Abstract
CD8+CD25+ cells, which expressed high levels of Foxp3, glucocorticoid-induced tumor necrosis factor receptor (GITR), CCR8, tumor necrosis factor receptor 2 (TNFR2), and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) mRNAs, were identified in the fibrous septa and medullary areas of human thymus. Activated CD8+CD25+ thymocytes did not produce cytokines, but most of them expressed surface CTLA-4 and transforming growth factor β1 (TGF-β1). Like CD4+CD25+, CD8+CD25+ thymocytes suppressed the proliferation of autologous CD25-T cells via a contact-dependent mechanism. The suppressive activity of CD8+CD25+ thymocytes was abrogated by a mixture of anti-CTLA-4 and anti-TGF-β1 antibodies and it was mediated by their ability to inhibit the expression of the interleukin 2 receptor α chain on target T cells. These results demonstrate the existence of a subset of human CD8+CD25+ thymocytes sharing phenotype, functional features, and mechanism of action with CD4+CD25+ T regulatory cells. (Blood. 2003;102:4107-4114)
Introduction
Several types of T regulatory (Treg) cells have been described in both mice and humans.1-4 Oral antigen administration or stimulation of CD4+CD25- T cells in vitro in the presence of exogenous transforming growth factor β1 (TGF-β1) induces the outcome of CD4+ T cells able to produce soluble TGF-β1 (Th3 cells), which are able to inhibit the outcome of some autoimmune diseases, including autoimmune experimental encephalomyelitis, insulin-dependent diabetes mellitus, and colitis.5-8 Repetitive stimulation of CD4+ T cells in vitro in the presence of exogenous interleukin 10 (IL-10) leads to generation of T regulatory 1 (TR1) cells able to produce high levels of IL-10 and to inhibit T-cell responses in vivo.9 Repetitive stimulation of naive T cells with allogeneic immature dendritic cells (DCs) induces the generation of poorly growing CD4+ T cells that primarily produce IL-10 and exhibit a suppressor phenotype.10 Another Treg population is characterized by the constitutive high expression of the IL-2 receptor α (IL-2Rα) chain (CD25high), which is known as the CD4+CD25+ Treg population.11 CD4+CD25+ Treg cells exist in the thymus and peripheral blood (PB) of both mice and humans.11-17 The mechanism of action of CD4+CD25+ Treg cells is still controversial although several findings suggest the possible involvement of cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), the glucocorticoid-induced tumor necrosis factor receptor (GITR), and membrane-bound or secreted TGF-β1.12,18-26 Recent reports have clearly demonstrated that Foxp3, the gene involved in the X-linked recessive disease, called IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome), is selectively expressed by mice CD4+CD25+ Treg cells and is the key factor for the development of T-cell suppressive activity.27-29
In this study, we demonstrate the existence in the human thymus of a previously unrecognized population of CD8+CD25+ cells sharing phenotype, functions, and mechanisms of action with CD4+CD25+ human regulatory thymocytes. In fact, CD8+CD25+ thymocytes could be detected in the same areas of human thymus, where CD4+CD25+ cells have been observed.12 Moreover, these cells exhibited poor, if any, proliferation in response to allogeneic stimulation and suppressed in a dose-dependent fashion the allogeneic response of both CD4+CD25- and CD8+CD25- autologous T cells. CD8+CD25+ thymocytes selectively expressed Foxp3 and GITR mRNAs and exhibited significantly higher CCR8, tumor necrosis factor receptor 2 (TNFR2), and CTLA-4 mRNA expression than CD8+CD25- population. CD8+CD25+ thymocytes constitutively expressed cytoplasmic CTLA-4, as well as surface CCR8 and TNFR2 proteins, whereas they did not exhibit the presence of either perforin or granzyme A. Following prolonged stimulation with anti-CD3 plus anti-CD28 monoclonal antibodies (mAbs), CD8+CD25+ thymocytes did not produce IL-2, IL-4, IL-5, IL-10, IL-13, and interferon γ (IFN-γ), whereas a remarkable proportion of CD8+CD25- cells produced IL-2 and IFN-γ. Under the same experimental conditions, most CD8+CD25+, but not CD8+CD25-, thymocytes expressed both CTLA-4 and TGF-β1, but not GITR, on their surface. Finally, the suppressive activity of CD8+CD25+ thymocytes was dependent on contact and appeared to be related to their ability to down-regulate the IL-2Rα chain expression in target T cells via the combined action of TGF-β1 and CTLA-4, thus making them unresponsive to IL-2, but not to IL-15.
Materials and methods
Reagents and antibodies
The medium used was RPMI 1640 (Seromed, Berlin, Germany), supplemented with 2 mM l-glutamine, 1% nonessential amino acids, 1% pyruvate, 2 × 10-5 M 2-mercaptoethanol (all from Gibco Laboratories, Grand Island, NY), and 10% fetal calf serum (FCS; Hyclone, Logan, Utah). Unconjugated and fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, allophycocyanin (APC)- or peridin chlorophyll protein (PerCP)-conjugated anti-CD3 (SK7, mouse IgG1), anti-CD3 (UCHT1, mouse IgG1), anti-CD4 (SK3, mouse IgG1), anti-CD8 (SK1, mouse IgG1), anti-CD14 (Mϕ9, mouse IgG2b), anti-CD16 (3G8, mouse IgG1), anti-CD19 (4G7, mouse IgG1), anti-CD25 (2A3, mouse IgG1), anti-CD28 (CD28.2, mouse IgG1), anti-CD34 (My10, mouse IgG1), anti-CD56 (My31, mouse IgG1), anti-CD69 (L78, mouse IgG1), anti-TCRγδ (11F2, mouse IgG1), anti-CTLA-4 (BNI3, mouse IgG2a), anti-IL-2 (5344.111, mouse IgG1), anti-IL-4 (3010.211, mouse IgG1), anti-IL-10 (JES3-9D7, rat IgG1), anti-IL-13 (JES10-5A2, rat IgG1), anti-IFN-γ (4S.B3, mouse IgG1), anti-TNF-α (6401.1111, mouse IgG1), antiperforin (δG9, mouse IgG2b), and anti-granzyme A (CB9, mouse IgG1) mAbs, as well as FITC-conjugated annexin V were purchased from BD Biosciences (Mountain View, CA). The anti-glycophorin A, B (E3, mouse IgG1) mAb was from Sigma Chemical (St Louis, MO). The PE-conjugated anti-TNFR2 (22235.311, mouse IgG2a), the purified anti-TGF-β1 (9016.2, mouse IgG1) neutralizing mAb, and the purified and PE-conjugated anti-GITR (110416, mouse IgG1) neutralizing mAbs were purchased from R&D Systems (Minneapolis, MN). All conjugated and unconjugated isotype-matched control antibodies (mouse IgG1, clone 15H6; mouse IgG2a, clone HOPC-1; mouse IgG2b, clone A-1; rat IgG, goat IgG, and rabbit IgG) were purchased from Southern Biotechnology (Birmingham, AL). Human recombinant IL-2 (rIL-2) was a kind gift from Eurocetus (Milan, Italy). Human rIL-15 was from R&D Systems. The anti-CCR8 rabbit polyclonal antibody used for flow cytometry was from AMS Biotechnology (Abingdon Oxon, United Kingdom). The goat antimouse IgG and anti-CD25 antibodies conjugated with magnetic beads were obtained from Miltenyi Biotec (Bisley, Germany).
