Abstract
A20 binding inhibitor of NF-κB activation-2, ABIN-2, is a newly identified intracellular protein that interacts with the zinc finger protein A20. ABIN-2 inhibits nuclear factor-κB (NF-κB) activity and is a possible effector of A20 regulation of NF-κB. Although A20 is a potent inhibitor of endothelial apoptosis, the effect of ABIN-2 on apoptosis is not known. ABIN-2 also interacts with the endothelial receptor Tie2. This receptor is essential for blood vessel formation and promotes endothelial survival. Here we examine the effects of ABIN-2 on endothelial cell apoptosis and its potential involvement in Tie2-mediated endothelial survival. ABIN-2 was found to inhibit endothelial apoptosis and rescue cells from death following growth factor deprivation. The inhibitors of phosphatidylinositol-3 kinase, wortmannin and LY294002, suppressed ABIN-2 inhibition of endothelial cell death. Deletion of the carboxy-terminus of ABIN-2 removed its ability to inhibit apoptosis. Expression of truncated ABIN-2 prevented the Tie2-activating ligand angiopoietin-1 from inhibiting endothelial cell death. (Blood. 2003;102:4407-4409)
Introduction
The receptor tyrosine kinase Tie2 is expressed primarily in endothelial cells and is essential for blood vessel formation.1 A family of ligands, the angiopoietins, has been identified for Tie2. Angiopoietin-1 (Ang1) and angiopoietin-2 (Ang2) are the best-characterized members of this ligand family. Ang1 activates Tie2 whereas Ang2 can antagonize this effect.2,3 Activation of Tie2 stimulates endothelial cell migration and sprouting,4-6 and the receptor is required for correct organization and integrity of the vascular system during development.7 In addition, Ang1 stimulation of Tie2 inhibits vascular leakage in the adult and suppresses vascular inflammation.8,9 These effects point to an important vascular protective activity of the Ang1/Tie2 system. In accord with this, angiopoietin stimulation of Tie2 also inhibits endothelial cell apoptosis in response to growth factor deprivation, irradiation, and mannitol treatment.10-12
Recently Tie2 was found to interact with the protein A20 binding inhibitor of NF-κB activation-2 (ABIN-2) in a ligand-dependent manner.13 As the name suggests, ABIN-2 is an inhibitor of nuclear factor-κB 14 (NF-κB), and it appears to have a role in Ang1 suppression of NF-κB activity.13 As well as regulating inflammatory gene expression, NF-κB has antiapoptotic activity in many cell types. In endothelial cells NF-κB activity is necessary for prevention of apoptosis induced by tumor necrosis factor-α (TNF-α) and growth factor deprivation.15,16 Although ABIN-2 inhibits NF-κB, its effects on apoptosis are not known and its involvement in Tie2-mediated endothelial survival is unexplored.17
ABIN-2 and the related protein ABIN-1 were originally discovered as binding partners for the inducible protein A20. This zinc finger protein is a potent inhibitor of NF-κB activity with a key role in limiting the extent and duration of inflammatory activation.18 ABIN-2 has been postulated as one of the mediators of the inhibitory effect of A20 on NF-κB.18 A20 inhibits apoptosis in endothelial cells and some other cell types. Expression of A20 inhibits apoptosis induced by growth factor deprivation of human umbilical vein endothelial cells (HUVECs)19 and by TNF-α in HUVECs20-22 and lipopolysaccharide in human microvascular endothelial cells.23 It is possible, therefore, that ABIN-2, like A20, could both inhibit NF-κB and suppress cell death. Such activity would make ABIN-2 an attractive target for promoting cytoprotection of the endothelium. In the present study we examine the effects of ABIN-2 on apoptosis of endothelial cells and its potential involvement in Tie2-mediated endothelial survival.
