Abstract
The ability of viral or mutated cellular oncogenes to initiate neoplastic events and their poor immunogenicity have considerably undermined their potential use as immunotherapeutic tools for the treatment of human cancers. Using an Epstein-Barr virus-encoded oncogene, latent membrane protein 1 (LMP1), as a model, we report a novel strategy that both deactivates cellular signaling pathways associated with the oncogenic phenotype and reverses poor immunogenicity. We show that cotranslational ubiquitination combined with N-end rule targeting of LMP1 enhanced the intracellular degradation of LMP1 and total blockade of LMP1-mediated nuclear factor-κB (NF-κB) and signal transducer and activator of transcription (STAT) activation in human cells. In addition, although murine cells expressing LMP1 were uniformly tumorigenic, this oncogenicity was completely abrogated by covalent linkage of LMP1 with ubiquitin, while an enhanced CD8+ T cell response to a model epitope fused to the C-terminus of LMP1 was observed following immunization with ubiquitinated LMP1. These observations suggest that proteasomal targeting of tumor-associated oncogenes could be exploited therapeutically by either gene therapy or vaccination. (Blood. 2003;102:4535-4540)
Introduction
A major defense against many types of tumors resides in cytotoxic T-lymphocyte (CTL)-mediated immunity. To evoke a normal CTL response directed against tumors is often only partially effective and does not always prevent the eventual development of malignancy. To significantly enhance this natural response, a range of vaccination regimes have been proposed,1-4 all of which are aimed at the prevention and treatment of cancer through stimulation of the CTL response directed against tumor-associated antigens. Although it is likely that the most effective CTL response will be elicited by use of a complete viral or cellular oncogene, such an approach carries with it the potential to independently induce malignancies. To address this risk, several groups have suggested the use of partial sequences derived from oncogenic proteins, specifically the major immunogenic T-cell epitopes, either individually or in a linear, polyepitope fashion.5,6 Although in many cases, these studies have demonstrated induction of protective T-cell-mediated antitumor immunity, they require selection of specific epitopes based on knowledge of both their inherent immunogenicity and their functional importance in eliciting a protective response.
There are many potential cancer targets that researchers are using to try out novel immunotherapeutic treatments. Some examples are the unaltered self-protein melanoma antigen genes (MAGE) and B melanoma antigen genes (BAGE) expressed in human melanoma tumors,7,8 the cell cycle-related protein p53,9,10 and virally encoded proteins (eg, human papilloma virus 16 [HPV16]-encoded early region 6 [E6] and E7,11,12 or the Epstein-Barr virus [EBV]-encoded latent membrane protein 1 [LMP1]).13,14 Using the melanoma antigens in a vaccine carries a potential risk, as the consequences of overexpression of such genes remain unknown. Likewise, recombinant vaccines for cervical carcinoma are in themselves hazardous if such vaccines contain the functional HPV16 E6 and E7 oncogenes.11,12 In this study, we demonstrate an alternative approach to subvert the cell's natural catabolic and immunopresentation processes based on cotranslational ubiquitination of an EBV-encoded oncogene (LMP1) in conjunction with N-end rule targeting. This strategy not only increased the intracellular degradation rate of LMP1, but also resulted in complete inactivation of LMP1-mediated cellular signaling and oncogenic potential while at the same time it enhanced immunogenicity of a model epitope fused to this subdominant antigen.
