Abstract
Purine nucleotides acting through P2 receptors play key roles in platelet signaling. The P2X1 receptor is an adenosine triphosphate (ATP)-gated ion channel that mediates a rapid calcium influx signal, but can also synergize with subsequent adenosine diphosphate (ADP)-evoked P2Y1 receptor-mediated responses and thus may contribute to platelet activation during hemostasis. Recent studies have shown that P2X1 receptors contribute to the formation of platelet thrombi, particularly under conditions of high shear stress. Based on intracellular Ca2+ measurements a previous report has suggested that a splice variant of the P2X1 receptor, P2X1del, is expressed in platelets and, in contrast to the full-length P2X1WT receptor, is activated by ADP. In the present study we show that the P2X1del receptor fails to form functional ion channels and is below the limit of detection in human platelets. Furthermore, ADP does not contribute to the rapid ionotropic P2X receptor-mediated response in platelets. These results support the notion that ATP is the principal physiologic agonist at P2X1 receptors and that it plays a role in the activation of platelets. (Blood. 2003;102:3646-3651)
Introduction
Purine nucleotides acting through P2 receptors play key roles in platelet signaling and hemostasis. P2 receptors can be subdivided into P2X ionotropic and P2Y metabotropic receptors, and transgenic mice models have now firmly established roles of P2X1, P2Y1, and P2Y12 receptors in platelet function.1-6 P2X1 receptors for adenosine triphosphate (ATP) mediate rapid transient increases in calcium concentration that can synergize with the larger, more sustained response through the adenosine diphosphate (ADP)-dependent P2Y1 receptor, leading to speeding and amplification of the calcium increase.4 In addition, P2X1 receptor-deficient mice have recently been shown to have a decreased aggregation response and to exhibit protection against thromboembolism.7 A major problem that has complicated the understanding and characterization of P2 receptors is the use of agonists to define P2 receptors because impurity or interconversion of the agonist has led to the pharmacologic misclassification of receptors. For example uridine diphosphate (UDP) appears as a weak agonist at the uridine triphosphate (UTP)-sensitive P2Y4 receptor; however, when contaminating UTP is removed with hexokinase, agonist activity is essentially abolished.8 The same problem has also influenced the study of P2 receptors in platelets and characterization of the specificity of action of different purines at individual receptors. For the P2X1 receptor we have shown that the apparent activity of ADP at the receptor can be accounted for by ATP contamination of commercially available ADP.9,10 This has important implications for the physiologic activation of platelet purinoceptors because ATP acts as a competitive antagonist rather than an agonist at platelet P2Y1 receptors as a result of their low level of expression.11,12 Thus, in platelets ATP and ADP are selective stimuli at P2X1 and P2Y1 receptors, respectively, and synergy between these 2 receptors may be important during hemostasis. However, the inability of ADP to activate platelet P2X receptors has been questioned by a study of a deletion variant, P2X1del. This variant, cloned from platelets and megakaryocytic cell lines, is deficient in 17 amino acids in the extracellular ligand-binding loop and has been reported to act as an ADP receptor in calcium measurement studies.13 Significant controversy exists as to whether the P2X1del receptor is expressed at high levels in platelets and exerts a functional role.4,14,15
In this study we have (1) characterized the expression and properties of recombinantly expressed P2X1del receptors, both as homomeric P2X1del receptors and in heteromeric assemblies with full-length P2X1 receptors; (2) determined in human platelets the relative concentrations of expression of P2X1 and P2X1del receptors; and (3) investigated whether P2X1del receptors contribute to generating P2X receptor-mediated signals. We provide conclusive evidence that platelet P2X1del receptors are not functional in cell lines or platelets and that ATP and ADP separately stimulate the native platelet P2X and P2Y receptors during hemostasis.
Materials and methods
Cell culture and transfections
Human embryonic kidney 293 (HEK293) cells were maintained in minimal essential medium with Earle salts (with GlutaMAX I) supplemented with 10% fetal bovine serum, 1% nonessential amino acid (Invitrogen, Paisley, United Kingdom) at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Native 1321-N1 astrocytoma cells (a gift from Dr T.E. Webb, De Montfort University, Leicester, United Kingdom) and 1321-N1 cells subcloned after transfection of either wild-type (WT) P2X1 or P2X1del (clone 4) (1321-N1 G P2X1WT or 1321-N1 G4 P2X1del, respectively; kindly provided by Dr N.J. Greco, Red Cross Research Laboratories, Rockville, MD) were maintained in Dulbecco modified Eagle medium (with GlutaMAX I, 4500 mg/L D-glucose) supplemented with 10% fetal bovine serum (Invitrogen). Stably transfected 1321-N1 cells were maintained under permanent selection in 240 μg/mL G418 (Invitrogen). HEK293 and 1321-N1 cells were transiently transfected with P2X1WT, P2X1del, or both receptors using LipofectAMINE 2000 Reagent/Opti-MEM (both from Invitrogen). For patch-clamp studies, as a control to identify cells that were efficiently transfected, the pEGFP-N1 (Clontech, Palo Alto, CA) vector (10% of total plasmid transfected) was cotransfected with P2X1 plasmids, and recordings were made only from fluorescent cells expressing the pEGFP. After 24 hours, the cells were either lysed, for Western blotting or were attached to glass coverslips and kept in culture for a maximum of 3 days for use in confocal microscopy and patch-clamp experiments.