Study design
The expression vector encoding green fluorescent protein (GFP) was obtained from BD Biosciences Clontech (Palo Alto, CA). Isolation and culture of HUVECs, preparation of the recombinant version of Ang1, Ang1*, and the generation of cDNA encoding human ABIN-2 were as described previously.13 Endothelial cells were transfected using Targefect F2 transfection reagent (Targeting Systems, Santee, CA). For examination of endothelial apoptosis and survival, HUVECs were grown to 80% to 90% confluence on gridded tissue culture dishes and transfected with control or test plasmids together with GFP. Twenty-four hours after transfection, cells were washed and incubated in growth factor- and serum-free medium for 18 hours before analysis of the transfected cells—assessed by expression of GFP—for apoptotic index or cell survival. Apoptotic index was determined essentially as described by Mejillano et al.24 Briefly, after growth factor deprivation, media were removed and cells fixed for 10 minutes at room temperature in 4% formaldehyde in phosphate-buffered saline (PBS). Nuclei were stained with 4,6 diamidino-2-phenylindole (DAPI) at 0.1 μg/mL in 10 mM Tris (tris(hydroxymethyl)aminomethane) (pH 7), 10 mM EDTA (ethylenediaminetetraacetic acid), and 100 mM NaCl for 5 minutes at room temperature. The fraction of GFP-expressing cells with condensed and fragmented nuclei was determined by fluorescence microscopy. For cell survival assays the numbers of viable transfected HUVECs were counted at the end of the serum-free incubation period and calculated as percentage of starting cell number in the same 5 grids in each dish. Cell viability was routinely determined by exclusion of 0.2% trypan blue. Cleaved active caspase-3, phospho-Akt, and total Akt were detected in lysates from adherent and floating cells by Western blotting with antibodies from Cell Signaling Technology (Beverly, MA). Preparation of cell lysates and Western blotting were performed as described previously.13
Results and discussion
Although A20 has been shown to inhibit cell death in a number of cell types, including growth factor-deprived endothelial cells, the effects of the A20 binding protein and putative NF-κB effector ABIN-2 on apoptosis have not been explored. To examine the potential antiapoptotic activity of ABIN-2, HUVECs were transfected with expression plasmids encoding GFP and control plasmid or with GFP and ABIN-2. Twenty-four hours after transfection, endothelial cells were growth factor-deprived for 18 hours before assessment of apoptotic index in transfected cells (Figure 1). As previously documented,10,16 growth factor deprivation of HUVECs induces substantial apoptotic cell death. However, cells expressing ABIN-2 exhibited a significant 2-fold decrease in apoptotic index (Figure 1B). Consistent with this, the cleaved 17 and 19 kDa active forms of caspase-3 were present in growth factor-deprived HUVECs and decreased by expression of ABIN-2 (Figure 1C). As might be expected from its effects on apoptotic index, expression of ABIN-2 also caused a significant increase in percentage survival of growth factor-deprived endothelial cells (Figure 1C). The protein kinases phosphatidylinositol-3 kinase (PI-3K) and Akt have central roles in protecting endothelial and other cells from death.25 Expression of ABIN-2 increased the activated phospho-Ser473 form of Akt (Figure 1E), and the PI-3K inhibitors wortmannin and LY294002 inhibited ABIN-2-induced endothelial survival (Figure 1F), suggesting a role for the PI-3K/Akt pathway in the prosurvival activity of ABIN-2.
