Abstract
We sought to enhance the selective toxicity of tumor necrosis factor α (TNFα) to permit its systemic use in cancer therapy. Because ligand-targeted therapeutics have proven successful in improving the selective toxicity of drugs, we prepared a fusion protein (L19mTNFα) composed of mouse TNFα and a high-affinity antibody fragment (L19 scFv) to the extradomain B (ED-B) domain of fibronectin, a marker of angiogenesis. L19mTNFα was expressed in mammalian cells, purified, and characterized. L19mTNFα was an immunoreactive and biologically active homotrimer. Radiolabeled L19mTNFα selectively targeted tumor neovasculature in tumor-bearing mice, where it accumulated selectively and persistently (tumor-to-blood ratio of the percentage of injected dose per gram [%ID/g] of 700, 48 hours from injection). L19mTNFα showed a greater anticancer therapeutic activity than both mTNFα and TN11mTNFα, a control fusion protein in which an antibody fragment, irrelevant in the tumor model used, substituted for L19. This activity was further dramatically enhanced by its combination with melphalan or the recently reported fusion protein L19-IL2. In conclusion, L19mTNFα allows concentrating therapeutically active doses of TNFα at the tumor level, thus opening new possibilities for the systemic use of TNFα in cancer therapy. (Blood. 2003;102:4384-4392)
Introduction
During tumor progression the microenvironment surrounding tumor cells undergoes extensive modifications that generate a “tumoral environment” which could ultimately represent a suitable target for antibody-based tumor therapy.1 In fact, the concept that the altered tumor microenvironment is itself a carcinogen that can be targeted is increasingly gaining consensus. Molecules that are able to effectively deliver therapeutic agents to the tumor microenvironment thus represent promising and important new tools for cancer therapy.1-3
Fibronectin is an extracellular matrix (ECM) component that is widely expressed in a variety of healthy tissues and body fluids. Different fibronectin (FN) isoforms can be generated by the alternative splicing of the FN pre-mRNA, a process modulated by cytokines and extracellular pH.4-7 The complete type III repeat extradomain B (ED-B) may be entirely included or omitted in the FN molecule.8 ED-B is highly conserved in different species, having 100% homology in all mammalians thus far studied (human, rat, mouse) and 96% homology with a similar domain in chicken. The FN isoform containing ED-B (B-FN) is undetectable immunohistochemically in healthy adult tissues, with the exception of tissues undergoing physiologic remodeling (eg, endometrium and ovary) and during wound healing.5,9 By contrast, its expression in tumors and fetal tissues is high.5 Furthermore, it was demonstrated that B-FN is a marker of angiogenesis10,11 and that endothelial cells invading tumor tissues migrate along ECM fibers containing B-FN.12
We reported on the possibility to selectively target tumoral vasculature, both in experimental tumor models and in patients with cancer, using a human recombinant antibody, L19 scFv, specific for B-FN.12-19 This observation paved the way for the antibody's use in both in vivo diagnostic (immunoscintigraphy) and therapeutic approaches entailing the selective delivery of radionuclides or toxic agents to tumoral vasculature. In addition, Birchler et al20 showed that L19, chemically coupled to a photosensitizer, selectively accumulates in the newly formed blood vessels of the angiogenic rabbit cornea model and, after irradiation with near infrared light, mediates the complete and selective occlusion of ocular neovasculature. More recently, Nilsson et al21 reported that the immunoconjugate of L19 with the extracellular domain of tissue factor mediates selective infarction in different types of murine tumor models. Furthermore, the cytokines interleukin 2 (IL-2) and IL-12 have both shown an enhanced therapeutic efficacy if delivered as fusion proteins with L19.22,23 Finally, because L19 reacts equally well with mouse and human ED-B, it can be used for both preclinical and clinical studies.
Tumor necrosis factor α (TNFα) is a pleiotropic cytokine with antitumoral activity24 composed of 3 noncovalently linked TNFα monomers, each of about 17.5 kDa, that yield a compact bell-shaped homotrimer.25 TNFα exerts its effects in tumors mainly on the endothelium of the tumor-associated vasculature,26,27 with increased permeability, up-regulation of tissue factor, fibrin deposition and thrombosis, and massive destruction of the endothelial cells.28-33 Moreover, treatment of tumor-bearing mice with intravenous injection of TNFα induces a significant reduction of the interstitial fluid pressure of the tumor,34 a process which is instrumental to increasing the concentration of antitumoral agents at the tumor site.28,35-37 The systemic administration of large, therapeutically effective, doses of TNFα is not possible, however, because of the unacceptably high levels of systemic toxicity it induces. For this reason, until today, only loco-regional therapies, such as “isolated limb perfusion” (ILP),38 have been used. Improved tumor response rates were achieved in patients with in transit melanoma metastases, using local perfusions of TNFα in combination with γ-interferon and melphalan.38 Moreover, ILP with melphalan and TNFα resulted in limb salvage in patients with soft-tissue sarcoma.39 A number of approaches are presently under investigation to improve the therapeutic effects and to reduce the toxic side effects of systemically administered TNFα. These strategies include the production of engineered TNFα mutants,40 the encapsulation of TNFα in long circulating liposomes,41 and the selective delivery to and concentration in tumors of TNFα through its coupling to specific ligands.42-44
Here we report the ligand-targeting treatment of tumor-bearing mice using the fusion protein L19mTNFα.