Protein expression analysis
Western blots. Human platelets from 13 healthy donors were prepared as described16 and then pelleted at 10 000 rpm for 10 minutes. Platelets, human embryonic kidney 293 (HEK293), and 1321-N1 cells were homogenized in 20 mM Tris (tris[hydroxymethyl]aminomethane)-HCl (pH 8), 250 mM NaCl, 3 mM EDTA (ethylenediaminetetraacetic acid), 3 mM EGTA (ethylene glycol-bis (b-aminoethylether)-N,N,N′,N′-tetraacetic acid), and 0.5% Triton X-100 complemented with protease inhibitor cocktail (1:100) (Sigma-Aldrich, Poole, United Kingdom). Samples were separated under nonreducing conditions on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels. After electrophoretic transfer to nitrocellulose (100 V, 1 hour), the membrane was blocked overnight in TTBS (20 mM Tris-HCl [pH 7.6], 145 mM NaCl, 0.05% Tween 20) and 10% dry skim milk. The membrane was then incubated with anti-P2X1 antibody (1:1000) (Alomone, Jerusalem, Israel) in TTBS and 10% dry skim milk for 2 hours, washed in TTBS, and incubated for 2 hours in antirabbit horseradish peroxidase secondary antibody (1:1500 dilution) (Sigma). After washing, the visualization of the protein bands was achieved with an ECL (Plus) kit (Amersham Biosciences, Little Chalfont, United Kingdom) according to the manufacturer's instructions.
Surface cell expression and immunoprecipitations. All cell surface proteins were biotinylated with Sulfo-NHS-LC-Biotin (0.5 mg/mL in phosphate-buffered saline (PBS) (Pierce, Perbio Science, Tattenhall, United Kingdom) for 30 minutes. After washing with PBS, cells were homogenized in RIPA buffer (10 mM Tris-HCl [pH 7.4], 2 mM EDTA, 160 mM NaCl, 1% Nonidet P-40, and 0.5% deoxycholic acid) for 10 minutes on ice and centrifuged at 13 000 rpm for 5 minutes. The supernatant was incubated in the presence of 300 μL anti-P2X1 antibody (Alomone) diluted at 1: 200 in TE (10 mM Tris-HCl, 2 mM EDTA [pH 7.4]) for 2 hours on ice. Protein A Sepharose CL-4B (180 μL of 3% gel, reconstituted in 10 mM Tris-HCl, 2 mM EDTA [pH 7.4]) (Amersham Biosciences) was added, and the samples were homogenized for 15 minutes at 4°C, washed successively in RIPA buffer and TE, and resuspended in the gel sample buffer (180 mM Tris-HCl [pH 6.8], 5.7% SDS, 29% glycerol) and heated at 60°C for 3 minutes. The samples were separated on SDS-PAGE gel and were transferred onto nitrocellulose membrane as described, and the membrane was blocked overnight in TTBS and 3% bovine serum albumin (BSA). The membrane was then incubated with Immunopure streptavidin/horseradish peroxidase conjugated (0.5 μg/mL) (Pierce) in TTBS + 3% BSA for 30 minutes and washed; the visualization of the protein bands was achieved as described above.
Patch-clamp recordings
Conventional whole-cell, patch-clamp experiments were performed at a holding potential of -60 mV at room temperature, as described previously.4 Agonists were rapidly applied through a U-tube.
Intracellular calcium measurements
From cell lines. 1321-N1 cells (1321-N1 G P2X1WT or 1321-N1 G4 P2X1del), attached to glass coverslips, were incubated with 1 to 3 μM fluo-3-AM for 30 to 45 minutes, then washed gently and added to the imaging chamber. The standard bath saline contained (in mM) 150 NaCl, 2.5 KCl, 1 MgCl2, 2.5 CaCl2, 10 HEPES (N-2-hydroxethylpiperazine-N'-2-ethanesulfonic acid), pH 7.3 (NaOH); CaCl2 was omitted for experiments in nominally Ca2+-free saline. Fluorescence recordings (488 nm excitation, greater than 505-nm emission) were made from fields of approximately 20 to 30 cells using a Zeiss LSM 510 confocal microscope or an Olympus Fluoview FV 300 (Solent Scientific, Portsmouth, United Kingdom). Fluorescent signals were background-subtracted, and F/F0 ratios were used to normalize fluorescence levels (F) against starting fluorescence (F0). Figures show the Ca2+ responses from individual cells representative of 15 to 30 cells within the field of view. ATP and ADP were applied either through a U-tube system or by perfusion of the entire chamber. Thapsigargin (Calbiochem, Meik Biosciences, Nottingham, United Kingdom) was added by dilution directly to the chamber.
Human platelets. Blood was taken with informed consent from 13 donors, and fura-2 ratiometric fluorescence measurements of [Ca2+]i in stirred, washed platelet suspensions were performed as described previously.17 Platelet saline contained (in mM) 145 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, 10 D-glucose, pH 7.35 (NaOH). The cuvette temperature was lowered to 13°C to slow the start of the P2Y receptor-dependent Ca2+ response and to more clearly distinguish P2X1-evoked Ca2+ increases.9 The background-corrected F340/F380 fluorescence ratio was used as a direct indication of [Ca2+]i to avoid errors in application of the standard calibration methods in platelets at this temperature (for a discussion, see Mahaut-Smith et al9 ). Agonists were added after insertion of Hamilton syringes into a custom-built holder to avoid artefacts introduced by opening the cuvette lid, and additions were marked electronically to allow comparison of timing between different experiments.