ABIN-2 inhibits endothelial cell death. HUVECs were cotransfected with GFP and control vector (CV) or with GFP and vector encoding human ABIN-2. Twenty-four hours after transfection, cells were subject to growth-factor deprivation for 18 hours. (A) Representative fluorescence photomicrographs showing nuclear morphology of GFP-expressing cells cotransfected with CV (upper panel) or ABIN-2 (lower panel). An example of an apoptotic (arrow) and nonapoptotic (arrowhead) transfected cell is indicated. Original magnification, × 200. (B) Apoptotic index of cells was determined as described in “Study design” for HUVECs transfected with CV or ABIN-2 as indicated. Data are presented as means and SEM for 3 independent experiments, *P < .01 versus CV. (C) Activated p17/19 caspase-3 was detected by Western blotting in HUVECs transfected with CV or ABIN-2 as indicated. Similar protein loading was confirmed by probing for β-actin and the presence of expressed ABIN-2 confirmed by probing for the FLAG-epitope tag present on the N-terminus of expressed ABIN-2. (D) Survival of cells was determined as described in “Study design” for HUVECs transfected with CV or ABIN-2 as indicated. Data are presented as means and SEM for 5 independent experiments, *P < .01 versus CV. (E) Phospho-Ser473 Akt and total Akt were detected by Western blotting in HUVECs transfected with CV or ABIN-2 as indicated. The presence of expressed ABIN-2 confirmed by probing for the FLAG-epitope tag. (F) Survival of HUVEC-transfected CV or ABIN-2 and incubated with control vehicle, 30 nM wortmannin, or 10 μm LY294002 as indicated during growth factor deprivation. Data are presented as means and SEM for at least 3 independent experiments. *P < .01 versus CV, control.
ABIN-2 inhibits endothelial cell death. HUVECs were cotransfected with GFP and control vector (CV) or with GFP and vector encoding human ABIN-2. Twenty-four hours after transfection, cells were subject to growth-factor deprivation for 18 hours. (A) Representative fluorescence photomicrographs showing nuclear morphology of GFP-expressing cells cotransfected with CV (upper panel) or ABIN-2 (lower panel). An example of an apoptotic (arrow) and nonapoptotic (arrowhead) transfected cell is indicated. Original magnification, × 200. (B) Apoptotic index of cells was determined as described in “Study design” for HUVECs transfected with CV or ABIN-2 as indicated. Data are presented as means and SEM for 3 independent experiments, *P < .01 versus CV. (C) Activated p17/19 caspase-3 was detected by Western blotting in HUVECs transfected with CV or ABIN-2 as indicated. Similar protein loading was confirmed by probing for β-actin and the presence of expressed ABIN-2 confirmed by probing for the FLAG-epitope tag present on the N-terminus of expressed ABIN-2. (D) Survival of cells was determined as described in “Study design” for HUVECs transfected with CV or ABIN-2 as indicated. Data are presented as means and SEM for 5 independent experiments, *P < .01 versus CV. (E) Phospho-Ser473 Akt and total Akt were detected by Western blotting in HUVECs transfected with CV or ABIN-2 as indicated. The presence of expressed ABIN-2 confirmed by probing for the FLAG-epitope tag. (F) Survival of HUVEC-transfected CV or ABIN-2 and incubated with control vehicle, 30 nM wortmannin, or 10 μm LY294002 as indicated during growth factor deprivation. Data are presented as means and SEM for at least 3 independent experiments. *P < .01 versus CV, control.
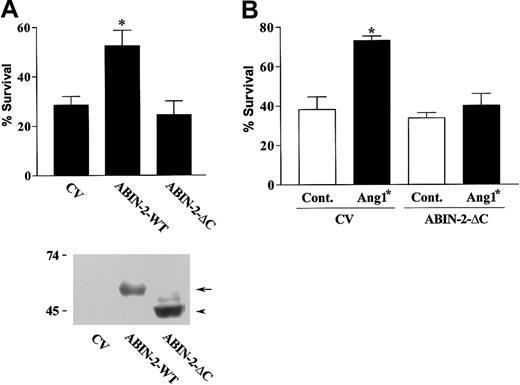
Deletion of the carboxy-terminus of ABIN-2 prevents it from inhibiting stimulated NF-κB activity when expressed in human embryonic kidney cells.14 We therefore examined whether deletion of the carboxy-terminal 85 amino acid residues affects the ability of ABIN-2 to prevent endothelial cell death. This deleted form of ABIN-2 was expressed in endothelial cells and its ability to promote survival examined as before. The ability of expressed ABIN-2 to rescue endothelial cells from death was lost on removal of the carboxy-terminal sequence of the protein (Figure 2A).