Materials and methods
Construction of ubiquitin-LMP1 chimeras
To generate noncleavable ubiquitin conjugates of LMP1 in pcDNA3.1, a vector was constructed expressing the LMP1 coding sequence, fused in-frame to the carboxy-terminus of the human ubiquitin-coding sequence (Figure 1A). The carboxy-terminal glycine residue (Gly76) of human ubiquitin was mutated to an alanine residue (Ala76), which diminishes cleavage of the fusion protein,15 and the amino terminal residue of LMP1 was modified from Met1 to Arg1, facilitating N-end rule targeting that decreases the in vivo half-life of a protein.16 For comparison, Gly1-LMP1 was also fused in frame to the C-terminus of modified ubiquitin-Ala76 to generate the expression vector Ub-Ala/Gly-LMP1. Similarly, Met1-LMP1 was also fused in frame to the C-terminus of unmodified ubiquitin-Gly76 to generate the expression vector (Ub-Gly/Met-LMP1). Since there are no previously defined Balb/c (H-2Kd)-restricted CTL epitopes within LMP1, an H-2Kd-restricted EBV nuclear antigen 1 CTL epitope (VYGGSKTSL) was fused to the carboxy-terminal of the pcDNA3.1 constructs for assessment of immunogenicity following in vivo DNA vaccinations. In addition, LMP1 and Ub-LMP1 inserts from the pcDNA3.1 constructs (minus the VYGGSKTSL epitope) were also subcloned in-frame with a sequence coding for green fluorescent protein (pEGFP-N1; Clontech, Palo Alto, CA) (Figure 1A).
Schematic description and localization of LMP1-GFP and Ub-LMP1-GFP expression constructs. (A) Four plasmids expressing LMP1 or LMP1 as a covalent fusion with the ubiquitin gene were generated in either pcDNA3.1 (LMP1; ubiquitin-LMP1 [Ub-LMP1]), or plasmid-enhanced green fluorescent protein-N1 (pEGFP-N1) (LMP1-GFP; Ub-LMP1-GFP) as described in “Materials and methods.” The LMP1/pcDNA3.1 constructs include insertion of an H-2Kd-restricted CTL epitope (VYGGSKTSL) at the LMP1 carboxy-terminal, which allows analysis of endogenous processing of LMP1. (B) GFP fluorescence of LMP1-GFP or Ub-LMP1-GFP-expressing Hela cells. Hela cells transiently transfected with pEGFP-N1, LMP1-GFP, or Ub-LMP1-GFP were examined by means of a laser-scanning Bio-Rad (Hercules, CA) MRC600 confocal microscope. Original magnification × 63.
Schematic description and localization of LMP1-GFP and Ub-LMP1-GFP expression constructs. (A) Four plasmids expressing LMP1 or LMP1 as a covalent fusion with the ubiquitin gene were generated in either pcDNA3.1 (LMP1; ubiquitin-LMP1 [Ub-LMP1]), or plasmid-enhanced green fluorescent protein-N1 (pEGFP-N1) (LMP1-GFP; Ub-LMP1-GFP) as described in “Materials and methods.” The LMP1/pcDNA3.1 constructs include insertion of an H-2Kd-restricted CTL epitope (VYGGSKTSL) at the LMP1 carboxy-terminal, which allows analysis of endogenous processing of LMP1. (B) GFP fluorescence of LMP1-GFP or Ub-LMP1-GFP-expressing Hela cells. Hela cells transiently transfected with pEGFP-N1, LMP1-GFP, or Ub-LMP1-GFP were examined by means of a laser-scanning Bio-Rad (Hercules, CA) MRC600 confocal microscope. Original magnification × 63.
Transfection and localization of LMP1 and Ub-LMP1 expression constructs
LMP1-GFP and Ub-LMP1-GFP constructs were transiently transfected into either a Hela cell line, an EBV- Burkitt lymphoma B-cell line (DG75), an EBV- keratinocyte cell line (HaCaT), or an NIH3T3 fibroblast cell line. Exponentially growing DG75 cells (5 × 106) were transfected in growth medium with 10 μg DNA by means of the Bio-Rad Gene Pulser (960-μF, 250-V, 0.4-cm gap electrode, 300 μL assay volume, 25°C). Hela cells, HaCaT keratinocytes, and NIH3T3 cells were transfected with the LMP1-GFP or Ub-LMP1-GFP expression constructs with the use of Effectene (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Stable GFP+ transfectants in NIH3T3 cells were selected with Geneticin (500 μg/mL) (Invitrogen, Carlsbad, CA) for 4 weeks. The efficiency of transfection was assessed by FACscan (Becton Dickinson, San Jose, CA) set to measure GFP fluorescence and analyzed with CellQuest software (Becton Dickinson). GFP fluorescence in cells was detected by means of a laser-scanning Bio-Rad confocal microscope with an argon laser at 488 nm, and the resulting images were exported to graphics software for further analysis. The proteasome inhibitor Lactacystin (a kind gift from Prof Emmanuel Wiertz Universiteit Leiden, Leiden, The Netherlands) was added to the cells at a final concentration of 10 μg/mL at 24 hours after transfection.