Materials and methods
Preparation of L19mTNFα and TN11mTNFα fusion proteins
Mouse TNFα cDNA covering the sequence coding for the 156 amino acid (aa)-long secreted form of mTNFα (Swissprot accession no. P06804)45 was obtained by reverse transcription-polymerase chain reaction (RT-PCR; Titan One Tube RT-PCR System; Roche Diagnostics, Mannheim, Germany) by using Balb/C mouse spleen total RNA as template and the primers BC-742 and BC-752. The forward BC-742 primer (sequence, ctcgaattctcttcctcatcgggtagtagctcttccggctcatcgtccagcggcctcagatcatcttctcaaaattcg) contained the EcoRI restriction enzyme sequence, a 45-base pair (bp) sequence encoding for a 15 amino acid linker (SSSSG)3, and the sequence coding for the first 8 amino acids of the mature murine TNFα. The reverse BC-752 primer (sequence, ctcgcggccgctcatcacagagcaatgactccaaagta) contained the sequence of the last 7 amino acids of murine TNFα, 2 stop codons, and the NotI restriction enzyme sequence (Figure 1B). The genomic sequence of the signal secretion leader peptide was obtained by HindIII and ApaLI digestion of the vector pUT-SEC,46 kindly provided by Dr Oscar Burrone (ICGEB, Trieste, Italy). The cDNA of the scFv L1912 was obtained by using Pwo enzymes (Roche Diagnostics), the primers BC-618 (containing the ApaLI restriction enzyme sequence) and BC-679 (containing the EcoRI restriction enzyme sequence) already reported by Carnemolla et al,22 and the DNA vector pDNEK-L19 as template. The cDNA sequence of the scFv TN1147 was obtained using Pwo enzymes (Roche Diagnostics) and the primers BC-773 (forward sequence, ctcgtgtgcactcgcaggtgcagctggtgcagtct) containing the ApaLI restriction enzyme sequence and BC-774 (reverse sequence, ctcgaattcacctaggacggtcagcttggt) containing the EcoRI restriction enzyme sequence, and the DNA vector reported by Carnemolla et al47 as template. The cDNA constructs depicted in Figure 1B (signal peptide, scFv L19 or TN11, and linker-mTNFα) were cloned in pCDNA3.1 mammalian expression vector (Invitrogen, Groningen, The Netherlands). The clones were sequenced on both strands using the ABI PRISM dRhodamine Terminator Cycle Sequencing Ready Reaction Kit (Perkin Elmer, Foster City, CA). All restriction enzymes (REs) were from Roche Diagnostics.
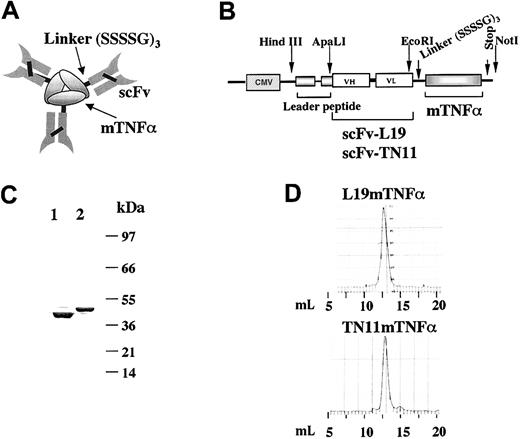
Preparation and characterization of L19mTNFα fusion protein and its control fusion protein TN11mTNFα (A) Scheme of the homotrimeric form of the fusion protein scFv-mTNFα in which the mTNFα monomers are held together through hydrophobic interactions. (B) Scheme of the construct for L19mTNFα and TN11mTNFα inserted in the mammalian expression vector pCDNA3.1, under the control of the cytomegalovirus (CMV) promoter. (C) SDS-PAGE analysis of the purified fusion proteins L19mTNFα (lane 1) and TN11mTNFα (lane 2). As expected, a major band corresponding to the monomer of about 45 kDa was detected. Numbers on the right are the apparent molecular masses of the standard, in kilodaltons. (D) Gel filtration profile of the purified L19mTNFα and TN11mTNFα (Superdex 200). The retention volume (milliliter) of the peaks corresponds to an apparent molecular mass of about 140 kDa, consistent with the homotrimeric format of the 2 fusion proteins.
Preparation and characterization of L19mTNFα fusion protein and its control fusion protein TN11mTNFα (A) Scheme of the homotrimeric form of the fusion protein scFv-mTNFα in which the mTNFα monomers are held together through hydrophobic interactions. (B) Scheme of the construct for L19mTNFα and TN11mTNFα inserted in the mammalian expression vector pCDNA3.1, under the control of the cytomegalovirus (CMV) promoter. (C) SDS-PAGE analysis of the purified fusion proteins L19mTNFα (lane 1) and TN11mTNFα (lane 2). As expected, a major band corresponding to the monomer of about 45 kDa was detected. Numbers on the right are the apparent molecular masses of the standard, in kilodaltons. (D) Gel filtration profile of the purified L19mTNFα and TN11mTNFα (Superdex 200). The retention volume (milliliter) of the peaks corresponds to an apparent molecular mass of about 140 kDa, consistent with the homotrimeric format of the 2 fusion proteins.
Expression, purification, and characterization of the L19mTNFα and TN11mTNFα fusion proteins
SP2/0 murine myeloma cells (American Tissue Type Culture Collection, ATCC, Rockville, MD) were transfected with the use of Fugene 6 Transfection Reagent (Roche Diagnostics) following the manufacturer's recommendations and selected in the presence of 500 μg/mL G418 (Calbiochem, San Diego, CA). The supernatants of the G418 resistant clones were screened for the production of the fusion proteins by using the enzyme-linked immunoabsorbent assay (ELISA) and the recombinant peptide composed of the type III homology repeats 7B8915 for L19, or the recombinant peptide Tenascin-C (TN-C)(A-D) for TN11.47 A rabbit anti-mTNFα polyclonal (PeproTech EC, London, England) was used as secondary antibody and a peroxidase-conjugated antirabbit immunoglobulin G (IgG) polyclonal (Pierce, Rockford, IL) as tertiary antibody. The biologic activity of TNFα was determined by using the cytotoxicity test on mouse L-M fibroblasts (ATCC), in the presence of 2 μg/mL actinomycin D (Sigma Chemical, St Louis, MO) described by Corti et al48 and 0.07 to 53 pM recombinant mouse TNFα (rmTNFα; 2 × 107 U/mg; kindly provided by Dr Corti, Dibit, Milano, Italy). Quadruplicates were done, and the results were expressed as the percentage of cell viability compared with the controls (cells treated with actinomycin D only). Immunoaffinity chromatography on ED-B15 and TN-C(A-D)47 conjugated to Sepharose 4B (Amersham Pharmacia Biotech, Uppsala, Sweden) was used to purify L19mTNFα and TN11mTNFα, respectively, from the conditioned media of the cells expressing the fusion proteins. The homotrimers (Figure 1A) of both fusion proteins were further purified by molecular exclusion chromatography (Superdex 200; Amersham Pharmacia Biotech) and subsequently analyzed under reducing conditions by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 4%-18% gradient) (Figure 1C) and in native conditions by fast-protein liquid chromatography on a Superdex 200 column (Figure 1D). The radius (nanometers) and the molecular weight (MW; kilodaltons) of the purified fusion proteins in phosphate-buffered saline (PBS; 20 mM NaH2PO4, 150 mM NaCl, pH 7.4) were determined at 25°C, using the DynaPro Molecular Sizing Instrument (ProteinSolutions, Lakewood, NJ).