Human thymuses
Normal postnatal thymus specimens were obtained from 12 children, aged 5 days to 3 years, who underwent corrective cardiac surgery at the Apuano Pediatric Hospital (Massa Carrara, Italy). The procedures followed in the study were in accordance with the ethical standards of the Regional Committee on Human Experimentation.
Real-time quantitative RT-PCR
Total RNA was extracted and treated with DNase I (Qiagen, Hilden, Germany) to eliminate possible gDNA contamination. TaqMan reverse transcription-polymerase chain reaction (RT-PCR) was performed as described elsewhere.30 Primers and probes were the following: GITR: VIC-TAMRA probe, 5′-CGAGGAGTGCGTTTCCGAGTGGGA-3′; forward 5′-GCCGCGATTACCCGG-3′; reverse 5′-CAGTGGAATTCAGGCTGGACA-3′. mRNA levels were quantitatively analyzed by comparing experimental levels to standard curves generated using serial dilutions of the same positive sample. Foxp3, TGF-β1, CCR8, CTLA-4, and TNFR2 quantitative analysis was performed using Assay on Demand (Applied Biosystems, Warrington, United Kingdom). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) quantitative analysis was performed using predeveloped TaqMan assay reagents target kits (Applied Biosystems). GAPDH was used for normalization. All primers and probes did not react with DNA.
Flow cytometry analysis
Flow cytometry analysis on thymic cell suspensions was performed as detailed elsewhere.31 To assess the expression of cytoplasmic CTLA-4, TGF-β1, perforin, and granzyme A cells were washed twice with phosphate-buffered saline (PBS), pH 7.2, fixed 15 minutes with formaldehyde (2% in PBS, pH 7.2), washed twice with 0.5% bovine serum albumin (BSA) in PBS, pH 7.2, permeabilized with PBS, pH 7.2, containing 0.5% BSA and 0.5% saponin, and then incubated for 15 minutes at room temperature with the specific mAbs. For indirect staining, cells were incubated for additional 15 minutes with a fluorochrome-conjugated anti-isotype mAb. Cells were then washed and analyzed on a BDLSR cytofluorimeter using the CellQuest software (BD Biosciences). The area of positivity was determined using an isotype-matched mAb; 104 events for each sample were acquired.
Isolation of CD4+CD25-, CD4+CD25+, CD8+CD25-, and CD8+CD25+ thymocytes
Negative selection of CD4+ and CD8+ single-positive (SP) thymocytes was first performed by high-gradient magnetic-activated cell sorting (MACS), as described elsewhere.31 Briefly, thymic mononuclear cell (MNC) suspensions were incubated for 20 minutes with anti-CD14, anti-CD16, anti-CD19, anti-CD34, anti-CD56, anti-TCRγδ, anti-glycophorin A, B, anti-CD8, or anti-CD4 mAbs, extensively washed, and then incubated for additional 20 minutes with goat antimouse polyclonal antibody conjugated to colloidal super-paramagnetic microbeads, according to the MACS system (Milteny Biotec). After washing, cells were separated on a (CS+) columns. The remaining SP CD4+ and CD8+ T cells were then separated into CD25+ and CD25- by positive selection by the use of anti-CD25 MACS microbeads. The positive selections were performed on (LS+) columns.
Immunohistochemistry
Immunohistochemical staining of thymic sections with anti-CD8 (10 μg/mL), anti-CD25 (10 μg/mL) mAbs was performed as detailed previously.31 Double immunostaining was performed by using the avidin-biotin-peroxidase system with 2 different substrates. To identify on the same specimen 2 different proteins, the 3-amino-9-ethyl-carbazole (AEC; red color) and the vector SG (bluish gray) substrates were used, respectively.
Polyclonal stimulation of CD8+CD25+ and CD8+CD25- thymocytes
CD8+CD25+ and CD8+CD25- purified thymocytes were stimulated in RPMI medium containing 10% FCS serum on plates coated with anti-CD3 (5 μg/mL) plus soluble anti-CD28 (10 μg/mL) mAbs. The cells were monitored every 2 days for CTLA-4 and TGF-β1 expression and stimulated at day 6 for cytokine production.
Flow cytometry analysis of intracellular cytokines
Flow cytometry analysis at single-cell level of intracellular IL-2, IL-4, IL-10, IL-13, TNF-α, and IFN-γ was performed as described.32 Briefly, 1 × 106 cells were stimulated with phorbol myristate acetate (PMA; 10 ng/mL) plus ionomycin (1 μM) for 6 hours, the last 2 in the presence of brefeldin A (5 μg/mL). After stimulation, cells were washed twice with PBS, pH 7.2, fixed 15 minutes with formaldehyde (2% in PBS, pH 7.2), washed twice with 0.5% BSA in PBS, pH 7.2, permeabilized with PBS, pH 7.2, containing 0.5% BSA and 0.5% saponin, and then incubated for 15 minutes at room temperature with the specific mAb. Cells were then washed and analyzed on a BDLSR cytofluorimeter using the CellQuest software (BD Biosciences). The area of positivity was determined using an isotype-matched mAb; 104 events for each sample were acquired.
Cloning of thymocytes
For the cloning procedure, CD4+CD25+, CD4+CD25-, CD8+CD25+, and CD8+CD25- thymocytes from one postnatal human thymus were seeded under limiting-dilution conditions (0.5 cell/well) in round-bottom microwell plates (Nunc, Rochester, NY), containing 105 irradiated (6000 rad) allogeneic peripheral blood mononuclear cells (PBMNCs) as feeder cells, 1% phytohemagglutinin (PHA; vol/vol), and rIL-2 (20 U/mL), as reported.33
Proliferation assays
To assess the proliferative response of human CD4+CD25+, CD4+CD25-, CD8+CD25+, CD8+CD25- thymocytes, allogeneic PBMNCs, depleted of T cells by the use of anti-CD3 MACS microbeads, were used. A total of 105 irradiated (6000 rad) T-cell-depleted PBMNCs were cultured with 5 × 104 purified CD4+CD25+, CD4+CD25-, CD8+CD25+, or CD8+CD25- thymocytes. On day 5, after 8 hours of pulsing with 0.5 μCi (0.0185 MBq) 3H-TdR/well (Amersham, Little Chalfont, United Kingdom), cultures were harvested and radionuclide uptake measured by scintillation counting.
