAM-1 is an important negative regulator of HITS-mediated platelet aggregation via its potent inhibition of FcγRIIa-mediated signaling events.
There are several examples of physical associations of platelet receptors on the platelet membrane that have been described, including GPIbα association with FcγRIIa18 and GPIbα association with protein-disulfide isomerase.19 On the basis of this study, we have shown the existence of a close physical relationship between FcγRIIa and PECAM-1 with potent inhibition of aggregated IgG binding with all 4 anti-PECAM-1 mAb Fab fragments directed to various Ig-domains. In contrast, no physical relationship between FcγRIIa and CD151 was established with anti-CD151 mAb Fab, 11B1.G4. Other physical studies using FRET and coimmunoprecipitation further supported the concept of a physical relationship and colocalization of these 2 receptors on the platelet plasma membrane. The FRET-based analysis indicated that FcγRIIa and PECAM-1 are located in close physical proximity as required for energy transfer to occur. The coimmunoprecipitation studies with and without chemical cross-linking using membrane impermeable, DTSSP (3,3′-dithiobis(sulfosuccinimidyl propionate)), revealed that FcγRIIa and PECAM-1 are constitutively associated on the platelet membrane under resting conditions. This interaction on an activated platelet may lead to FcγRIIa influencing conformational changes in PECAM-1, or vice versa, to modulate ligand binding properties. Again, no such relationship appears to exist between FcγRIIa and CD151.
The sensitivity of FRET in a system in which both donor and acceptor fluorochromes are used is known to be dependent on a number of factors. These factors include relative receptor density of the 2 receptors under analysis, brightness of donor and acceptor fluorochromes with preferential overlapping of the emission, and absorption peaks of the donor and acceptor fluorochromes, respectively, and relative distribution of receptors on the platelet membrane. The Forster upper limit of critical distance for energy transfer (8-10 nm) is dependent upon the donor and acceptor pair and on the distance between fluorochromes attached to respective antigens. On the basis of estimates of copy number, the density difference between PECAM-1 and FcγRIIa is approximately 5 to 1. Taking these factors into consideration, we used the lower density FcγRIIa as the donor and the higher density PECAM-1 as the acceptor.
One could also argue that the significant FRET observed with PECAM-1 and FcγRIIa labeled acceptor and donor fluorochromes may be due to an artifact of high receptor density, whereby FRET is due to chance association rather than true physical proximity. This association can be ruled out, as it can be calculated that one molecule of PECAM-1 would be found in an area of 1800 nm2 from the statistics of 10 000 copies of PECAM-1 on 22 μm2 area of a platelet surface. On the assumption of even receptor distribution in a lattice-like arrangement, receptors are, therefore, estimated to be 60 nm apart, much greater than the required Forster upper limit of 8 to 10 nm for FRET. The chance of random FRET with respect to FcγRIIa is even lower given the 5-fold lower density. In addition, our results of FRET between the same receptor were significantly lower than that between FcγRIIa and PECAM-1, and FcγRIIa and GPIbα, indicating that homoassociation of receptors leading to FRET was not significant.
Functional studies using 3 different approaches involving aggregated IgG, biotinylated IV.3 mAb IgG cross-linking of FcγRIIa, and HITS antibody-induced platelet aggregation indicated the potent inhibitory functional effects of anti-PECAM-1 Fab fragments in negatively regulating FcγRIIa-mediated platelet aggregation. In contrast, the tetraspanin family member, CD151, was observed to have no functional relationship with FcγRIIa. These 3 different approaches have different mechanisms of activation of FcγRIIa to induce platelet aggregation. The use of a more specific and potent method involving the cross-linking of FcγRIIa with or without PECAM-1 engagement using biotinylated anti-FcγRIIa Fab and biotinylated anti-PECAM-1 Fab and Neutravidin have shown consistent inhibitory activity of PECAM-1 on FcγRIIa-mediated platelet aggregation. For this reason, this method was used in subsequent Ca2+ mobilization and signaling studies.
Numerous in vitro studies have concluded that antibodies to the heparin:PF4 complex activate human platelets via FcγRIIa.30-33 In this study, we provide evidence that platelet aggregation induced by HITS antibodies is mediated by FcγRIIa in vitro as revealed by the inhibition of platelet aggregation by intact and Fab anti-FcγRIIa mAb, IV.3, in both washed platelets and plasma-rich plasma. More significantly, activation of PECAM-1 using anti-PECAM-1 intact mAb and Neutravidin biotinylated anti-PECAM-1 Fab inhibited platelet aggregation, whereas anti-CD151 Fab fragments had no effect. Because PECAM-1 can negatively modulate collagen GPVI-dependent signaling events involving the FcR-γ chain1,2 and FcγRIIa as revealed in this study, it is possible that the same mechanism of inhibition involving activation of PECAM-1 and its ITIM coinhibitory signaling pathways is involved in inhibition of HITS antibody-induced ITAM-containing FcγRIIa-mediated platelet aggregation.