Histologic analyses
Tissue samples were both paraffin embedded for histologic analysis performed as reported by Carnemolla et al22 and snap-frozen for immunohistochemistry. For immunocytochemistry, L-M murine fibroblasts were grown in Chamberslide (Nunc, Roskilde, Denmark) and then methanol fixed. Immunohistochemistry on 4-μm cryostat sections and immunocytochemistry were performed as reported by Carnemolla et al.22 The following primary antibodies were used: monoclonal rat antimouse Ki-67 (clone TEC-3; DAKO A/S, Glostrup, Denmark), monoclonal rat antimouse CD31 (clone 13.3; kindly supplied by A. Mantovani; Mario Negri Institute, Milan, Italy), and the fusion proteins L19mTNFα and TN11mTNFα. To reveal immunoreactivity the following antibodies were used: rabbit anti-mTNFα polyclonal (PeproTech) followed by biotinylated goat antimouse IgG polyclonal (BioSpa, Milan, Italy) and mouse antirat IgG2b and IgG1/2a (BD Biosciences, Heidelberg, Germany). Sections were then counterstained with hematoxylin and mounted permanently. Quantitative image analysis for the area of vital (non-necrotic) tumor tissue (tumor cell viability) and quantitative immunohistochemistry for blood vessel density were carried out by computer-aided image analysis by using the image processing and analysis system Quantimet 600 and Qwin software (Leica, Heidelberg, Germany), and the results were expressed as percentage of the whole measurement area, as reported by Carnemolla et al.22 For each animal group the mean value and SE of all measurement results were calculated. The tumor cell viability (percentage) was obtained by dividing the tumor cell viability after treatments by the tumor cell viability of the respective controls, considered 100%. The blood vessel density was assessed following the criteria of Weidner.49 Apoptotic cells were detected on dewaxed paraffin sections by using the TUNEL (terminal deoxynucleotidyl transferase mediated dUTP-fluorescein nick-end labeling) according to Gavriely et al,50 applying the In Situ Cell Death Detection Kit/AP (Roche). A negative control was included in each experimental setup by omitting the terminal deoxynucleotidyl transferase from the TUNEL reaction mixture.51
Animal tumor models
F9 mouse embryonal teratocarcinoma cells (ATCC), WEHI-164 mouse fibrosarcoma cells (ECACCC; Sigma Aldrich, Milan, Italy), and C51 mouse colon adenocarcinoma cells52 (kindly provided by Dr M. P. Colombo, Milan, Italy) were subcutaneously implanted (3 × 106) in immunocompetent syngeneic mice (129 strain for F9 and Balb/C for WHEI-164 and C51). All mice were 8 to 10 weeks old and purchased from Harlan UK (Oxon, United Kingdom).
All studies were performed when the tumors reached a volume of 0.3 to 0.4 cm3. The tumor volume was determined with the following formula: (d)2 × D × 0.52, where d and D are the short and long dimensions (centimeters) of the tumor, respectively, measured with a caliper.12 Housing, treatment, and killing of animals followed national legislative provisions (Italian law no. 116 of 27 January 1992) for the protection of animals used for scientific purposes.
Biodistribution experiments, microautoradiography, and blood clearance rate
Radioiodination of the fusion proteins was achieved as reported by Borsi et al.18 The radioactivity was established by using a RIASTAR γ-counter (Packard Instruments, Milan, Italy). After labeling, the Superdex 200 profile and the immunoreactivity test were performed.18 Nonspecific accumulation of 125Iodine in the stomach and concentration in thyroid was blocked as reported by Borsi et al.18 Tumor-bearing mice were injected in the tail vein with the radioiodinated fusion proteins in 100 μL PBS. Groups of 3 animals were killed at 3, 6, 24, and 48 hours after injection. The different organs, including tumor and blood, were taken and weighed, and the radioactivity was counted to determine the percentage of injected dose per gram (%ID/g). Tissue samples were then fixed with 5% formaldehyde in PBS, pH 7.4, and processed for microautoradiographies, according to Tarli et al.12 The blood clearance parameters of the radioiodinated antibodies were fitted with a least squares minimization procedure, using the Macintosh Program Kaleidagraph (Synergy Software, Reading PA) and the following equation: X(t) = Aexp(-(alpha t)) + Bexp(-(beta t)), where X(t) is the %ID/g of radiolabeled antibody at time t, as reported by Borsi et al.18 X0 was assumed to be equal to 40%, corresponding to a blood volume of 2.5 mL in each mouse.
In vivo treatments
For treatments, groups of 6 tumor-bearing mice (6-7 days after subcutaneous injection, when the tumors reached a volume of about 0.3-0.4 cm3) received injections in their tail vein of rmTNFα and the purified fusion proteins in 100 μL PBS. The control group received PBS only. Lyophilized melphalan (Alkeran; Glaxo Smith Kline, Research Triangle Park, NC) was reconstituted (5 mg/mL) in the solvent provided by the manufacturer immediately before use and, after further dilution in PBS, administered intraperitoneally (4.5 μg/g of mouse in 400 μL). The weight of the animals and the tumor size were recorded at 24-hour intervals before and after treatments. The doubling time of the tumor size, (T - C)200%, in the treated (T) versus the untreated (C) mice, was calculated as reported by Bosslet et al.53 Toxicity was evaluated on the basis of weight loss. We considered a severe toxicity to be a more than 10% weight loss within 48 hours after a single intravenous injected dose, and an acceptable toxicity to be a lower than 5% weight loss within 48 hours after a single intravenous injected dose. We found that the weight loss was 0 or lower than 5% up to 1.5 pmol/g, with no significant differences between the 2 fusion proteins and rmTNFα. In the therapeutic protocols with a single compound, 1 pmol/g L19mTNFα, TN11mTNFα, or mTNFα was used, whereas 0.7 pmol/g was used in therapies combined with other drugs.