Data analysis
Data are presented throughout as mean ± SEM; n represents the number of observations. Differences between means were determined by the appropriate Student t test and were considered significant when P < .05.
Reagents
αβ-Methylene ATP (αβ-meATP), ATP, and ADP were all obtained from Sigma. ADP was treated with hexokinase in a high-glucose-containing saline at pH 8, as described previously,9 to remove contaminating levels of ATP. Fluo-3-AM and fura-2-AM were from Molecular Probes (Leiden, the Netherlands) and made as stocks of 1 mM in 20% (wt/vol) Pluronic in dimethyl sulfoxide (DMSO).
Results
Electrophysiologic properties of P2X1 WT and P2X1del receptors transiently expressed in HEK cells
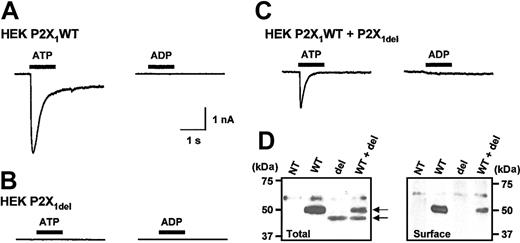
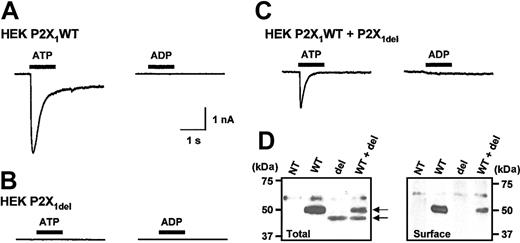
ATP (100 μM) is a full agonist at P2X1 WT receptors and evokes rapidly desensitizing inward currents through these receptors transiently expressed in HEK293 cells (mean amplitude, 4629 ± 700 pA; n = 18) (Figure 1A). Commercially available ADP (100 μM) evoked an inward current (mean amplitude, 1514 ± 502 pA; n = 7; data not shown); however, after removing contaminating ATP with hexokinase, the response to ADP was reduced by more than 95%, to -71 ± 44 pA (n = 10; Figure 1A). We failed to detect any ATP or ADP (both 100 μM) receptor-activated currents in HEK293 cells transfected with the P2X1del receptor (n = 14, 18) (Figure 1B). Total expression levels of P2X1WT and P2X1del were determined using Western blot analysis with an anti-P2X1 receptor antibody. The anti-P2X1 receptor antibody identified an approximately 50-kDa band in cells transfected with the P2X1WT plasmid and an approximately 46-kDa band in cells transfected with the P2X1del plasmid (Figure 1D) (in addition, a nonspecific band of approximately 60 kDa was present in nontransfected HEK293 cells, but not in 1321-N1 cells; see Figure 2B). The total level of expression of the P2X1del was approximately 20% compared with the P2X1WT. Cell surface biotinylation studies demonstrated that the P2X1WT receptor was trafficked to the cell surface; however, surface expression of P2X1del receptors was below the limit of detection (Figure 1D). These results indicate that the lack of agonist-evoked membrane current in cells transfected with P2X1del receptors is at least in part caused by poor surface expression of the receptor.
Expression and properties of recombinant P2X1WT and P2X1delreceptors in HEK293 cells. (A) ATP (100 μM; drug application is indicated by bar) induced a transient inward current in HEK293 cells transiently transfected with P2X1 WT receptors, whereas ADP (100 μM) was ineffective. (B) ATP or ADP (both 100 μM) failed to evoke a change in holding current from HEK293 cells transiently transfected with P2X1 del receptors. (C) When P2X1 WT and P2X1 del receptors were transiently coexpressed, ATP (100 μM) induced inward currents; however, ADP (100 μM) was ineffective. (D) Western blot analysis on nontransfected HEK293 cells (NT) and cells transiently transfected with either P2X1 WT (WT), P2X1 del (del), or both receptors (WT + del). Total cell protein analysis (left panel) showed that P2X1WT and P2X1del receptors were expressed. However, only P2X1 WT receptor was detected at the cell surface (right panel). A weak nonspecific band was observed around 60 kDa in nontransfected HEK293 cells.
Expression and properties of recombinant P2X1WT and P2X1delreceptors in HEK293 cells. (A) ATP (100 μM; drug application is indicated by bar) induced a transient inward current in HEK293 cells transiently transfected with P2X1 WT receptors, whereas ADP (100 μM) was ineffective. (B) ATP or ADP (both 100 μM) failed to evoke a change in holding current from HEK293 cells transiently transfected with P2X1 del receptors. (C) When P2X1 WT and P2X1 del receptors were transiently coexpressed, ATP (100 μM) induced inward currents; however, ADP (100 μM) was ineffective. (D) Western blot analysis on nontransfected HEK293 cells (NT) and cells transiently transfected with either P2X1 WT (WT), P2X1 del (del), or both receptors (WT + del). Total cell protein analysis (left panel) showed that P2X1WT and P2X1del receptors were expressed. However, only P2X1 WT receptor was detected at the cell surface (right panel). A weak nonspecific band was observed around 60 kDa in nontransfected HEK293 cells.