Degradation of LMP1 and Ub-LMP1 proteins in DG75 cells
DG75 cells were transfected with LMP1-GFP or Ub-LMP1-GFP expression vectors. At 36 hours after transfection, 8 × 106 cells were subjected to either 35[S]-metabolic labeling for pulse-chase analysis or cycloheximide degradation as described previously.17 For pulse-chase analysis, cells were lysed at the indicated times, and proteins were immunoprecipitated with an LMP1-specific CS1-4 antibody (Dako, Glostrup, Denmark) followed by the addition of an equal combination of protein A (Sigma, St Louis, MO) and protein G (Roche Diagnostics, Mannhiem, Germany) beads. Beads were washed, and proteins eluted and resolved under reducing conditions on a 7.5% sodium dodecyl sulfate (SDS)-polyacrylamide gel. For cycloheximide degradation experiments, cycloheximide (Sigma, Sydney, New South Wales, Australia) (50 μg/mL) was added to 8 × 106 cells. Equal aliquots of cells were removed at time points 0 minutes, 1.5 hours, 3 hours, and 6 hours; lysed in SDS-polyacrylamide gel electrophoresis sample dye; and resolved under reducing conditions on a 7.5% SDS-polyacrylamide gel.
Luciferase reporter assay
HaCaT cells were cotransfected with luciferase reporter plasmids for either nuclear factor-κB (NF-κB) (3Enh.κB-ConALuc) or signal transducer and activator of transcription (STAT) (pGRR5-Luc)18 and LMP1-GFP expression constructs (pEGFP-N1, LMP1-GFP, Ub-LMP1-GFP, Ub-Ala/Gly-LMP1-GFP, and Ub-Gly/Met-LMP1-GFP) in a 1:2 ratio. For the NF-κB cotransfections, a total of 0.8 μg DNA was added to 2 × 105 HaCaT cells with the use of Effectene (Qiagen, Clifton Hill, Victoria, Australia). Electroporation was used for the STAT activation assays where 9 μg total DNA was used for 5 × 106 HaCaT cells. At 24 hours after transfection, cells were harvested and resuspended in 2% fetal calf serum (FCS)/phosphate-buffered saline (PBS) for FACScan analysis of GFP expression, allowing for normalization of transfection efficiencies. Transfection efficiencies were generally between 10% and 30%. To determine the transcription factor activity, cells were pelleted and lysed with 120 μL Cell Culture Lysis Reagent (Promega, Madison, WI), and the luciferase activity was measured on 20 μL triplicates by addition of Luciferase Assay Reagent (Promega). Results were analyzed relative to the LMP1-induced cell-signaling activity.
Analysis of cell growth kinetics
To assess the effect of ubiquitination on the LMP1-mediated growth kinetics, 3T3 cells transfected with LMP1-GFP or Ub-LMP1-GFP expression constructs were cultured in 6-well plates containing RPMI 1640 medium supplemented with 1% FCS. Cell growth was assessed at 48-hour intervals for 6 days, and viable cell numbers were determined by trypan blue exclusion. In addition, a clonogenic assay was used to measure the growth rate of these LMP1-transfected cells. Cells were plated in a 6-well plate at a density of 1 × 103 cells per well and grown for 10 days in RPMI 1640/10% serum with media changed every 2 days. Resulting colonies were stained with crystal violet.
Assessment of oncogenic potential of LMP1-transfected primary fibroblasts
NIH3T3 cells (1 × 107) transfected with LMP1-GFP or Ub-LMP1-GFP were injected subcutaneously in 5-week-old nude mice. Mice were monitored every third day and killed when the tumor mass reached 1 cm3.