For the assessment of suppressive properties, 5 × 104 CD4+CD25- thymocytes were cultured with 105 irradiated T-cell-depleted allogeneic PBMNCs in the presence of different numbers of CD4+CD25+, CD8+CD25+, or CD8+CD25- autologous thymocytes. In some experiments, anti-TGF-β1 (10 μg/mL), anti-CTLA-4 (10 μg/mL), anti-GITR (10 μg/mL), anti-TGF-β1 plus anti-CTLA-4, or isotype control (IgG1 + IgG2a, 10 μg/mL each) mAbs were added to the cultures. On day 5, after 8 hours of pulsing with 0.5 μCi (0.0187 MBq) 3H-TdR/well (Amersham), cultures were harvested and radionuclide uptake measured by scintillation counting. In some experiments, the expression of CD25 and CD69 in both the CD8+CD25+ regulatory population and CD4+CD25- target population was also evaluated. In parallel, mixed lymphocyte cultures (MLCs) were performed in the presence or absence of IL-2 (10 IU/mL) or IL-15 (10 ng/mL) and thymidine incorporation was evaluated on day 5.
Transwell experiments
Transwell experiments were performed in 24-well transwell plates (0.22-μm pore size; Costar, Corning, NY). Then, 5 × 105 CD4+CD25- thymocytes were stimulated with 106 irradiated T-cell-depleted allogeneic PBMNCs in the lower chamber, in the absence or presence of 5 × 105 CD8+CD25+ thymocytes placed in the same or in the upper chamber. CD8+CD25+ thymocytes placed in the upper chamber were cultured in medium alone or stimulated with 106 irradiated T-cell-depleted allogeneic PBMNCs in presence or in the absence of 5 × 105 CD4+CD25- cells.
On day 5, after 8 hours of pulsing with 2 μCi/mL (0.074 MBq) 3H-TdR (Amersham), the cells of the lower chambers were harvested and radionuclide uptake was measured by scintillation counting.
Detection of cytokine production by ELISA
The ability of CD8+CD25- and CD8+CD25+ T cells to produce cytokines was also evaluated in cell-free supernatants after a 36-hour stimulation of 106/mL cells with PMA (20 ng/mL) and anti-CD3 mAb (100 ng/mL), as previously described.33 To this end, in-house-made enzyme-linked immunosorbent assays (ELISAs) were used. Anti-IL-2, anti-IL-4, anti-IL-10, anti-IL-13, anti-IFN-γ, and anti-TNF-α mAbs used in these assays were purchased from BD Biosciences.
Statistical analysis
Statistical analysis was performed using the Student t test.
Results
Identification, localization, and characterization of CD8+CD25+ T cells
Freshly isolated thymocytes from 12 postnatal human thymuses were assessed by fluorescence-activated cell sorting (FACS) analysis for the expression of CD3, CD4, CD8, and CD25. As reported in a previous study,12 a small population of SP CD4+ cells (on average 7%) exhibited high CD25 expression (Figure 1A). In the same thymuses a small population of SP CD8+CD25+ thymocytes (on average 4%) was also consistently observed (Figure 1A). By using immunohistochemical staining with anti-CD25 and anti-CD8 mAbs, it was found that CD8+CD25+ thymocytes, as CD4+CD25+, were prevalently detected in the thymic septa (Figure 1B) and in medullary areas (Figure 1D).
Identification, localization, and purification of CD8+CD25+ human thymocytes. (A) Freshly isolated human thymocytes were assessed for expression of CD3, CD4, CD8, and CD25 by flow cytometry. Gated CD4+ (R1) or CD8+ (R2) SP thymocytes were assessed for CD3 and CD25. (B) Staining of a thymic section with anti-CD8 (bluish gray) plus anti-CD25 (red) mAbs. Arrows indicate cells showing double staining in the fibrous septa. (C) A consecutive section stained with anti-CD8 (bluish gray) plus isotype control (red) mAbs. (D) Staining of a thymic section with anti-CD8 (bluish gray) plus anti-CD25 (red) mAbs. Arrows indicate cells showing double staining in the medulla. (E) A consecutive section stained with anti-CD8 (bluish gray) plus isotype control (red) mAbs. Original magnification, ×400 for panels B-E.
Identification, localization, and purification of CD8+CD25+ human thymocytes. (A) Freshly isolated human thymocytes were assessed for expression of CD3, CD4, CD8, and CD25 by flow cytometry. Gated CD4+ (R1) or CD8+ (R2) SP thymocytes were assessed for CD3 and CD25. (B) Staining of a thymic section with anti-CD8 (bluish gray) plus anti-CD25 (red) mAbs. Arrows indicate cells showing double staining in the fibrous septa. (C) A consecutive section stained with anti-CD8 (bluish gray) plus isotype control (red) mAbs. (D) Staining of a thymic section with anti-CD8 (bluish gray) plus anti-CD25 (red) mAbs. Arrows indicate cells showing double staining in the medulla. (E) A consecutive section stained with anti-CD8 (bluish gray) plus isotype control (red) mAbs. Original magnification, ×400 for panels B-E.
To better characterize the CD8+CD25+ population, CD4+CD25+ and CD8+CD25+ thymocytes were purified by MACS, as described in “Materials and methods.” The CD4+ Treg population consistently contained more than 98% CD4+CD25+ thymocytes, whereas the purity of the CD8+CD25+ thymocyte fraction ranged from 90% to 95% (data not shown).