Results
Expression, purification, and characterization of L19mTNFα and TN11mTNFα fusion proteins
For the preparation of L19mouse(m)TNFα, we used the cDNA of the scFv L1912 and, for the control fusion protein TN11mTNFα, the cDNA of the scFv TN11 (Figure 1A-B). TN11 is directed to the C repeat of human TN-C,47 which is not expressed in the tumor models used. As shown in Figure 1B, the cDNA corresponding to the open reading frame of mature murine TNFα was appended to the 3′ end of the cDNA of the scFvs by a linker of 45 bp. The cDNA constructs were cloned into the pcDNA3.1 mammalian expression vector used to transfect SP2/0 murine myeloma cells.
The 2 fusion proteins were purified from the conditioned media of the transfected cells by affinity chromatography (described in “Materials and methods”), yielding about 3 mg/L for each fusion protein. The homotrimers were further purified by gel filtration. The fusion proteins were then analyzed in SDS-PAGE (Figure 1C), in which they migrated as monomers of the expected size of about 45 kDa. The gel filtration profiles (Superdex 200) showed a single peak for both, with a retention volume corresponding to the apparent molecular mass of homotrimers of about 140 kDa (Figure 1D). By dynamic light scattering measurements we obtained a molecular radius of 4.819 nm for L19mTNFα, corresponding to a molecular mass of 133.5 kDa, and of 4.835 nm for TN11mTNFα, corresponding to a molecular mass of 134.4 kDa, confirming as expected that both fusion proteins were homotrimers.
Both fusion proteins were tested for immunoreactivity of the scFv moiety by immunohistochemistry and ELISA, and the same immunoreactivity of the original scFvs with the respective antigens was found. Both fusion proteins were also evaluated for the biologic activity of the mTNFα component by using a cytotoxic assay on L-M mouse fibroblasts. In this test we found that L19mTNFα showed a 5 to 6 times higher activity than equimolar amounts of recombinant mTNFα and of the fusion protein TN11mTNFα (Figure 2A). This enhanced activity was explained by the fact that mouse L-M cells express B-FN, which leads to a concentration of TNFα on the B-FN surrounding the cells, as demonstrated by immunocytochemistry using L19mTNFα as primary antibody and an antibody to mTNFα as secondary antibody (Figure 2Ba). When the binding of L19mTNFα to the endogenous B-FN (Figure 2Bb) was inhibited by adding a large excess (150 μg/mL) of L19scFv to L19mTNFα, the biologic activity of equimolar amounts of L19mTNFα, TN11mTNFα, and rmTNFα then became identical (Figure 2A).
Cytotoxic activity of L19mTNFα and TN11mTNFα (A) Cytotoxic activity on L-M mouse fibroblasts, expressed as a percentage of viability compared with controls (mean ± SD), of different concentrations (picomolar) of mTNFα, TN11mTNFα, and L19mTNFα without and with an excess of scFv L19 in the culture medium (150 μg/mL). (B) Immunocytochemistry (see “Materials and methods”) on L-M mouse fibroblasts using L19mTNFα (i) and TN11mTNFα (iii). Although no staining was visible by using TN11mTNFα (iii), a strong reactivity was found by using L19mTNFα (i) that, as shown in Bii, was completely abolished by an excess of scFv L19. Original magnification, × 440.
Cytotoxic activity of L19mTNFα and TN11mTNFα (A) Cytotoxic activity on L-M mouse fibroblasts, expressed as a percentage of viability compared with controls (mean ± SD), of different concentrations (picomolar) of mTNFα, TN11mTNFα, and L19mTNFα without and with an excess of scFv L19 in the culture medium (150 μg/mL). (B) Immunocytochemistry (see “Materials and methods”) on L-M mouse fibroblasts using L19mTNFα (i) and TN11mTNFα (iii). Although no staining was visible by using TN11mTNFα (iii), a strong reactivity was found by using L19mTNFα (i) that, as shown in Bii, was completely abolished by an excess of scFv L19. Original magnification, × 440.
Biodistribution experiments
For in vivo biodistribution experiments, L19mTNFα and TN11mTNFα were radioiodinated with I-125, as reported in “Materials and methods.” On gel filtration (Superdex 200) the profile of the radiolabeled fusion proteins was identical to that of unlabeled fusion proteins (Figure 1D), and the radioactivity recovery from the column was 100%, indicating that no molecular aggregation occurred.18
After radioiodination the immunoreactivity of L19mTNFα was always more than 90%, as established by using ED-B/Sepharose affinity chromatography.18 Both L19mTNFα and TN11mTNFα retained more than 90% of the cytotoxic activity of the starting materials.