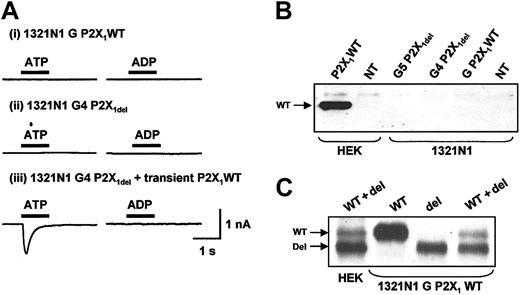
Expression and properties of recombinant P2X1WT and P2X1delreceptors in 1321-N1 cells. (A) ATP and ADP (both 100 μM, application indicated by bar) had no effect on the holding current in 1321-N1 cells stably transfected with either (i) P2X1WT (1321-N1 G P2X1 WT) or (ii) P2X1del (1321-N1 G4 P2X1del) receptors.13 When transiently transfected with P2X1WT receptors (iii), these cells gave a transient inward current in response to ATP (100 μM) but not to ADP (100 μM). (B) Western blot showing that no P2X1 receptor signal was detected in 1321-N1 G5 P2X1del, 1321-N1 G4 P2X1del, or 1321-N1 G P2X1WT cell clones13 and in nontransfected native 1321-N1 or HEK293 cells (NT). We used HEK293 cells transiently transfected with P2X1 WT as a positive control of expression of the P2X1 receptor. (C) However, bands corresponding to P2X1 receptors were detected when P2X1WT (WT), P2X1del (del), or P2X1 WT + P2X1del (WT + del) receptors (ratio 1:9) were transiently expressed in 1321-N1 G P2X1WT cells.
Expression and properties of recombinant P2X1WT and P2X1delreceptors in 1321-N1 cells. (A) ATP and ADP (both 100 μM, application indicated by bar) had no effect on the holding current in 1321-N1 cells stably transfected with either (i) P2X1WT (1321-N1 G P2X1 WT) or (ii) P2X1del (1321-N1 G4 P2X1del) receptors.13 When transiently transfected with P2X1WT receptors (iii), these cells gave a transient inward current in response to ATP (100 μM) but not to ADP (100 μM). (B) Western blot showing that no P2X1 receptor signal was detected in 1321-N1 G5 P2X1del, 1321-N1 G4 P2X1del, or 1321-N1 G P2X1WT cell clones13 and in nontransfected native 1321-N1 or HEK293 cells (NT). We used HEK293 cells transiently transfected with P2X1 WT as a positive control of expression of the P2X1 receptor. (C) However, bands corresponding to P2X1 receptors were detected when P2X1WT (WT), P2X1del (del), or P2X1 WT + P2X1del (WT + del) receptors (ratio 1:9) were transiently expressed in 1321-N1 G P2X1WT cells.
Heteromeric assembly of different P2X receptor subunits (eg, P2X2 and P2X318 ) or WT and mutant P2X1 receptor subunits can give rise to channels with altered properties.19 Both P2X1 WT and P2X1del receptor RNAs are made by platelets and a range of megakaryocytic cells,13 which raises the possibility that heteromeric channels may be formed with novel properties. We therefore cotransfected P2X1WT and P2X1del receptors in HEK293 cells with a DNA ratio of 1:9 to determine whether we could detect any regulation of the properties of P2X1WT receptors. Agonist-evoked currents from cells cotransfected with P2X1WT and P2X1del were indistinguishable from those of WT P2X1 receptors—that is, there was a rapidly desensitizing response to ATP, whereas ADP was ineffective as an agonist (Figure 1C; n = 15, 10) (similar results were found when the receptors were transiently cotransfected in 1321-N1 cells, ratio P2X1WT/P2X1del 1:9; n = 10; for Western blot see Figure 2C). Western blot analysis indicated that the total level of P2X1WT and P2X1del receptors was similar in HEK293 cells (Figure 1D). However, cell surface biotinylation again showed that only P2X1WT was detected at the cell surface (Figure 1D).
Functional properties of P2X1 receptors expressed in 1321-N1 cells
In the study by Greco et al,13 P2X1del receptors were stably expressed in the 1321-N1 cell line, and changes in calcium levels were monitored from populations of cells. Because Ca2+ increases evoked by ATP and ADP can result from both P2X and P2Y receptors, we used patch-clamp recordings to detect P2X receptor currents in individual cells from the lines used in the original study by Greco et al.13 Applying ATP or ADP to 1321-N1 G P2X1 WT or 1321-N1 G4 P2X1del cell lines gave no change in holding current, demonstrating that these cells do not express functional P2X receptors (n = 19, 8, and 15, 15 respectively) (Figure 2A). This was consistent with Western blotting results in which both P2X1 WT and P2X1del receptors were below the limit of detection in these cell lines (Figure 2B).
When P2X1WT receptors were transiently transfected into a 1321-N1 cell background, ATP (100 μM) evoked inward currents (in either the 1321-N1 G P2X1WT or the 1321-N1 G4 P2X1del mean amplitude, -1674 ± 470 pA; n = 19) (Figure 2A), demonstrating that P2X1WT receptors can form functional channels in this cell line as described previously.20 As shown for the recombinant P2X1 receptors expressed in HEK293 cells, purified ADP was ineffective as an agonist at activating P2X1WT receptor-mediated inward currents in 1321-N1 cells (Figure 2Aiii, -32 ± 12 pA; n = 9). No inward current was evoked by either ATP or ADP (100 μM) when applied to native 1321-N1 cells transiently expressing P2X1del receptors (data not shown; n = 8, 9). Western blotting was used to estimate the total levels of expression of transiently transfected P2X1WT and P2X1del receptors in 1321-N1 cells (Figure 2C). The anti-P2X1 receptor antibody detected bands in total cellular lysates corresponding to the P2X1WT and P2X1del receptors. These results confirm that the P2X1del receptor can be expressed but that it fails to form functional ion channels in 1321-N1 cells.