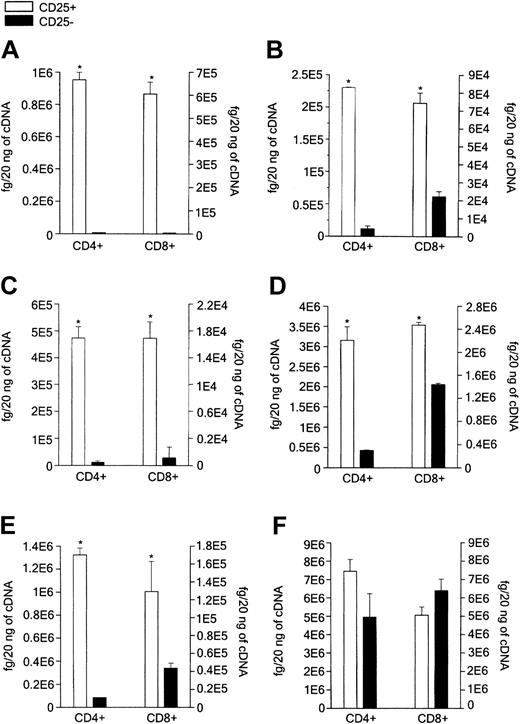
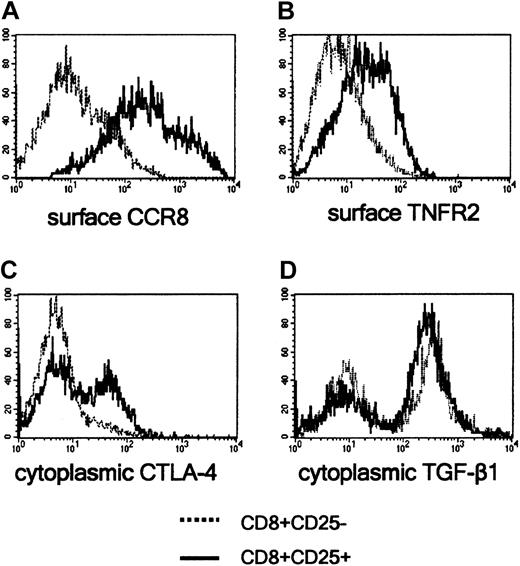
Because Foxp3 and GITR have been considered as markers of CD4+CD25+ Treg cells, mRNA concentrations of GITR and Foxp3 were measured in purified CD8+CD25+ versus CD8+CD25- thymocytes, as well as in purified CD4+CD25+ versus CD4+CD25- thymocytes, by using quantitative RT-PCR. Both CD4+CD25+ and CD8+CD25+ thymocytes expressed high levels of GITR and Foxp3 mRNAs, whereas both CD4+CD25- and CD8+CD25- cells had poor, if any, GITR and Foxp3 mRNA expression (Figure 2A-B). In our previous study, TNFR2 and CCR8 were selectively expressed on the surface of the majority of CD4+CD25+ thymocytes and CTLA-4 and TGF-β1 appeared to be involved in their suppressive activity.12 Therefore, mRNA levels for TNFR2, CCR8, CTLA-4, and TGF-β1 were assessed in both CD4+CD25+ and CD8+CD25+ purified thymocytes, as well as in their CD25- counterparts. As shown in Figure 2C-E, both CD4+CD25+ and CD8+CD25+ thymocytes exhibited significantly higher levels of CCR8, TNFR2, and CTLA-A mRNAs than CD4+CD25- or CD8+CD25- autologous thymocytes, the differences between CD25+ and CD25- cells being lower in CD8+ than in CD4+ subsets. By contrast, both CD4+CD25+ and CD8+CD25+ populations expressed high TGF-β1 mRNA concentrations, although no significant differences in comparison with their CD25- counterparts were observed (Figure 2F). The expression of GITR, TNFR2, CCR8, CTLA-4, and TGF-β1 proteins was also compared in CD8+CD25+ and CD8+CD25- thymocytes using flow cytometry. Only few cells expressed GITR in both cell populations (data not shown), whereas most CD8+CD25+ thymocytes, but not CD8+CD25- thymocytes, expressed both TNFR2 and CCR8 (Figure 3A-B). By contrast, neither CTLA-4 nor TGF-β1 was expressed on the surface of both CD8+ thymocyte subsets. Of note, both molecules were detectable on CD8+CD25+ and CD8+CD25- at cytoplasmic level (Figure 3C-D). The expression of scurfin, the protein encoded by Foxp3 gene, was not assessed due to the unavailability of antihuman scurfin antibodies.
Measurement of mRNAs. mRNA was measured for Foxp3 (A), GITR (B), CCR8 (C), TNFR2 (D), CTLA-4 (E), and TGF-β1 (F) in purified CD4+CD25+ versus CD4+CD25- or CD8+CD25+ versus CD8+CD25-thymocytes. mRNA was obtained from CD4+CD25+, CD4+CD25-, CD8+CD25+, or CD8+CD25-thymocytes purified by MACS. Columns represent mean values (± SD) of mRNA levels obtained in 4 separate experiments by using real-time quantitative RT-PCR. *P < .01.
Measurement of mRNAs. mRNA was measured for Foxp3 (A), GITR (B), CCR8 (C), TNFR2 (D), CTLA-4 (E), and TGF-β1 (F) in purified CD4+CD25+ versus CD4+CD25- or CD8+CD25+ versus CD8+CD25-thymocytes. mRNA was obtained from CD4+CD25+, CD4+CD25-, CD8+CD25+, or CD8+CD25-thymocytes purified by MACS. Columns represent mean values (± SD) of mRNA levels obtained in 4 separate experiments by using real-time quantitative RT-PCR. *P < .01.
Detection of CCR8, TNFR2, CTLA-4, and TGF-β1 proteins in CD8+CD25+ and CD8+CD25- human thymocytes. CCR8 (A) and TNFR2 (B) were detected by flow cytometry on the surface of freshly prepared thymocytes, and CTLA-4 (C) and TGF-β1 (D) in the cytoplasm of fixed thymocytes. A representative experiment is shown.
Detection of CCR8, TNFR2, CTLA-4, and TGF-β1 proteins in CD8+CD25+ and CD8+CD25- human thymocytes. CCR8 (A) and TNFR2 (B) were detected by flow cytometry on the surface of freshly prepared thymocytes, and CTLA-4 (C) and TGF-β1 (D) in the cytoplasm of fixed thymocytes. A representative experiment is shown.