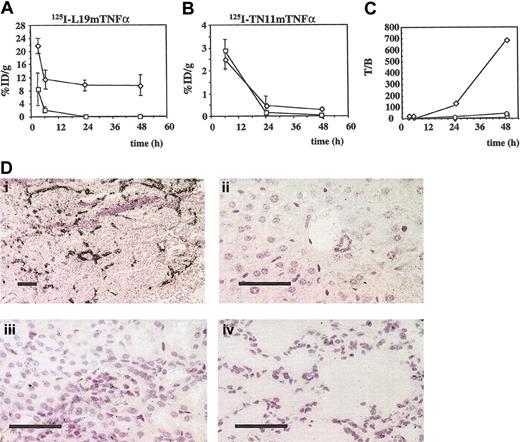
The radioiodinated fusion proteins were intravenously injected (0.125 μCi [0.004625 MBq], 1.0 pmol/g, in 100 μL PBS) in groups of F9 tumor-bearing mice; groups of 3 animals were killed at 3, 6, 24, and 48 hours after injection. The biodistribution results of the fusion proteins, expressed as the %ID/g of tissue, are summarized in Table 1 and compared with those of the dimeric scFv L19. Although 125I-L19mTNFα showed a very stable and high-level accumulation in tumors (Figure 3A), no accumulation of 125I-TN11mTNFα was detected at any time point of the experiment (Figure 3B; Table 1). Microautoradiographies of tumors and different organs showed specific and selective accumulation of L19mTNFα on tumor vasculature (Figure 3D), whereas no accumulation of TN11mTNFα was detectable in the tumor or in any other organs. Figure 3C shows the curves of the F9 tumor-to-blood ratios of the %ID/g after intravenous injection of 125I-L19mTNFα, 125I-TN11mTNFα, and 125I-L19(scFv)2. Forty-eight hours after intravenous injection of 125I-L19mTNFα, this ratio had a value of 700, nearly 14 times higher than the value obtained using the dimeric scFv L19.
Biodistribution experiments in F9 tumor-bearing mice by using radioiodinated L19mTNFα and TN11mTNFα (A) The %ID/g of tumor (⋄) and blood (□) at different times from intravenous injection of 125IL19mTNFα (mean ± SD). (B) The %ID/g of tumor (⋄) and blood (□) at different times after intravenous injection of 125I-TN11mTNFα (mean ± SD). (C) Tumor-to-blood ratio of the %ID/g after injection of 125I-L19mTNFα (⋄), 125I-TN11mTNFα (□) and 125I-L19 (scFv)2 (○). (D) Microautoradiographies of F9 tumor (i), liver (ii), kidney (iii), and lung (iv) 6 hours after intravenous injection of 125IL19mTNFα. L19mTNFα accumulates selectively in the F9 tumor vasculature. Bars = 200 μm (i), and 50 μm (ii-iv).
Biodistribution experiments in F9 tumor-bearing mice by using radioiodinated L19mTNFα and TN11mTNFα (A) The %ID/g of tumor (⋄) and blood (□) at different times from intravenous injection of 125IL19mTNFα (mean ± SD). (B) The %ID/g of tumor (⋄) and blood (□) at different times after intravenous injection of 125I-TN11mTNFα (mean ± SD). (C) Tumor-to-blood ratio of the %ID/g after injection of 125I-L19mTNFα (⋄), 125I-TN11mTNFα (□) and 125I-L19 (scFv)2 (○). (D) Microautoradiographies of F9 tumor (i), liver (ii), kidney (iii), and lung (iv) 6 hours after intravenous injection of 125IL19mTNFα. L19mTNFα accumulates selectively in the F9 tumor vasculature. Bars = 200 μm (i), and 50 μm (ii-iv).
Blood clearance of L19mTNFα was mediated mainly by way of the kidney, as determined by counting the urine samples, and showed a biphasic curve with an α and a β phase. Despite a molecular size of L19mTNFα of nearly 140 kDa, its α and β phase half-lives (T1/2α = 0.67 hour; T1/2β = 3.9 hours) were similar to those found for dimeric scFv L19 (T1/2α = 0.53 hour; T1/2β = 8 hours), which has a molecular mass of about 60 kDa, and much shorter than the complete L19IgG (T1/2α = 1.48 hours; T1/2β = 106.7 hours) which has a molecular mass of about 150 kDa.
Therapeutic effects of L19mTNFα
Groups of 3 F9 tumor-bearing mice were intravenously injected with 0.04, 0.25, and 1.0 pmol/g of the fusion proteins L19mTNFα and TN11mTNFα. The control group received PBS. The animals were killed 1 hour, 5 hours, and 24 hours after treatment. Healthy organs (lung, spleen, liver, kidney, intestine, and heart) were studied histologically to reveal side effects as a result of TNFα toxicity. With the exception of small foci of hemorrhages in the lung of all treated mice 5 hours and 24 hours after treatment, no morphologic side effects were found.
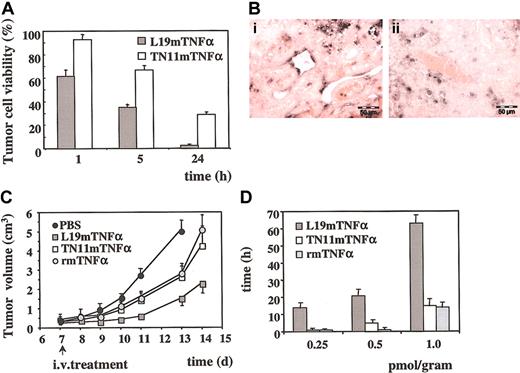
One hour from injection of 1.0 pmol/g, the tumor cell viability (described in “Materials and methods”) was 90% in the tumors treated with TN11mTNFα and 62% in the tumors treated with L19mTNFα. These values decreased after 24 hours to about 30% in the tumors treated with TN11mTNFα and to less than 6% in the tumors treated with L19mTNFα (Figure 4A). In the remaining vital tumor tissue, the vascular density and the cellular proliferation (Ki-67 index) were not affected. Twenty-four hours after L19mTNFα treatment, numerous apoptotic endothelial cells were demonstrated in the vessels of the vital tumor tissue (described in “Materials and methods”), whereas no apoptotic endothelial cells were detected in the vessels of the untreated control tumors (Figure 4B).
Effects of the systemic treatment of F9 tumor-bearing mice with L19mTNFα (A) Results of the quantitative analysis of F9 tumor cell viability at different times after intravenous injection of 1.0 pmol/g of mouse of L19mTNFα and TN11mTNFα, respectively. The resulting values are the average (± SE) of the percentage of tumor cell viability. (B) TUNEL analysis in the vital part of F9 tumors. (i) Twenty-four hours after treatment with L19mTNFα, numerous apoptotic endothelial cells are visible; (ii) no endothelial cell labeling of tumor vessels is found in the tumor of untreated mice in which only few apoptotic tumor cells are detected. Bars = 50 μm. (C) F9 tumor growth curves (mean ± SD) in mice treated with a single injection of PBS and 1.0 pmol/g TN11mTNFα, rmTNFα, and L19mTNFα, respectively. The number of days after subcutaneous inoculum of F9 cells is reported in the abscissa. An arrow indicates the day of the treatment. (D) Dose-dependent effect on tumor doubling time of a single intravenous injection with 0.25, 0.5, and 1.0 pmol/g L19mTNFα, TN11mTNFα, and rmTNFα. The doubling time results are expressed in hours (± SD).