Characteristics of the P2 receptor-evoked [Ca2+]i responses in cell lines used for previous P2X1del studies
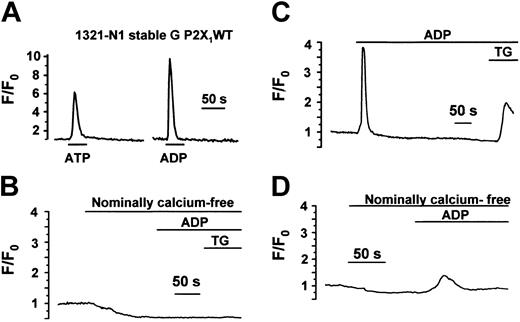
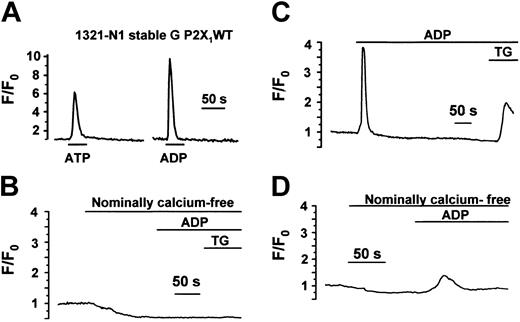
Even though we could not detect P2X1 receptor current or protein in the cell lines provided by Greco et al,13 we were able to detect rapid increases in intracellular calcium in response to ATP and ADP (Figure 3A) in 50% and 67% of cells tested (70 cells from 3 different studies) (Figure 3A). These results can be explained by the presence of native P2Y receptors in the subclonal 1321-N1 G P2X1WT and 1321-N1 G4 P2X1del cell lines. The standard approach to assess the relative contribution of P2Y versus P2X receptors is to examine the Ca2+ response in the absence of external Ca2+. P2X receptors rely entirely on Ca2+ influx, whereas the response to P2Y receptors was at least partly caused by inositol 1, 4,5-triphosphate (IP3)-dependent Ca2+ release. However, this proved difficult in 1321-N1 cells because of the rapid depletion of the intracellular Ca2+ stores in Ca2+-free medium. Thus, after only 2 to 3 minutes in nominally Ca2+-free saline, ADP failed to evoke a response, though this resulted from the depletion of the internal stores because the subsequent addition of the endomembrane Ca-ATPase inhibitor Thapsigargin had no effect on the cytosolic Ca2+ level (Figure 3B). The extremely rapid depletion of Ca2+ stores in Ca2+-free medium in this cell line highlights the difficulty of using this condition as a definitive test for P2X or P2Y receptors. In Ca2+-containing medium, when the stores are replenished after agonist exposure, Thapsigargin causes its expected effect of a substantial increase in cytosolic Ca2+ (Figure 3C). However, ADP did evoke a small Ca2+ response after shorter exposures to Ca2+-free medium (for example, 60 seconds; see Figure 3D) in both 1321-N1 G P2X1WT and 1321-N1 G4 P2X1 del, confirming the presence of P2Y responses in these cell lines.
Intracellular Ca2+measurements in subclones of 1321-N1 cells indicate the presence of P2Y receptor responses. Intracellular Ca2+ responses in single 1321-N1 G P2X1 WT (A) and 1321-N1 G4 P2X1del cells (B-D)13 loaded with the fluorescent calcium indicator fluo-3. Similar results were obtained in the 2 cell lines. (A) In the presence of extracellular Ca2+, ATP (30 μM) and ADP (30 μM) evoked a transient Ca2+ increase. (B) Loss of the response to ADP (100 μM) after only a short period (120 seconds in this experiment) in nominally Ca2+-free medium. This lack of response was caused by the rapid depletion of Ca2+ from intracellular stores because Thapsigargin (TG) had no effect in Ca2+-free medium. (C) Demonstration that TG evoked an expected large increase in Ca2+-containing medium after ADP exposure. (D) Adding ADP earlier (60 seconds) after exposure to Ca2+-free medium evoked a small, delayed Ca2+ increase.
Intracellular Ca2+measurements in subclones of 1321-N1 cells indicate the presence of P2Y receptor responses. Intracellular Ca2+ responses in single 1321-N1 G P2X1 WT (A) and 1321-N1 G4 P2X1del cells (B-D)13 loaded with the fluorescent calcium indicator fluo-3. Similar results were obtained in the 2 cell lines. (A) In the presence of extracellular Ca2+, ATP (30 μM) and ADP (30 μM) evoked a transient Ca2+ increase. (B) Loss of the response to ADP (100 μM) after only a short period (120 seconds in this experiment) in nominally Ca2+-free medium. This lack of response was caused by the rapid depletion of Ca2+ from intracellular stores because Thapsigargin (TG) had no effect in Ca2+-free medium. (C) Demonstration that TG evoked an expected large increase in Ca2+-containing medium after ADP exposure. (D) Adding ADP earlier (60 seconds) after exposure to Ca2+-free medium evoked a small, delayed Ca2+ increase.