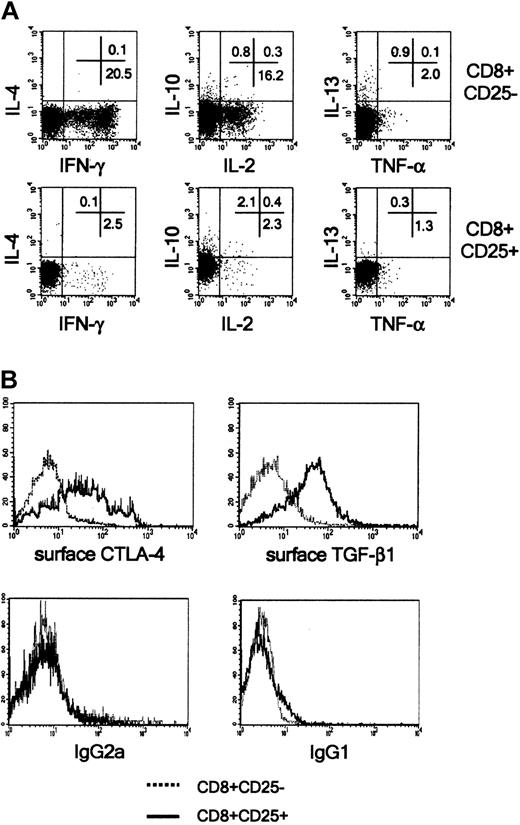
Activated CD8+CD25+ thymocytes do not produce cytokines, and most of them express CTLA-4 and TGF-β1 on their membrane
The ability of freshly isolated CD8+CD25+ thymocytes to produce IL-2, IL-4, IL-10, IL-13, TNF-α, and IFN-γ in response to polyclonal stimulation with PMA plus ionomycin was then investigated. Neither CD8+CD25- nor CD8+CD25+ thymocytes produced any of the these cytokines (data not shown). However, following a 6-day preactivation with insolubilized anti-CD3 plus anti-CD28 mAbs, CD8+CD25- cells acquired the ability to produce IL-2 and IFN-γ in response to stimulation with PMA plus ionomycin. Of note, under the same experimental conditions, the production of IL-2, IFN-γ, IL-4, IL-10, TNF-α, and IL-13 by CD8+CD25+ cells was never observed (Figure 4A). The assessment of the CD8+CD25+ culture supernatants (following stimulation with PMA plus anti-CD3 mAb) by ELISA, confirmed their inability to produce the cytokines (data not shown).
Activated CD8+CD25+ human thymocytes do not produce cytokines, but express CTLA-4 and TGF-β1 on their surface. (A) CD8+CD25- or CD8+CD25+ thymocytes were incubated in the presence of insolubilized anti-CD3 and anti-CD28 mAbs. On day 6, cells were stimulated for 6 hours with PMA plus ionomycin, and the last 2 hours of stimulation occurred in the presence of brefeldin A, and cytokine synthesis at the single-cell level was analyzed by flow cytometry. (B) Detection of CTLA-4 and TGF-β1 by flow cytometry on the surface of the same cell populations. Histograms in the bottom panels represent stainings obtained with isotype-matched mAbs. A representative experiment is shown.
Activated CD8+CD25+ human thymocytes do not produce cytokines, but express CTLA-4 and TGF-β1 on their surface. (A) CD8+CD25- or CD8+CD25+ thymocytes were incubated in the presence of insolubilized anti-CD3 and anti-CD28 mAbs. On day 6, cells were stimulated for 6 hours with PMA plus ionomycin, and the last 2 hours of stimulation occurred in the presence of brefeldin A, and cytokine synthesis at the single-cell level was analyzed by flow cytometry. (B) Detection of CTLA-4 and TGF-β1 by flow cytometry on the surface of the same cell populations. Histograms in the bottom panels represent stainings obtained with isotype-matched mAbs. A representative experiment is shown.
Of note, the great majority of activated CD8+CD25+ thymocytes also expressed CTLA-4 and TGF-β1 on their surface, whereas CD8+CD25- did not (Figure 4B). No remarkable expression of GITR was observed on both thymocyte populations (data not shown).
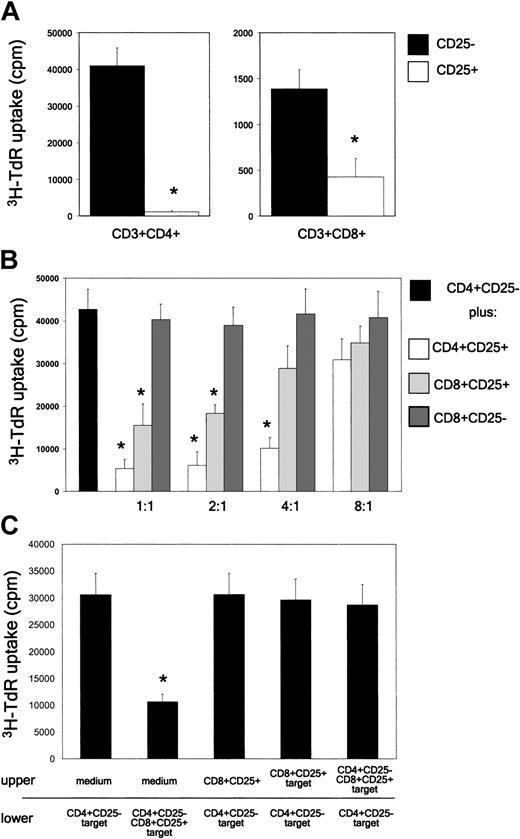
CD8+CD25+ human thymocytes do not proliferate, but suppress the proliferation of both autologous CD4+CD25- and CD8+CD25- T cells by a contact-dependent mechanism
Having established that CD8+CD25+ thymocytes have similar phenotypic features of CD4+CD25+ Treg cells, subsequent experiments were devoted to compare the ability of CD8+CD25+ and CD4+CD25+ thymocytes to proliferate in response to irradiated allogeneic T-cell-depleted human PBMNCs. Whereas CD4+CD25- cells showed high and CD8+CD25- cells low (even if detectable) proliferation, CD8+CD25+ and CD4+CD25+ thymocytes did not proliferate in response to irradiated allogeneic T-cell-depleted PBMNCs (Figure 5A). The suppressive activity of CD4+CD25+, CD8+CD25+, and CD8+CD25- thymocytes was then assessed by adding each population to autologous CD4+CD25- cells, which were stimulated with allogeneic irradiated T-cell-depleted PBMNCs. The results indicated that both CD4+CD25+ and CD8+CD25+, but not CD8+CD25-, thymocytes, exerted a dose-dependent inhibition of the proliferative response of autologous CD4+CD25- cells (Figure 5B).
CD8+CD25+ thymocytes do not proliferate in MLCs and suppress the proliferation in MLCs of CD4+CD25- thymocytes via a contact-dependent mechanism. (A) Proliferative response of purified CD4+CD25-, CD4+CD25+, CD8+CD25-, or CD8+CD25+ human thymocytes to allogeneic stimulation. On day 5, cells were harvested and their proliferation assessed by measuring 3H-TdR uptake. Mean values (± SD) obtained in 9 separate experiments are reported. (B) Suppression by CD4+CD25+ or CD8+CD25+ thymocytes, but not by CD8+CD25- thymocytes, of the proliferative response of autologous CD4+CD25- thymocytes to allogeneic T-cell-depleted PBMNCs. On day 5, cells were harvested and proliferation assessed by measuring 3H-TdR uptake. Mean values (± SD) obtained in 9 separate experiments are reported. (C) Cell contact dependency of suppressive activity exerted by CD8+CD25+ thymocytes. CD4+CD25- thymocytes were stimulated with irradiated T-cell-depleted allogeneic PBMNCs in the lower chamber of a transwell plate in the absence or presence of CD8+CD25+ thymocytes, which were placed in the same chamber or in the top chamber. CD8+CD25+ thymocytes placed in the upper chamber were cultured in medium alone or stimulated with 106 irradiated T-cell-depleted allogeneic PBMNCs in the presence or absence of 5 × 105 CD4+CD25- cells. On day 5, cells present in the bottom chamber were harvested and their proliferation assessed by measuring 3H-TdR uptake. The mean values (± SD) obtained in 4 separate experiments are reported. *P < .01.