DNA immunization
DNA was purified by means of the Qiagen endotoxin-free maxi-prep kit. Six- to 8-week-old female BALB/c (H-2Kd) mice were immunized intramuscularly 3 times, at 14-day intervals, with 100 μg plasmid DNA. This was dissolved in endotoxin-free PBS at a concentration of 1 μg/mL, and 50 μL was injected into each of the rear quadricep muscles with a 28-gauge needle. For the constructs LMP1, Ub-Ala/Arg-LMP1, and pcDNA3.1 vector control, 5 mice were used in each treatment group. At 4 weeks after the final DNA immunization, mice were killed by cervical dislocation and spleens removed for in vitro restimulation of CTLs.
Assay for LMP1-specific CTL response
Splenocytes (16 × 106) from each mouse were seeded into 4 wells of a 24-well plate and mixed with peptide-coated (10 μg/mL) gamma-irradiated (2000 rads) lipopolysaccharide (LPS) blasts (derived from naive Balb/c splenocytes) at a responder-to-stimulator ratio of 3:1. The H-2Kd-restricted, Epstein-Barr virus nuclear antigen 1 (EBNA1) peptide epitope VYGGSKTSL was used to sensitize the LPS blasts. After 5 days, the responder cells were harvested and viable cells counted. In 96-well round-bottom plates, 51Cr-labeled P815 target cells (an H-2Kd-positive mastocytoma cell line) were incubated in the presence or absence of the VYGGSKTSL peptide epitope and plated in duplicate in 200 μL volumes with restimulated T cells at graded effector-target ratios for 5 hours. Then, 25 μL supernatant per well was harvested, and specific chromium release was calculated by the following formula: (test release - spontaneous release) × 100/(total release - spontaneous release).
Assessment of T-cell responses by interferon-γ enzyme-linked immunospot assay
The interferon-γ (IFN-γ) enzyme-linked immunospot (ELISPOT) assay has been described in detail elsewhere.13 Briefly, splenocytes were harvested from the immunized mice and placed in sterile tubes containing 10 mL growth medium supplemented with 10-5 2-mercaptoethanol and 2 mM glutamine. These cells were plated in ELISPOT plates (106 per well), and then the synthetic peptide epitope VYGGSKTSL was added into the wells at a final concentration of 1 mg/mL. For negative controls, splenocytes were incubated without peptide. Spots were automatically counted by means of image analysis software and are expressed as spot-forming cells (SFCs) per 106 peripheral blood mononuclear cells (PBMCs). The number of IFN-γ-secreting T cells were calculated by subtracting the negative control value from the number of SFCs.
Results
Effect of ubiquitination and N-end rule targeting on LMP1 localization and intracellular turnover
Previous studies have suggested that the transmembrane domains of LMP1 spontaneously aggregate, enabling 2 sites within the carboxy-terminal activator domains to interact with cellular proteins and initiate signaling events. We hypothesized that any disruption of this aggregation may abrogate the oncogenic potential of LMP1. One possible strategy to achieve this would be to target LMP1 for rapid intracellular degradation. It has been well documented that proteasomal degradation of intracellular proteins is critically dependent on the covalent linkage of ubiquitin to the protein substrate.19,20 Data from localization studies presented in Figure 1B show LMP1 expression forming distinct perinuclear aggregated patches. In contrast, although ubiquitinated LMP1 similarly localized to the perinuclear region, the expression pattern was significantly more diffuse, with the aggregates being smaller than those observed for LMP1 expression.