Lack of evidence for ADP-evoked Ca2+ responses through native P2X receptors in human platelets
The lack of functional expression of recombinant P2X1del receptors may reflect the absence of some accessory factor necessary for efficient trafficking/processing of the receptor in HEK293 and 1321-N1 cell lines. Given that the P2X1del receptor was originally isolated from platelets, we determined the levels of P2X1del receptor expression in human platelets and the functional role of P2X and P2Y receptor-mediated calcium signaling. In Western blot analysis of platelet lysates (from 13 donors) the anti-P2X1 receptor antibody detected a single band at approximately 50 kDa, corresponding to the P2X1WT receptor, but no lower molecular weight band corresponding to the P2X1del receptor was detected (Figure 4A). To estimate the detection threshold for the anti-P2X1 antibody, we diluted the amount of platelet sample run on the gel. P2X1WT receptors could still be detected at a 1:24 dilution (Figure 4B). Assuming that the antibody has the same sensitivity for P2X1WT and P2X1del (which is valid given that the deletion is in the extracellular portion of the receptor and the 2 variants share the identical epitope at the C-terminal), this indicates that the P2X1del receptor accounts for less than 4% of the total protein. Given the additional reduction in efficiency of transfer to the cell membrane, this indicates that the P2X1del accounts for a small proportion, if any, of the cell surface P2X1 receptors. Studies of P2 receptor Ca2+ responses were also conducted in platelets from 13 donors, including 6 of those used for the Western blot analyses of Figure 4A. The temperature was lowered to 13°C to clearly distinguish rapid P2X1 increases from the slower P2Y1 response.9 In all donors tested (n = 13), the P2X1 receptor agonist αβ-meATP evoked a rapid transient calcium increase (Figure 4C), whereas the P2Y1 receptor agonist, ADP (40 μM, hexokinase purified) evoked Ca2+ increases with a prolonged delay of 2 to 3 seconds. The lack of evidence for an ADP-induced P2X receptor-mediated rise in calcium demonstrates that ADP-dependent P2X1del receptors do not contribute to calcium signaling in human platelets.
Lack of detection of P2X1delprotein or ADP evoked P2X responses in human platelets. (A) Ten μg platelet total protein from 13 healthy human donors was analyzed by Western blotting (i) and (ii) as a control. Samples of recombinant P2X1WT and P2X1del receptors in HEK293 cells are shown. P2X1WT receptors were detected in the platelets from each donor; the P2X1 del receptor, however, was below the limit of detection. (B) To overcome the possibility that P2X1 del receptor was not detected because of the weak expression level in platelets, dilution of one of the platelet protein samples (donor 4) was analyzed by Western blotting. The band corresponding to the P2X1 WT receptor was still detectable when only 0.625 μg platelet total proteins were separated (the small box represents the 3 last lanes for which the autoradiography has been submitted to a longer exposure). Therefore, if P2X1 del receptor is also expressed in human platelets, the ratio P2X1del/P2X1WT is less than 1:24. (C) [Ca2+]i measurements in suspensions of fura-2-loaded human platelets, as indicated by the 340/380 fluorescence excitation ratio, in response to either 10 μM αβmeATP or 10 μM hexokinase-treated ADP (arrow). Traces from 2 donors (10 and 11 in panel Aii) are shown at 2 different time scales and are representative of the results from 13 donors. The cuvette temperature was set at 13°C to allow clear separation of P2X and P2Y receptor-evoked responses, as described in Mahaut-Smith et al.9
Lack of detection of P2X1delprotein or ADP evoked P2X responses in human platelets. (A) Ten μg platelet total protein from 13 healthy human donors was analyzed by Western blotting (i) and (ii) as a control. Samples of recombinant P2X1WT and P2X1del receptors in HEK293 cells are shown. P2X1WT receptors were detected in the platelets from each donor; the P2X1 del receptor, however, was below the limit of detection. (B) To overcome the possibility that P2X1 del receptor was not detected because of the weak expression level in platelets, dilution of one of the platelet protein samples (donor 4) was analyzed by Western blotting. The band corresponding to the P2X1 WT receptor was still detectable when only 0.625 μg platelet total proteins were separated (the small box represents the 3 last lanes for which the autoradiography has been submitted to a longer exposure). Therefore, if P2X1 del receptor is also expressed in human platelets, the ratio P2X1del/P2X1WT is less than 1:24. (C) [Ca2+]i measurements in suspensions of fura-2-loaded human platelets, as indicated by the 340/380 fluorescence excitation ratio, in response to either 10 μM αβmeATP or 10 μM hexokinase-treated ADP (arrow). Traces from 2 donors (10 and 11 in panel Aii) are shown at 2 different time scales and are representative of the results from 13 donors. The cuvette temperature was set at 13°C to allow clear separation of P2X and P2Y receptor-evoked responses, as described in Mahaut-Smith et al.9
Discussion
In the present study we have investigated the expression and function of the P2X1del receptor because it has been suggested that this receptor may be sensitive to ADP and may play a functional role in the activation of platelets. We have demonstrated that the P2X1del receptor fails to form functional channels when expressed in HEK293 and 1321-N1 cell lines and that this most likely results from poor trafficking of the receptor to the cell surface. In addition, in human platelets, P2X1del protein was below the limit of detection. These results demonstrate that the P2X1del receptor does not play a significant role in platelet function and that the P2X1 receptor in human platelets is activated by ATP not ADP.