Effects of the systemic treatment of F9 tumor-bearing mice with L19mTNFα (A) Results of the quantitative analysis of F9 tumor cell viability at different times after intravenous injection of 1.0 pmol/g of mouse of L19mTNFα and TN11mTNFα, respectively. The resulting values are the average (± SE) of the percentage of tumor cell viability. (B) TUNEL analysis in the vital part of F9 tumors. (i) Twenty-four hours after treatment with L19mTNFα, numerous apoptotic endothelial cells are visible; (ii) no endothelial cell labeling of tumor vessels is found in the tumor of untreated mice in which only few apoptotic tumor cells are detected. Bars = 50 μm. (C) F9 tumor growth curves (mean ± SD) in mice treated with a single injection of PBS and 1.0 pmol/g TN11mTNFα, rmTNFα, and L19mTNFα, respectively. The number of days after subcutaneous inoculum of F9 cells is reported in the abscissa. An arrow indicates the day of the treatment. (D) Dose-dependent effect on tumor doubling time of a single intravenous injection with 0.25, 0.5, and 1.0 pmol/g L19mTNFα, TN11mTNFα, and rmTNFα. The doubling time results are expressed in hours (± SD).
Figure 4C shows the F9 tumor growth curves in mice treated with intravenous injection of 1.0 pmol/g L19mTNFα, TN11mTNFα, and mTNFα. The animals were treated when the tumors were about 0.4 cm3. In the PBS-treated group of control mice, the tumor volume doubling time was about 20 hours, whereas the tumor volume of the animals treated with L19mTNFα remained stable for about 4 days, and then the growth curve presented a slope similar to that of the tumors of untreated animals. This finding is consistent with the results shown in Figure 4A, showing that a single injection of 1 pmol/g L19mTNFα reduces the vital part of the tumor to about 5%. In fact, considering that this 5% of the vital tumors still conserves a doubling time of 20 hours, it should reach the original volume in about 4 days, after which time it resumes the growth curve of the untreated controls.
Tumor growth rate in the 3 groups of treated mice was evaluated by calculating the tumor doubling time53 with respect to the PBS-treated controls. The results, depicted in Figure 4D, clearly show no significant differences between TN11mTNFα and rmTNFα, whereas L19mTNFα was at least 4 times more active. In fact, a 4-fold higher dose of both rmTNFα and TN11mTNFα was necessary to achieve a response similar to that of L19mTNFα. No significant weight loss was observed in the treated animals.
Therapeutic effects of L19mTNFα in combination with L19-IL2
L19-IL2 is a recently reported fusion protein composed of the scFv L19 and interleukin 2.22 Because of the targeting ability of L19, L19-IL2 concentrates IL2 in tumors and, therefore, enhances the cytokine's therapeutic index.22
We performed biodistribution studies in F9 tumor-bearing mice by using 0.5 μg/g radioiodinated L19-IL2, administered intravenously with and without 0.7 pmol/g unlabeled L19mTNFα or unlabeled TN11mTNFα. No specific accumulation was found in healthy organs at any time when 125I-L19-IL2 was injected alone or in combination with unlabeled L19mTNFα or TN11mTNFα (data not shown). 125I-L19-IL2 accumulation in F9 tumors was significantly more persistent and at high levels when it was co-injected with L19mTNFα (about 12%ID/g between 3 and 48 hours) than when given alone or in combination with TN11mTNFα (Figure 5A). This persistently high accumulation in tumors accounted for the tumor-to-blood ratio of the %ID/g of 250 at 48 hours when 125I-L19-IL2 was co-injected with unlabeled L19mTNFα, whereas, after the same time, it was less than 50 when 125I-L19-IL2 was injected alone or with unlabeled TN11mTNFα (Figure 5C).
Effects of L19mTNFα on biodistribution of 125I-L19-IL2 in tumor-bearing mice. The %ID/g (mean ± SD) of tumor (A) and blood (B) and tumor-to-blood ratios of the %ID/g (C) at different times after intravenous injection of 0.5 μg/g 125I-L19-IL2 alone (⋄) and in combination with 0.7 pmol/g unlabeled L19mTNFα (♦) or unlabeled TN11mTNFα (▵). (D) Effects on tumor growth of the treatments with L19mTNFα combined to L19-IL2. F9 tumor growth curves after 2 intravenous (IV) injections with PBS, 0.7 pmol/g L19mTNFα, 1.0 μg/g L19-IL2, and with the combinations of 1.0 μg/g L19-IL2 and 0.7 pmol/g L19mTNFα or TN11mTNFα. F9 tumor volume is expressed in cm3 (mean ± SD). The intravenous injections were carried out at the times indicated by arrows.
Effects of L19mTNFα on biodistribution of 125I-L19-IL2 in tumor-bearing mice. The %ID/g (mean ± SD) of tumor (A) and blood (B) and tumor-to-blood ratios of the %ID/g (C) at different times after intravenous injection of 0.5 μg/g 125I-L19-IL2 alone (⋄) and in combination with 0.7 pmol/g unlabeled L19mTNFα (♦) or unlabeled TN11mTNFα (▵). (D) Effects on tumor growth of the treatments with L19mTNFα combined to L19-IL2. F9 tumor growth curves after 2 intravenous (IV) injections with PBS, 0.7 pmol/g L19mTNFα, 1.0 μg/g L19-IL2, and with the combinations of 1.0 μg/g L19-IL2 and 0.7 pmol/g L19mTNFα or TN11mTNFα. F9 tumor volume is expressed in cm3 (mean ± SD). The intravenous injections were carried out at the times indicated by arrows.