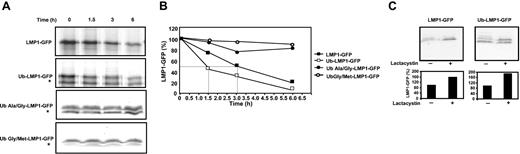
To determine the impact of loss of aggregation on the intracellular turnover of LMP1, DG75 cells were transiently transfected with expression vectors pEGFP-N1, LMP1-GFP, or Ub-LMP1-GFP, and protein expression was analyzed by pulse-chase analysis. Representative data from one such analysis are shown in Figure 2A. The native LMP1-GFP protein displayed a half-life of 3.1 hours, while covalent linkage of ubiquitin and N-end rule targeting of LMP1 resulted in enhanced proteasomal degradation, leading to an increase in LMP1-GFP turnover with a half-life of 1.5 hours (Figure 2B). We also observed a truncated LMP1-GFP protein band on the Ub-LMP1-GFP immunoblot. This band may represent native LMP1 after cleavage from ubiquitin. The degradation rate of this truncated lower band was determined and observed to occur with slower kinetics (half-life 4.3 hours) compared with the upper band, confirming that enhanced proteasomal degradation results from both modifications: N-end rule targeting and covalent ubiquitin modification. Interestingly, the expression level of ubiquinated LMP1 was consistently lower than native LMP1 at the 0-minute chase time point, further supporting the evidence that proteasomal targeting of LMP1 can reduce its intracellular stability. Since LMP1 is biologically active only following correct membrane insertion and dimerization,14,21 we included 2 control ubiquitin-LMP1 fusion proteins in our study to confirm that our observed results were in fact due to proteasomal degradation. The first control fusion protein was constructed with a glycine instead of an arginine at the amino terminal residue of LMP1 (Ub-ala/Gly-LMP1-GFP). For our second control fusion construct, we changed the alanine residue at the C-terminus of ubiquitin back to the native glycine residue and also changed the N-terminal LMP1 arginine residue back to its native methionine residue (Ub-Gly/Met-LMP1-GFP). These amino acid changes at the junction of the ubiquitin-LMP1 fusion protein should prolong the half-life of the LMP1 fusion protein.16 Degradation studies performed with the use of these control ubiquitin-LMP1 fusion proteins (Ub-Ala/Gly-LMP1-GFP) and (Ub-Gly/Met-LMP1-GFP) are illustrated in Figure 2A-B and demonstrate a marked increase in stability. The stabilizing effect of the N-terminal ubiquitin when it is not used in conjunction with N-end rule targeting is most likely due to the blocking of an essential recognition motif at the N-terminal region of the LMP1 molecule. Wild-type LMP1 belongs to a unique group of proteins that is ubiquitinated through the alpha amino group of their N-terminal residue.22 Indeed, this same stabilizing effect on LMP1 was also observed when a Myc tag was fused to the amino terminal end of LMP1.22 As further evidence that the enhanced degradation observed in Figure 2A-B is due to proteasomal degradation, we used the proteasomal inhibitor Lactacystin to see if we could stabilize both the LMP1-GFP and the Ub-LMP1-GFP constructs. Figure 2C shows that following the addition of Lactacystin to the LMP1-GFP- and Ub-LMP1-GFP-transfected cells, there was a significant increase in LMP1-GFP and Ub-LMP1-GFP stability, confirming proteasomal dependent degradation.
Ubiquitin enhancement of intracellular degradation of LMP1. (A) Pulse-chase analysis of LMP1-GFP and Ub-LMP1-GFP expression. DG75 cells were transfected with expression constructs LMP1-GFP or Ub-LMP1-GFP, and at 30 hours after transfection, the cells were metabolically labeled for 10 minutes and processed for pulse-chase analysis as described in “Materials and methods.” * denotes a truncated Ub-LMP1-GFP protein band. (B) Densitometric analysis of LMP1-GFP and Ub-LMP1-GFP expression. Band intensities were quantified by analysis of the imaging data and plotted as a relative percentage of the signal at time 0 for LMP1-GFP, Ub-LMP1-GFP, and 2 control ubiquitin-LMP1 fusion proteins: Ub-Ala/Gly-LMP1-GFP and Ub-Gly/Met-LMP1-GFP. (C) Effect of the proteasomal inhibitor Lactacystin on the stability of LMP1. Duplicate aliquots of DG75 cells were transfected with expression constructs LMP1-GFP and Ub-LMP1-GFP; 36 hours later, the proteasome inhibitor Lactacystin was added at a final concentration of 10 μg/mL for 12 hours to one of each pair of duplicates. Cell lysates were separated by electrophoresis for immunoblotting with the GFP-specific antibody. The absence (-) or presence (+) of Lactacystin is indicated. Densitometric analysis of the LMP1-GFP and Ub-LMP1-GFP (upper band) expression products are shown.