P2X1WT receptors were transiently expressed in HEK293 and 1321-N1 cells as a positive control. Activation of this channel by ATP evoked rapidly inactivating responses, and purified ADP was ineffective as an agonist, confirming results described by several independent laboratories.9,10,18,20-24 It is anomalous that in the study of Greco et al,13 purified ADP was found to be an equi-effective agonist at the P2X1WT receptor. Even when commercially available ADP (impure) has been used, it only acts as a weak partial agonist with more than 100-fold reduced potency compared with ATP in electrophysiologic9,21 or calcium-imaging studies.20 In the study by Greco et al,13 ADP evoked an intracellular calcium increase of amplitude comparable with that of ATP when these agents were added sequentially, with an interval of only 10 to 20 seconds in the P2X1WT stable cell line (designated 1321-N1 G P2X1 WT in this study).13 This is unusual because native and recombinant P2X1 receptors show profound desensitization to agonist application, such that long wash/recovery periods between applications are required to generate reproducible responses. Furthermore, agonist desensitization is used routinely to remove “contaminating” P2X1 receptor-mediated responses in pharmacologic studies.18,25 For example, the role of native P2X1 receptors in HL60 cells and platelets had been overlooked because of problems associated with receptor desensitization during cell preparation.17,26 For these reasons the pharmacology of the P2X1WT responses reported by Greco et al13 appear inconsistent with the consensus of those reported for wild-type P2X1 receptors (for recent review, see North18 ).
The patch-clamp technique provides a highly sensitive measure of changes in membrane current and detection of functional ion channels. The single-channel amplitude of the P2X1WT receptor under the recording conditions used is approximately 0.6 pA (R.J.E., unpublished observations, 2001), and signals of approximately 2 to 3 pA can be readily discriminated; therefore, theoretically the opening of as few as 5 channels can be detected. The P2X1del receptor failed to produce functional ion channel currents when transiently expressed in either HEK293 or 1321-N1 cells. Using Western blot analysis (Figures 1, 2), we show that the P2X1del receptor is produced in these cells and associates to form trimeric channels (data not shown), as for the wild type. However, the total P2X1del receptor level was reduced compared with equivalent transfection of P2X1WT (for a discussion, see also Oury et al14 ), indicating that the P2X1del receptor is either inefficiently made or broken down. In addition, we showed the P2X1del receptor is below the limit of detection at the cell surface, and this lack of receptor trafficking most likely accounts for the lack of functional P2X1del receptor-mediated currents.
There was an approximately 3- to 4-kDa decrease in the molecular mass of the P2X1del receptor monomer compared with the full-length P2X1WT receptor, as described previously after in vitro translation.13,14 This is to be expected because the deletion removes 17 amino acids that include a glycosylation site (N184, which contributes 2-3 kDa).27 The poor surface expression of the P2X1del receptor in the present study is unlikely to result solely from the removal of glycosylation at N184.22,27 Interestingly, for the rP2X1a deletion mutant, which has a larger deletion (amino acids 175-201 compared with 176-192 for P2X1del), the coexpression of P2X1WT can in part rescue cell surface channel expression in HEK293 cells.22 This was not the case for the P2X1del receptor. The reason the extra 10-amino acid deletion of rP2X1a encourages cotrafficking to the cell surface remains to be determined.
We were unable to detect P2X1 or P2X1del receptor expression either electrophysiologically or by Western blotting using the stable cell lines expressing P2X1 and P2X1del receptors provided by Greco et al.13 When the P2X1WT receptor was transiently transfected into these 1321-N1 stable cell lines, ATP evoked robust P2X receptor currents, and receptor protein of the appropriate molecular weight was detected, confirming that the cells can produce functional P2X1WT receptors.20 The fact that calcium responses to ADP and ATP can be recorded from these cells in the absence of detectable P2X1 receptor currents or protein indicates that endogenous P2 receptors other than P2X1 are expressed in these cells.13
Results following the expression of recombinant receptors indicate that P2X1del receptors are not functional, though it is possible that HEK293 and 1321-N1 cell lines lack some accessory factor necessary for efficient trafficking/processing of these receptors. Previous estimates of relative levels of P2X1WT and P2X1del in platelets have been made based on reverse transcription-polymerase chain reaction (RT-PCR). In this study we measured protein levels directly with Western analysis and investigated the role of ADP-activated P2X1 receptors in functional assays. Western blotting failed to detect the P2X1del receptor protein in total platelet lysates from 13 donors (in assays that could detect a 24-fold dilution of the WT receptor). Given the likely additional inefficient trafficking to the cell surface, P2X1del accounts for a small, if any, proportion of the P2X1 receptors at the cell surface. Because the P2X1del receptor failed to be expressed on the cell surface of HEK or 1321-N1 cells, it is still uncertain whether ADP is an agonist, as shown for the WT receptor. Nevertheless, functional studies failed to detect a rapid ADP-mediated P2X receptor Ca2+ response; thus, ADP-activated receptors, whether P2X1del or other naturally occurring mutants, do not contribute to the P2X1 receptor phenotype, as also shown in kinase assays.24 Previous work has shown that the P2X1 receptor in human platelets synergizes with functional responses through P2Y1 and collagen receptors.4,5 Thus, our present study supports the concept that ATP, released from damaged vascular cells or cosecreted from platelets with ADP and other agonists, is an agonist through P2X1 receptors during hemostasis. A recent study using P2X1-/- mice has suggested that the main role of this receptor is to enhance platelet activation under conditions of high shear stress.7 This functional importance of P2X1 receptors may be a consequence of the rapid nature of the ATP-activated Ca2+ influx or its ability to potentiate P2Y1 and collagen-receptor signaling.4,5
In summary, we can find no evidence to support a functional role for an ADP-activated P2X1del receptor in human platelets and conclude that ATP is the principal natural agonist at P2X receptors in platelets mediating a rapid increase in intracellular calcium. This calcium increase also synergizes with subsequent ADP-mediated P2Y receptor signaling events associated with hemostasis, and blockade of the ATP-sensitive P2X1 receptor may provide a novel drug target for protection against thromboembolism.7
Prepublished online as Blood First Edition Paper, August 7, 2003; DOI 10.1182/blood-2003-06-1963.