To define the molecular mechanisms of PECAM-1-mediated negative regulation of FcγRIIa-mediated platelet responses, we analyzed the profiles of signaling molecules associated in FcγRIIa-mediated signaling pathways. We hypothesized that PECAM-1 may negatively regulate the FcγRIIa-mediated signaling pathway on its activation, phosphorylation of its dual ITIMs, recruitment and activation of SHP-2 protein-tyrosine phosphatase, and subsequent dephosphorylation of multiple key proteins involved in FcγRIIa-mediated signaling pathway. Our studies show that PECAM-1 coengagement in FcγRIIa-mediated signaling results in inducible tyrosine phosphorylation of the dual PECAM-1 ITIMs, recruitment of SHP-2,34 and dephosphorylation of protein-tyrosine signaling species known to be involved in this signaling pathway, including PLCγ2,35 Akt, and inhibition of Ca2+ mobilization. These signaling events are a prerequisite for integrin αIIbβ3 activation and platelet aggregation. Taken together, these results provide further evidence of the inhibitory role of PECAM-1 in FcγRIIa-mediated platelet aggregation.
However, there is current controversy in relation to recent findings about the actual role of SHP-2. Unlike SHP-1 protein-tyrosine phosphatase, which primarily acts as a negative regulator, SHP-2 can act as a positive or negative regulator in cell signaling, depending on the cell type and signaling complexes formed.36,37 These 2 closely related SH2 domain-containing protein-tyrosine phosphatases have been demonstrated to display differential substrate specificity, including the Ig-ITIM family member, SIRPα.38 Recently, SHP-2 has been shown to negatively regulate the kinetics and amplitude of PI 3-kinase activation in a receptor-specific manner.39 Several studies have highlighted differences in the functional regulation mediated by SHP-2. In fibroblast cell lines, SHP-2 acts as a positive regulator of PI 3-kinase activation by insulin growth factor 1 (IGF-1) or insulin. In contrast, in similar cell lines, SHP-2 can act as a negative regulator of epidermal growth factor (EGF)-induced PI 3-kinase activation. In platelet studies, SHP-2 appears to play a negative regulatory role in collagen GPVI activation. PECAM-1-deficient platelets showed hyperresponsive features of collagen GPVI/FcR-γ chain-mediated responses and increased platelet thrombus formation on type I collagen and preferential recruitment of SHP-2 by phosphorylated PECAM-1 ITIMs in platelet signaling.1,2
Overall, on the basis of our findings, there is strong evidence that a physical and functional relationship between FcγRIIa and PECAM-1 exists on the surface of human platelets. The implications of these findings are of relevance in scientific and clinical terms. First and foremost, FcγRIIa, with its ITAM associated with the ligand-binding polypeptide, has not been demonstrated to be regulated by PECAM-1 on platelets. In addition, the novel findings of the colocalization of FcγRIIa and PECAM-1 on human platelets, albeit probably only existing in approximately 10% of the PECAM-1 receptor population, on human platelets is quite unexpected. FcγRIIa has been demonstrated in our and numerous in vitro studies to be a very potent mediator of platelet activation, secretion, and aggregation. From this perspective, PECAM-1 may have a role in functional regulation of FcγRIIa-mediated events in platelets in vivo. In particular, FcγRIIa has been demonstrated to be involved in immune complex-mediated phagocytosis of platelets by macrophages and in high IgG content sites such as in inflammation.40,41 From a clinical point of view, FcγRIIa has been implicated in immune complex-mediated diseases such as systemic lupus erythematosus (SLE) and heparin-induced thrombocytopenia and/or thrombosis (HITTS).42,43 Specifically, our findings show that PECAM-1 can abrogate FcγRIIa-mediated HITS antibody-induced platelet aggregation. These in vitro studies provide a foundation to explore the consequences of PECAM-1-negative regulation of FcγRIIa-mediated signaling events within the context of in vivo disease models, including HITS.
Prepublished online as Blood First Edition Paper, July 31, 2003; DOI 10.1182/blood-2003-02-0496.
Supported by grant no. 250398 (D.E.J.) from the National Health and Medical Research Council of Australia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.