Ubiquitin enhancement of intracellular degradation of LMP1. (A) Pulse-chase analysis of LMP1-GFP and Ub-LMP1-GFP expression. DG75 cells were transfected with expression constructs LMP1-GFP or Ub-LMP1-GFP, and at 30 hours after transfection, the cells were metabolically labeled for 10 minutes and processed for pulse-chase analysis as described in “Materials and methods.” * denotes a truncated Ub-LMP1-GFP protein band. (B) Densitometric analysis of LMP1-GFP and Ub-LMP1-GFP expression. Band intensities were quantified by analysis of the imaging data and plotted as a relative percentage of the signal at time 0 for LMP1-GFP, Ub-LMP1-GFP, and 2 control ubiquitin-LMP1 fusion proteins: Ub-Ala/Gly-LMP1-GFP and Ub-Gly/Met-LMP1-GFP. (C) Effect of the proteasomal inhibitor Lactacystin on the stability of LMP1. Duplicate aliquots of DG75 cells were transfected with expression constructs LMP1-GFP and Ub-LMP1-GFP; 36 hours later, the proteasome inhibitor Lactacystin was added at a final concentration of 10 μg/mL for 12 hours to one of each pair of duplicates. Cell lysates were separated by electrophoresis for immunoblotting with the GFP-specific antibody. The absence (-) or presence (+) of Lactacystin is indicated. Densitometric analysis of the LMP1-GFP and Ub-LMP1-GFP (upper band) expression products are shown.
Effect of ubiquitination and N-end rule targeting of LMP1 on cellular signaling and growth kinetics
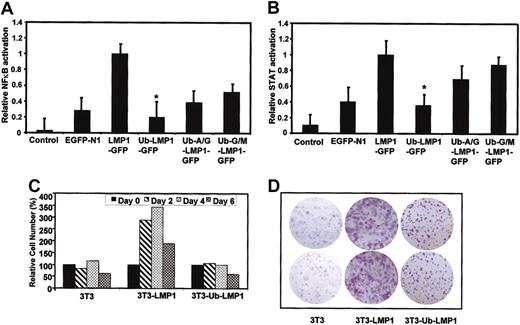
Since targeting of the LMP1 protein for proteasomal degradation resulted in its increased intracellular turnover as well as decreased membrane aggregation, we investigated whether these altered parameters may affect LMP1-mediated NF-κB and STAT cellular signaling pathways, potentially leading to impaired oncogenicity. EGFP-N1, LMP1-GFP, Ub-LMP1-GFP, Ub-Ala/Gly-LMP1-GFP, and Ub-Gly/Met-LMP1-GFP expression plasmids were cotransfected with specific reporter plasmids in the HaCaT keratinocyte cell line. At 24 hours after transfection, the luciferase activity was measured and expressed relative to an LMP1 reference. Our results confirm earlier studies18 showing that LMP1 activates both NF-κB and STAT-signaling pathways (Figure 3A-B). In contrast, NF-κB and STAT-signaling activation was completely blocked following expression of ubiquitinated LMP1 (Figure 3A-B). Following expression of control ubiquitin-LMP1 fusion proteins Ub-Ala/Gly-LMP1-GFP and Ub-Gly/Met-LMP1-GFP where the junction amino acids were altered to increase stability of the fusion proteins, we observed a corresponding increase in NF-κB and STAT activation when compared with the Ub-LMP1 construct where cotranslational ubiquitination was combined with N-end rule targeting of LMP1 (Figure 3A-B), demonstrating that the observed loss of LMP1 function is indeed proteasomal dependent.