Supported by a Wellcome Trust Programme Grant (R.J.E.) and the British Heart Foundation (BS/10, FS/02/033, and FS/97052) (M.P.M.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr N. Greco for providing the P2X1del plasmid and transfected 1321-N1 cell lines, Dr T. Webb for providing native 1321-N1 cells, ProfessorA. Goodall and Dr J.Appleby for providing samples of human platelets, and J. Holdich for technical assistance.

![Figure 4. Lack of detection of P2X1del protein or ADP evoked P2X responses in human platelets. (A) Ten μg platelet total protein from 13 healthy human donors was analyzed by Western blotting (i) and (ii) as a control. Samples of recombinant P2X1WT and P2X1del receptors in HEK293 cells are shown. P2X1WT receptors were detected in the platelets from each donor; the P2X1 del receptor, however, was below the limit of detection. (B) To overcome the possibility that P2X1 del receptor was not detected because of the weak expression level in platelets, dilution of one of the platelet protein samples (donor 4) was analyzed by Western blotting. The band corresponding to the P2X1 WT receptor was still detectable when only 0.625 μg platelet total proteins were separated (the small box represents the 3 last lanes for which the autoradiography has been submitted to a longer exposure). Therefore, if P2X1 del receptor is also expressed in human platelets, the ratio P2X1del/P2X1WT is less than 1:24. (C) [Ca2+]i measurements in suspensions of fura-2-loaded human platelets, as indicated by the 340/380 fluorescence excitation ratio, in response to either 10 μM αβmeATP or 10 μM hexokinase-treated ADP (arrow). Traces from 2 donors (10 and 11 in panel Aii) are shown at 2 different time scales and are representative of the results from 13 donors. The cuvette temperature was set at 13°C to allow clear separation of P2X and P2Y receptor-evoked responses, as described in Mahaut-Smith et al.9](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/10/10.1182_blood-2003-06-1963/6/m_h82235216004.jpeg?Expires=1769288118&Signature=WNnwGsJlT1fFvp7uZ-xB-OfmV05JVZxypfJxGbxDnX0Dco0mjeZ6DP38I3LETfTc~CgJT6LNJbjkFpX2vHUmCjfxqPzqEyw72xeBw-C3PzgqL6x-7E-SngCo60rHCEMSh7riyS1FZpAhkPXvO~LojPg-fWlgEnjZValvy4K507iglleUXOwq4ITsonBkUcvWEhjRSyepbPjyYcXIa8kaQrDpUxxasPiRn27j04lnrE3g7ePSW~VVNjdMwYWPm5JOiyDIDHMd2Q6SGI~mVNQhM7G6DPMAXYyEzdX87yYIDSWGuNDhimqhw8~skWbsQjY96rdDwMyi5DgkimwGh7HJdg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 4. Lack of detection of P2X1del protein or ADP evoked P2X responses in human platelets. (A) Ten μg platelet total protein from 13 healthy human donors was analyzed by Western blotting (i) and (ii) as a control. Samples of recombinant P2X1WT and P2X1del receptors in HEK293 cells are shown. P2X1WT receptors were detected in the platelets from each donor; the P2X1 del receptor, however, was below the limit of detection. (B) To overcome the possibility that P2X1 del receptor was not detected because of the weak expression level in platelets, dilution of one of the platelet protein samples (donor 4) was analyzed by Western blotting. The band corresponding to the P2X1 WT receptor was still detectable when only 0.625 μg platelet total proteins were separated (the small box represents the 3 last lanes for which the autoradiography has been submitted to a longer exposure). Therefore, if P2X1 del receptor is also expressed in human platelets, the ratio P2X1del/P2X1WT is less than 1:24. (C) [Ca2+]i measurements in suspensions of fura-2-loaded human platelets, as indicated by the 340/380 fluorescence excitation ratio, in response to either 10 μM αβmeATP or 10 μM hexokinase-treated ADP (arrow). Traces from 2 donors (10 and 11 in panel Aii) are shown at 2 different time scales and are representative of the results from 13 donors. The cuvette temperature was set at 13°C to allow clear separation of P2X and P2Y receptor-evoked responses, as described in Mahaut-Smith et al.9](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/10/10.1182_blood-2003-06-1963/6/m_h82235216004.jpeg?Expires=1769288119&Signature=pi3NkwuvYMvB6O7HRzkdoGas-oL-m5w3TZyUa65F5H8TBkxCSWrhELI5hhTI7gVZ1Z1d-L1gw-jPvpb8JoGWDjkh0JbpltI8GwHsbgLpmNLs4HwFL5qZiOgzvyBIeSGJ~ibnyPZv5XZhfSHtHn267vjgc1Q~1txqkaI84qvoGXKF0ae8Fk8qkILbiFPqbpoR5gNwuy69e2GNFg64SO66bbjirO5MHYjTtzXnstMMr4JhZ2We88FPlWMnouYETx~mhEuYsR0jYmcL7yOgyRR-pRhfZX9~-C6AeKFJMt3rKR81awLCWUJjQIyuYHw3ocwLfJ9WqVb2N8SedWtMn6HyvA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)