CD8+CD25+ thymocytes do not proliferate in MLCs and suppress the proliferation in MLCs of CD4+CD25- thymocytes via a contact-dependent mechanism. (A) Proliferative response of purified CD4+CD25-, CD4+CD25+, CD8+CD25-, or CD8+CD25+ human thymocytes to allogeneic stimulation. On day 5, cells were harvested and their proliferation assessed by measuring 3H-TdR uptake. Mean values (± SD) obtained in 9 separate experiments are reported. (B) Suppression by CD4+CD25+ or CD8+CD25+ thymocytes, but not by CD8+CD25- thymocytes, of the proliferative response of autologous CD4+CD25- thymocytes to allogeneic T-cell-depleted PBMNCs. On day 5, cells were harvested and proliferation assessed by measuring 3H-TdR uptake. Mean values (± SD) obtained in 9 separate experiments are reported. (C) Cell contact dependency of suppressive activity exerted by CD8+CD25+ thymocytes. CD4+CD25- thymocytes were stimulated with irradiated T-cell-depleted allogeneic PBMNCs in the lower chamber of a transwell plate in the absence or presence of CD8+CD25+ thymocytes, which were placed in the same chamber or in the top chamber. CD8+CD25+ thymocytes placed in the upper chamber were cultured in medium alone or stimulated with 106 irradiated T-cell-depleted allogeneic PBMNCs in the presence or absence of 5 × 105 CD4+CD25- cells. On day 5, cells present in the bottom chamber were harvested and their proliferation assessed by measuring 3H-TdR uptake. The mean values (± SD) obtained in 4 separate experiments are reported. *P < .01.
To establish whether, CD8+CD25+ thymocytes exerted their suppressive activity via a contact-dependent mechanism, as CD4+CD25+ did, the proliferative response of CD4+CD25- T cells to irradiated allogeneic T-cell-depleted PBMNCs in the presence (direct contact) or in the absence (physical separation by a porous septum) of autologous CD8+CD25+ cells was assessed. As shown in Figure 5C, the proliferative response of CD4+CD25- T cells to irradiated allogeneic T-depleted cells was strongly inhibited by CD8+CD25+ T cells only when the 2 cell populations were present in the same chamber.
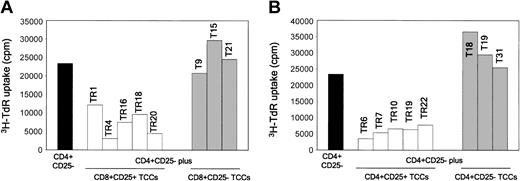
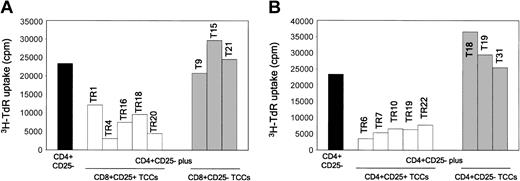
To verify whether the suppressive effect of either CD4+CD25+ or CD8+CD25+ thymocytes was a property of each cell in the suspensions, T-cell clones were derived from both thymocyte subpopulations, as well as from CD4+CD25- or CD8+CD25- cells obtained from the thymus of one donor. Five CD4+CD25+ or CD8+CD25+ and 3 CD4+CD25- or CD8+CD25- T-cell clones were assessed for their ability to suppress the proliferative response of autologous CD4+CD25- thymocytes to allogeneic stimulation. All CD25+ T-cell clones showed suppressive activity, whereas none of the CD25- ones did (Figure 6A-B).
CD4+CD25+ and CD8+CD25+ thymocyte-derived T-cell clones suppress the proliferation in MLCs of CD4+CD25- thymocytes. Suppression by CD4+CD25+ or CD8+CD25+, but not by CD4+CD25- or CD8+CD25-, T-cell clones of the proliferative response of autologous CD4+CD25- thymocytes to allogeneic T-cell-depleted PBMNCs. On day 5, cells were harvested and their proliferation assessed by measuring 3H-TdR uptake.
CD4+CD25+ and CD8+CD25+ thymocyte-derived T-cell clones suppress the proliferation in MLCs of CD4+CD25- thymocytes. Suppression by CD4+CD25+ or CD8+CD25+, but not by CD4+CD25- or CD8+CD25-, T-cell clones of the proliferative response of autologous CD4+CD25- thymocytes to allogeneic T-cell-depleted PBMNCs. On day 5, cells were harvested and their proliferation assessed by measuring 3H-TdR uptake.
The suppressive effect of CD8+CD25+ thymocytes is mediated by the inhibition of IL-2 responsiveness of target T cells induced by the combined action of CTLA-4 and membrane TGF-β1
To exclude that the suppressive activity of CD8+CD25+ thymocytes was related to their cytolytic potential, we assessed the expression of intracellular perforin and granzyme A in either CD8+CD25- or CD8+CD25+ thymocytes. Flow cytometry analysis revealed the absence of both perforin and granzyme A in both populations (data not shown). Furthermore, annexin V and propidium iodide evaluation was performed by flow cytometry on CD4+CD25- thymocytes stimulated with allogeneic T-cell-depleted PBMNCs, in the absence or presence of CD8+CD25+ thymocytes. Even by using this approach, no evidence for cytolytic activity by CD8+CD25+ was observed (data not shown).