The effects of a combined systemic therapy using L19mTNFα and L19-IL2 are shown in Figure 5D. Groups of 6 F9 tumor-bearing mice received intravenous treatments at day 7 and 10 after subcutaneous inoculation of the F9 tumor cells, when the tumor was about 0.3 cm3. The treatments entailed intravenous injections of 1 μg/g L19-IL2 combined with 0.7 pmol/g L19mTNFα or TN11mTNFα. The animals' weight loss was always less than 5%. As depicted in Figure 5D, the tumors in animals treated with the combination L19-IL2 and L19mTNFα grew at a much slower rate compared with the tumors in the other groups of mice. In fact, tumors in mice receiving the 2 L19 fusion proteins showed no increase in size up to 15 days after tumor grafting.
Therapeutic effects of L19mTNFα in combination with melphalan
Melphalan is an alkylating compound widely used in combination with TNFα in the isolated limb perfusion treatment of melanomas and soft tissue sarcomas.33,38,39,54 The aim of the experiment was to establish whether a higher therapeutic synergy than that already described could be achieved by substituting TNFα with L19mTNFα.
Groups of 6 F9 tumor-bearing mice were given a single intravenous injection of 0.7 pmol/g L19mTNFα, of the control fusion protein TN11mTNFα, and of rmTNFα on day 6 after tumor grafting, followed by intraperitoneal injection of 4.5 μg/g melphalan 24 hours later.
The tumor growth curves of animals treated with PBS, PBS and melphalan, rmTNFα and melphalan, TN11mTNFα and melphalan, and L19mTNFα and melphalan are depicted in Figure 6A. All the treatments, including melphalan alone, reduced the tumoral mass within 2 to 3 days. However, although treatment with melphalan alone or in combination with rmTNFα or TN11mTNFα induced tumor quiescence up to day 16 to 18 after tumor cell grafting and thereafter produced a growth slope similar to that seen in the PBS-treated control group, the tumors of mice treated with melphalan and L19mTNFα were quiescent up to 25 to 26 days after tumor cell grafting. Similar results were obtained by using different tumor models such as WHEI-164 fibrosarcoma (Figure 6B) and C51 colon adenocarcinoma (data not shown).
Effects on tumor growth of the treatments with L19mTNFα combined with melphalan. (A) F9 tumor growth curves after a single intravenous injection of PBS, 0.7 pmol/g L19mTNFα, TN11mTNFα, and rmTNFα, followed, after 24 hours, by melphalan 4.5 μg/g given intraperitoneally. The growth curve of animals treated with PBS only is also reported. F9 tumor volume is expressed in cm3 (mean ± SD). The treatments were performed at the times indicated by arrows. (B) WHEI-164 tumor growth curves after a single intravenous (IV) injection of PBS, 0.7 pmol/g L19mTNFα, and rmTNFα, followed, after 24 hours, by melphalan 4.5 μg/g given intraperitoneally (IP). The growth curve of animals treated with PBS only is also reported. WHEI-164 tumor volume is expressed in cm3 (mean ± SD). The treatments were performed at the times indicated by arrows.
Effects on tumor growth of the treatments with L19mTNFα combined with melphalan. (A) F9 tumor growth curves after a single intravenous injection of PBS, 0.7 pmol/g L19mTNFα, TN11mTNFα, and rmTNFα, followed, after 24 hours, by melphalan 4.5 μg/g given intraperitoneally. The growth curve of animals treated with PBS only is also reported. F9 tumor volume is expressed in cm3 (mean ± SD). The treatments were performed at the times indicated by arrows. (B) WHEI-164 tumor growth curves after a single intravenous (IV) injection of PBS, 0.7 pmol/g L19mTNFα, and rmTNFα, followed, after 24 hours, by melphalan 4.5 μg/g given intraperitoneally (IP). The growth curve of animals treated with PBS only is also reported. WHEI-164 tumor volume is expressed in cm3 (mean ± SD). The treatments were performed at the times indicated by arrows.
Discussion
TNFα is one of the most potent antitumor cytokines known. Therapeutically effective doses of TNFα cannot be given systemically, however, because of its unacceptably toxic side effects. As a result, the clinical use of TNFα has until now been limited to locoregional applications. In particular, the use of TNFα in ILP was authorized in combination with melphalan by the European Agency for the Evaluation of Medicinal Products (EMEA) for the treatment of nonresectable, high-grade sarcomas,39,54 and the American College of Physicians approved a phase 3 clinical trial using melphalan with or without TNFα for the ILP of melanomas.38,55 TNFα is also being evaluated for the therapy of nonresectable liver tumors by isolated hepatic perfusion.56
The selective targeted delivery of TNFα to tumors seems to provide an appealing strategy that could ultimately lead to its systemic use, because it would achieve the purpose of selectively concentrating the cytokine to elicit a significant antitumoral activity in primary and disseminated tumors while limiting systemic toxicity. The effective targeting of tumors, however, has 2 main requisites: (1) a target in the tumor that is specific, abundant, stable, and readily available for ligand molecules coming from the bloodstream; and (2) a ligand molecule with suitable pharmacokinetic properties to allow its diffusion from the bloodstream to the tumor and with a high affinity for the target to ensure its efficient and selective accumulation in the tumor.
We have chosen to selectively deliver therapeutic agents to tumor ECM components, which are generally more abundant and stable with respect to tumor-associated cell surface antigens.1 The tumor microenvironment can be considered a possible pantumoral target; in fact, tumor progression induces (and subsequently needs) significant modifications in tumor microenvironment components, particularly those of the tumor ECM, that differ both quantitatively and qualitatively from those of the healthy ECM. Many of these tumor ECM components are shared by all solid tumors, accounting for general properties and functions such as cell invasion (both healthy cells into tumor tissues and cancer cells into healthy tissues) and angiogenesis.