Expression of truncated ABIN-2 inhibits Ang1*-stimulated endothelial survival. (A) HUVECs were transfected with GFP and control vector (CV) or with vector encoding wild-type ABIN-2 (ABIN-2-WT) or ABIN-2 lacking 85 amino acids of the carboxy-terminus (ABIN-2-ΔC), as indicated. Cell survival of transfected cells was determined as described in “Study design.” The lower part of panel A depicts a Western blot showing expression of wild-type ABIN-2 (ABIN-2-WT) (arrow) and ABIN-2-ΔC (arrowhead) in HUVECs transfected with CV, ABIN-2-WT, or ABIN-2-ΔC as indicated. The presence of expressed forms of ABIN-2 was detected by probing for the N-terminal FLAG-epitope tag. Relative mobility of molecular mass markers is shown in kilodaltons. (B) HUVECs were transfected with GFP and CV or truncated ABIN-2 as indicated. Twenty-four hours after transfection, cells were subjected to growth factor withdrawal in the absence or presence of 400 ng/mL Ang1* and cell survival determined at 18 hours as detailed in “Study design.” Data are presented as means and SEM for 5 independent experiments (A) or 3 independent experiments (B). *P < .01 vs CV.
Expression of truncated ABIN-2 inhibits Ang1*-stimulated endothelial survival. (A) HUVECs were transfected with GFP and control vector (CV) or with vector encoding wild-type ABIN-2 (ABIN-2-WT) or ABIN-2 lacking 85 amino acids of the carboxy-terminus (ABIN-2-ΔC), as indicated. Cell survival of transfected cells was determined as described in “Study design.” The lower part of panel A depicts a Western blot showing expression of wild-type ABIN-2 (ABIN-2-WT) (arrow) and ABIN-2-ΔC (arrowhead) in HUVECs transfected with CV, ABIN-2-WT, or ABIN-2-ΔC as indicated. The presence of expressed forms of ABIN-2 was detected by probing for the N-terminal FLAG-epitope tag. Relative mobility of molecular mass markers is shown in kilodaltons. (B) HUVECs were transfected with GFP and CV or truncated ABIN-2 as indicated. Twenty-four hours after transfection, cells were subjected to growth factor withdrawal in the absence or presence of 400 ng/mL Ang1* and cell survival determined at 18 hours as detailed in “Study design.” Data are presented as means and SEM for 5 independent experiments (A) or 3 independent experiments (B). *P < .01 vs CV.
ABIN-2 has recently been shown to interact with the endothelial receptor tyrosine kinase Tie2.13 Activated Tie2 inhibits endothelial cell death.10-12 We were interested, therefore, to determine whether ABIN-2 has a role in the antiapoptotic activity of Tie2. To examine this we utilized the carboxy-terminal-deleted form of ABIN-2 as a putative dominant negative. The ability of the Tie2 agonist Ang1* to promote survival of growth factor-deprived endothelial cells was examined in cells expressing control vector or deleted ABIN-2. Ang1* caused a significant increase in survival of growth factor-deprived endothelial cells expressing control vector (Figure 2B). In contrast, expression of the truncated form of ABIN-2 interfered with the ability of Ang1* to promote endothelial survival consistent with ABIN-2 involvement in the prosurvival activity of Tie2.
The PI-3K/Akt pathway has been shown to have an essential role in Tie2-mediated endothelial survival.10-12 The current findings support a role also for ABIN-2 in the prosurvival activity of Tie2. The exact relationship between the PI-3K/Akt pathway and ABIN-2 in inhibition of endothelial apoptosis will require definition of the mechanism by which ABIN-2 promotes survival of endothelial cells.
In summary, these data demonstrate for the first time that ABIN-2 has antiapoptotic activity in endothelial cells and that this protein is involved in Ang1 inhibition of endothelial apoptosis. This prosurvival activity, together with its known antiinflammatory effects, suggests ABIN-2 may be an important vascular protective protein.
Prepublished online as Blood First Edition Paper, August 21, 2003; DOI 10.1182/blood-2003-05-1602.
Supported by the Wellcome Trust (project grant 062645).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.