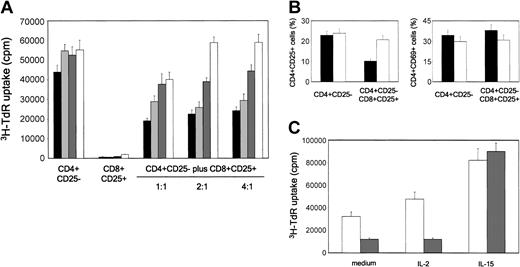
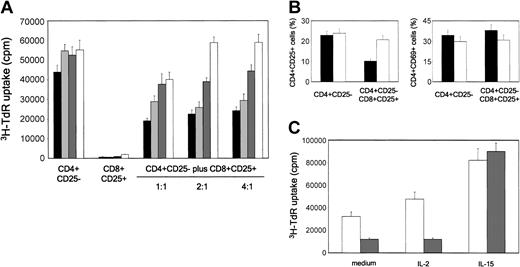
We then asked whether CD8+CD25+ thymocytes exerted their suppressive activity via the combined activity of CTLA-4 and TGF-β1 expressed on their surface membrane, as previously reported for CD4+CD25+ thymocytes.12 To this end, different numbers of purified CD8+CD25+ thymocytes were added to cultures of autologous CD4+CD25- cells, stimulated with irradiated allogeneic T-cell-depleted human PBMNCs, in the absence or presence of neutralizing anti-CTLA-4, anti-TGF-β1, anti-CTLA-4 plus anti-TGF-β1, or isotype control mAbs. As shown in Figure 7A, the addition in culture of isotype control mAbs did not exert any effect on the proliferative response of CD4+CD25- thymocytes. By contrast, the addition of either anti-CTLA-4 or anti-TGFβ1 mAbs consistently reduced, whereas the simultaneous addition in culture of the 2 mAbs completely abrogated, the suppressive activity of CD8+CD25+ thymocytes. By contrast, an anti-GITR mAb did not exhibit any effect on the suppressive activity of CD8+CD25+ thymocytes (data not shown).
Suppression mediated by CD8+CD25+ thymocytes is mediated by CTLA-4 and TGF-β1 and is due to the inhibition of IL-2Rα chain expression. (A) CD4+CD25- thymocytes were stimulated with allogeneic irradiated T-cell-depleted PBMNCs in the presence of different numbers of autologous purified CD8+CD25+ thymocytes without (IgG1 + IgG2a isotype control; black bars) or with anti-CTLA-4 (dark gray bars), anti-TGF-β1 (light gray bars), or a mixture of both (anti-mix; white bars) mAbs. Cell proliferation was measured by assessing 3H-TdR incorporation. Mean values (± SD) obtained in 4 separate experiments are reported. (B) Effect of the addition of CD8+CD25+ thymocytes on the expression of CD25 and CD69 by CD4+CD25- autologous thymocytes, as detected on day 5 of culture by flow cytometry. Mean values (± SD) obtained in 4 separate experiments are reported. ▪ indicates isotype mAb; and □ indicates anti-mix. (C) IL-15, but not IL-2, restores the proliferative response of CD4+CD25- thymocytes suppressed by CD8+CD25+ thymocytes. Cell proliferation was measured by assessing 3H-TdR incorporation. Mean values (± SD) obtained in 4 separate experiments are reported. □ indicates CD4+CD25- thymocytes; and ▦ indicates CD4+CD25- plus CD8+CD25+ (ratio 1:1).
Suppression mediated by CD8+CD25+ thymocytes is mediated by CTLA-4 and TGF-β1 and is due to the inhibition of IL-2Rα chain expression. (A) CD4+CD25- thymocytes were stimulated with allogeneic irradiated T-cell-depleted PBMNCs in the presence of different numbers of autologous purified CD8+CD25+ thymocytes without (IgG1 + IgG2a isotype control; black bars) or with anti-CTLA-4 (dark gray bars), anti-TGF-β1 (light gray bars), or a mixture of both (anti-mix; white bars) mAbs. Cell proliferation was measured by assessing 3H-TdR incorporation. Mean values (± SD) obtained in 4 separate experiments are reported. (B) Effect of the addition of CD8+CD25+ thymocytes on the expression of CD25 and CD69 by CD4+CD25- autologous thymocytes, as detected on day 5 of culture by flow cytometry. Mean values (± SD) obtained in 4 separate experiments are reported. ▪ indicates isotype mAb; and □ indicates anti-mix. (C) IL-15, but not IL-2, restores the proliferative response of CD4+CD25- thymocytes suppressed by CD8+CD25+ thymocytes. Cell proliferation was measured by assessing 3H-TdR incorporation. Mean values (± SD) obtained in 4 separate experiments are reported. □ indicates CD4+CD25- thymocytes; and ▦ indicates CD4+CD25- plus CD8+CD25+ (ratio 1:1).
Finally, we asked whether the activity of CD8+CD25+ thymocytes was related to their ability to down-regulate the expression of the IL-2Rα chain (CD25) on the surface of effector T cells, thus rendering them unresponsive to IL-2, as previously shown for CD4+CD25+ thymocytes.12 To do this, CD4+CD25- thymocytes were stimulated with allogeneic irradiated T-cell-depleted PBMNCs alone or with autologous CD8+CD25+ thymocytes, in the absence or presence of a mixture of anti-CTLA-4 and anti-TGF-β1, or isotype control mAbs. As shown in Figure 7B, the presence in culture of CD8+CD25+ cells consistently reduced CD25 expression on autologous CD4+CD25- thymocytes and, more importantly, CD25 expression was not affected when the mixture of the 2 mAbs was added in culture. By contrast, the expression of another T-cell activation marker, such as CD69, was not altered (Figure 7B).
To support the possibility that the suppressive effect on the proliferation of CD4+CD25- cells was due to their unresponsiveness to IL-2, the same cells were stimulated with allogeneic irradiated T-cell-depleted PBMNCs alone or with autologous CD8+CD25+ thymocytes, in the absence or presence of exogenous IL-2 or IL-15. As shown in Figure 7C, whereas IL-2 did not exert any effect, the addition of IL-15 completely abrogated the CD8+CD25+ inhibitory activity on the proliferation of autologous CD4+CD25- thymocytes.