Of the numerous molecules constituting the modified tumor ECM, we focused our attention on B-FN. B-FN is widely expressed in the ECM of all solid tumors thus far tested and is constantly associated with angiogenic processes,10,11 but it is otherwise undetectable in healthy adult tissues.5 These features make B-FN a potentially ideal tumor target, also because the targeted delivery of therapeutic agents to the subendothelial ECM overcomes problems associated with the interstitial hypertension of solid tumors.57
Today, some of the most promising ligands are the human antibody molecules that can be generated and customized by using molecular engineering technologies. We have produced L19,12-14 an scFv with a high affinity (Kd = 5.4 × 10-11 M) for the ED-B domain of FN, and demonstrated in vivo that it selectively and efficiently accumulates around tumor neovasculature and is able to selectively transport to and concentrate in the tumor mass any one of a number of therapeutic molecules to which it is conjugated.20-23 The ability of L19 to selectively target tumors has also been demonstrated in patients using scintigraphic techniques.19
Here we report the preparation, characterization, and preclinical therapeutic evaluation of the fusion protein L19mTNFα. We expressed this fusion protein in mammalian cells and purified it as an immunoreactive and biologically active homotrimer. Already in in vitro assays using L-M mouse fibroblasts we observed an increased toxic activity of L19mTNFα compared with rmTNFα and with the control fusion protein TN11mTNFα, because of the selective binding of L19mTNFα to B-FN present in the ECM of cultured L-M cells (Figure 2). Inhibition of L19mTNFα binding to B-FN present in the ECM of the cultured cells by competition with scFv L19 offset the higher toxicity of L19mTNFα (Figure 2). This experiment demonstrates that TNFα, in particular, and cytokines, in general, can exert their biologic activity if delivered directly to the ECM. Cells, both in vitro and in vivo, constitute a dynamic system in which they migrate along ECM structures that, in our case, are armed with TNFα; as such, cell receptors come into contact with the cytokine, which is thus able to exercise its biologic activity. In particular, it is known that TNFα is toxic for tumor endothelial cells and that, within a tumor, endothelial cells migrate along ECM structures containing B-FN.12 Thus, delivery of TNFα to the tumor ECM creates a sort of “minefield” that should prove lethal for invading endothelial cells, thus leading to antitumoral effects.
The results obtained in biodistribution experiments with the radiolabeled L19mTNFα in the syngeneic murine tumor model F9 showed that this fusion protein accumulates more persistently and at higher levels in the tumor than scFv L19 (more than 10% of the ID/g, 48 hours after injection of the radiolabeled fusion protein) (Table 1; Figure 3). Moreover, despite its molecular mass of nearly 140kDa, L19mTNFα shows a much faster blood clearance rate than molecules of similar size, such as the complete L19IgG (described in “Results”). Similar observations were reported by Gillies et al43 and Rosenblum et al,58 who found that when fused or conjugated to TNFα, a monoclonal antibody presented a more rapid α phase of clearance than the monoclonal antibody alone, very likely as a result of microvascular leakage59 and to reduced interstitial tumor pressure34 induced by TNFα.
In our syngeneic murine tumor models the antibody-mediated delivery of TNFα to the subendothelial ECM clearly enhanced the therapeutic performance of TNFα. F9 tumor-bearing mice, 24 hours after intravenous treatment with a single dose of L19mTNFα, showed a remarkable tumor necrosis (about 95% of the total tumor tissue, Figure 4A). Tumor necrosis was accompanied by thrombosis, vascular ectasia, and hemorrhaging, features that are produced by the activity of TNFα on the angiogenic endothelial cells as clearly demonstrated in earlier studies.26-33 Furthermore, we observed that in numerous vessels of the vital part of the tumor, many endothelial cells undergo apoptosis that was not detected in the vessels of the control tumors (Figure 4B). Induction of tumor necrosis as well as inhibition of tumor growth by L19mTNFα was at least 4 times higher compared with both the control fusion protein TN11mTNFα and rmTNFα. Because we used a human (L19)-mouse (TNFα) chimeric fusion protein, repeated intravenous injections induced production of large amounts of mouse antibodies to the human part of the fusion protein (L19) that correlated with a reduced antitumor activity of the fusion protein (data not shown). Studies to demonstrate the neutralizing activity of these antibodies are under way.
The most dramatic therapeutic results were achieved by treating tumor-bearing mice with L19mTNFα in combination with melphalan or L19-IL2.We recently reported on the enhancement of the antitumor properties of IL2 when delivered in the format of fusion protein L19-IL2.22 L19-IL2 administered in combination with L19mTNFα accumulates more persistently within the tumor than does L19-IL2 alone or in combination with TN11mTNFα (Figure 5A-C). Such higher and more persistent accumulations ultimately lead to enhanced therapeutic performance (Figure 5D).
The synergistic antitumor effect of TNFα and melphalan38,39 derives from the effects on tumor vasculature of TNFα, which reduces tumor interstitial pressure and increases34 vascular permeability,36 ultimately leading to a higher melphalan accumulation.35 Synergy between L19mTNFα and melphalan was dramatically higher than that between TN11mTNFα or rmTNFα and melphalan (Figure 6A-B), thus demonstrating that the therapeutic effects depend on TNFα concentration in the tumor.
In conclusion, we have shown that the L19mTNFα fusion protein is a potent bioactive molecule. Because B-FN is a naturally occurring marker of angiogenesis and of tissue remodeling, antigenically identical in mouse and human and present in similar amounts in human tumors and in murine tumor models,22 L19mTNFα alone or in combination with different antineoplastic drugs may open new perspectives for the systemic use of TNFα for anticancer therapy.
Prepublished online as Blood First Edition Paper, August 21, 2003; DOI 10.1182/blood-2003-04-1039.
Supported partially by the Associazione Italiana per la Ricerca sul Cancro (AIRC), the European Commission, the Italian Health Ministry, the Swiss National Science Foundation, and the Bundesamt für Bildung und Wissenschaft.
Two authors (L.Z. and D.N.) have declared a financial interest in a company (Philogen srl) whose potential product was studied in the present work. One of the authors (A. Birò) is employed by a company (Philogen srl) whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Angelo Corti (Dibit, Milano, Italy) for recombinant mouse TNFα and for useful discussions and Mr Thomas Wiley for manuscript revision.