Discussion
This study shows the existence of a previously unrecognized population of CD8+CD25+ cells with regulatory activity in postnatal human thymus. These cells share localization, phenotype, functions, and mechanisms of action with the recently described CD4+CD25+ human Treg thymocytes.12 CD4+CD25+ cells probably represent the most important members of a heterogeneous family of Treg cells, which includes Th3 cells5-8 and Tr1 cells.9 However, Treg activity is not only limited to CD4+ T cells, but is also a property of some CD8+ T-cell subsets. CD8+ T cells showing Treg activity via the production of TGF-β1 have been demonstrated in individuals infected with HIV.34 Another CD8+ T-cell population exerting suppressor activity via the production of IL-10 has been induced by priming naïve CD8+ T cells with DC2 cells.35 More recently, CD8+CD25-CD28- T cells were generated by multiple stimulations of human peripheral blood lymphocytes (PBLs) with allogeneic antigen-presenting cells (APCs).36 These CD8+CD25-CD28- T cells specifically recognized HLA-I antigens expressed by the stimulatory APCs and suppressed the proliferative response of alloreactive CD4+ T cells against APCs used for priming.37
The CD8+CD25+ thymocytes described in this study were different from the previously described CD8+ Treg cell populations and appeared to be quite similar to the CD4+CD25+ Treg human thymocytes. First, they localized in the same thymic areas (ie, the fibrous septa and medullary areas) and expressed Foxp3 and GITR mRNAs, which are considered as main markers of murine CD4+CD25+ Treg cells.25-29 They also expressed higher levels of CCR8, TNFR2, and CTLA-4 mRNAs than CD8+CD25- thymocytes. The differences between CD8+CD25+ and CD8+CD25-, however, were usually lower than those found between the CD4+CD25+ and CD4+CD25- subsets. This may be due to the difficulty in sorting out pure CD8+CD25+ cells, because the expression of the CD25 molecule on the surface of these cells is low. However, like their CD4+CD25+ counterpart, CD8+CD25+ thymocytes constitutively expressed cytoplasmic CTLA-4, as well as surface TNFR2 and CCR8 and they did not produce any cytokine. Moreover, CD8+CD25+, like CD4+CD25+ thymocytes, acquired the ability to express both CTLA-4 and TGF-β1 on their surface following activation, whereas neither CD4+CD25- nor CD8+CD25- thymocytes did. The reason why TGF-β1 mRNA levels were comparable in both CD25+ and CD25- thymocytes, whereas surface TGF-β1 was detectable only in CD25+ (either CD4+ or CD8+) subsets, is at present unclear. One possibility is that TGF-β1 may be carried only on the surface of CD25+ thymocytes or, alternatively, it is expressed on the surface of all populations, but cannot be detected on CD25- (either CD4+ or CD8+) subsets because of its continuous cleavage.
Of note, CD8+CD25+ thymocytes also exhibited the same functional properties as CD4+CD25+ thymocytes, since they did not proliferate in MLCs and inhibited in a dose-dependent fashion the proliferation of autologous CD4+CD25- thymocytes in response to allogeneic stimulation. This inhibitory effect was a property of every CD4+CD25+ or CD8+CD25+ thymocyte, as demonstrated by assessing the activity of a number of T-cell clones derived from these cell populations. More important, CD8+CD25+ thymocytes exerted their suppressive activity via the same mechanisms reported for CD4+CD25+ regulatory thymocytes.12 Indeed, their regulatory activity was mediated by cell-to-cell contact via the interaction of both CTLA-4 and TGF-β1 with their receptors. This interaction leads to the down regulation of the IL-2Rα chain expression on target T cells, making them unresponsive to IL-2. Moreover, the suppressive activity of CD8+CD25+ thymocytes, like that of CD4+CD25+ thymocytes, could be abrogated by the addition of IL-15, a T-cell growth factor that does not bind the IL-2Rα chain. By contrast, the possibility that the suppressor activity of CD8+CD25+ thymocytes was due to cytotoxicity could be excluded because these cells did not contain perforins or granzymes and did not induce apoptosis on target T cells.
The results of this study allow us to designate some focal points and to formulate one hypothesis on their functional meaning. First, CD8+CD25+ thymocytes represent a previously unrecognized population of Treg cells, as demonstrated by their selective expression of Foxp3 and GITR. The reason why GITR is highly expressed on the same cells at mRNA, but not at protein level, is presently unclear. Moreover, the functional relevance of GITR in human cells could not be evaluated because the commercially available antihuman GITR used in our experiments, which had no effect on the suppressive activity of CD8+CD25+ thymocytes, was a neutralizing antibody, whereas the role of GITR on mouse CD4+CD25+ Treg cells has been demonstrated by using anti-GITR antibodies with agonistic activity.25,26 This is in agreement with another paper reporting the lack of effect of the same antibody on the activity of CD4+CD25+ human T-cell clones.23 Thus, additional studies are required to better clarify the role of GITR in human Treg cell-mediated suppressive activity by using well-characterized agonistic and antagonistic antibodies, as well as natural ligands. Second, both CTLA-4 and TGF-β1, despite the nonselective expression of the latter at mRNA level, become prevalent at protein level on the surface of activated CD4+CD25+ and CD8+CD25+ Treg cells and seem to provide the major basis for the contact-dependent activity of these suppressor cells. This possibility has been made questionable by the observation that dominant-negative TGF-βR2 transgenic mice possess fully suppressible CD4+CD25- T cells, and that CD4+CD25+ T cells, isolated from young TGF-β1-deficient mice, are fully competent suppressors when mixed with CD4+CD25- T cells from wild-type mice.38 However, this finding has not been confirmed by others.24
Moreover, we have previously shown that human CD4+CD25+ thymocytes are able to express mTGF-β1 and that its neutralization abrogated their suppressive activity in vitro.12 In addition, the role of TGF-β1 in the suppressive activity of human CD4+CD25+ T-cell clones has been confirmed in a recent study, although the expression of mTGF-β1 on these cells could not be demonstrated.22-24
The physiologic meaning of CD8+CD25+ thymocytes sharing phenotypic and functional properties with CD4+CD25+ thymocytes is presently unclear. It has been suggested that CD4+CD25+ thymocytes could be educated during the process of negative selection to recognize self-antigens that are presented on major histocompatibility complex (MHC) class II molecules by medullary DCs in a process that is known as “altered negative selection,” after which they migrate directly to peripheral lymphoid tissues.39-41 Another possibility is that CD25 expression and suppressor function are acquired at much earlier stage of differentiation in the thymic cortex during the process of positive selection on cortical epithelial cells. Some of these cells then undergo a process of negative selection on DCs in the medulla and dye by apoptosis, but others are allowed to migrate to peripheral lymphoid tissues, according to the affinity of their receptors for self-antigens.42 A similar explanation might be provided for CD8+CD25+ thymocytes with the only difference that these cells are educated to recognize self-antigens associated with MHC class I molecules by cells present in the thymic microenvironment. After their migration to the peripheral lymphoid tissues, CD8+CD25+ Treg cells may be involved in the regulation of T-cell responses directed against viruses and transformed cells, which are usually exploited by cytotoxic CD8+ T lymphocytes. The demonstration and characterization of CD8+CD25+ Treg cells in the peripheral lymphoid tissues may help to better identify their exact functional meaning.
Prepublished online as Blood First Edition Paper, July 31, 2003; DOI 10.1182/blood-2003-04-1320.
Supported by funds provided by the Italian Ministry of Education (FIRB and 40%), the Italian Ministry of Health (AIDS Project), the Italian Association for Cancer Research (AIRC), and the Ministry of Health of the Tuscan Region.
L.C. and F.L. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.