Effect of covalent linkage of ubiquitin. Covalent linkage of ubiquitin blocks LMP1-mediated cellular signaling pathways. (A) (B) Summary of the relative NF-κB (A) and STAT (B) activity induced by either pEGFP-N1, LMP1-GFP, Ub-LMP1-GFP Ub-Ala/Gly-LMP1-GFP, or Ub-Gly/Met-LMP1-GFP as determined by quantitation of luciferase activity produced from a cotransfected reporter plasmid. The data were normalized for transfection efficiency by measuring GFP+ cells and then expressed relative to the activity obtained with the B95.8 LMP1 gene (100%) without subtracting the basal activity in control pEGFP-N1-transfected cells. Results are the mean standard deviation of at least 4 separate experiments; * denotes that values obtained for Ub-LMP1-GFP are statistically significant (P < .05) when compared with LMP1-GFP. (C) Kinetics of cell survival in control and LMP1-GFP transfectants of HaCaT cells. The percentage of cell viability was determined by trypan blue staining every second day for up to 6 days of culture in medium containing 1% FCS. Cell number is expressed as cells × 103/mL and is shown as the percentage of cell growth with counts at day 0 being considered 100%. (D) Clonogenic assay for cell growth. Cells (103) from control, LMP1-GFP-, and Ub-LMP1-GFP-expressing 3T3 cells were plated in each well of 6-well plates. After 10 days of cell growth, the resulting colonies were stained with crystal violet. The results shown are from a representative duplicate experiment. Original magnification × 10.
Effect of covalent linkage of ubiquitin. Covalent linkage of ubiquitin blocks LMP1-mediated cellular signaling pathways. (A) (B) Summary of the relative NF-κB (A) and STAT (B) activity induced by either pEGFP-N1, LMP1-GFP, Ub-LMP1-GFP Ub-Ala/Gly-LMP1-GFP, or Ub-Gly/Met-LMP1-GFP as determined by quantitation of luciferase activity produced from a cotransfected reporter plasmid. The data were normalized for transfection efficiency by measuring GFP+ cells and then expressed relative to the activity obtained with the B95.8 LMP1 gene (100%) without subtracting the basal activity in control pEGFP-N1-transfected cells. Results are the mean standard deviation of at least 4 separate experiments; * denotes that values obtained for Ub-LMP1-GFP are statistically significant (P < .05) when compared with LMP1-GFP. (C) Kinetics of cell survival in control and LMP1-GFP transfectants of HaCaT cells. The percentage of cell viability was determined by trypan blue staining every second day for up to 6 days of culture in medium containing 1% FCS. Cell number is expressed as cells × 103/mL and is shown as the percentage of cell growth with counts at day 0 being considered 100%. (D) Clonogenic assay for cell growth. Cells (103) from control, LMP1-GFP-, and Ub-LMP1-GFP-expressing 3T3 cells were plated in each well of 6-well plates. After 10 days of cell growth, the resulting colonies were stained with crystal violet. The results shown are from a representative duplicate experiment. Original magnification × 10.
In the next set of experiments, the growth of LMP1-GFP- and Ub-LMP1-GFP-expressing 3T3 cells and their survival in low-serum media (1%) were analyzed and are shown in Figure 3C. As shown previously,23 3T3 cells expressing LMP1-GFP grew to a higher cell density than did control 3T3 cells, displaying a 3-fold increase in cell number on days 2, 4, and 6 (Figure 3C). Interestingly, cells expressing ubiquitinated LMP1-GFP demonstrated a significantly slower growth rate than that observed for LMP1-GFP expressing cells and by day 8 had viability counts equal to those of control cells (Figure 3C). These results were also confirmed by a clonogenic assay in which LMP1-GFP transfectants formed colonies with a significantly higher cell density than was observed for colonies resulting from Ub-LMP1-GFP transfectants (Figure 3D). Taken together, these data demonstrate that intracellular targeting of LMP1 for enhanced proteasomal degradation results in deactivation of normal LMP1-mediated cellular signaling pathways and does not protect cells from low-serum-induced cell death.
Tumorigenicity of NIH3T3 cells expressing LMP1
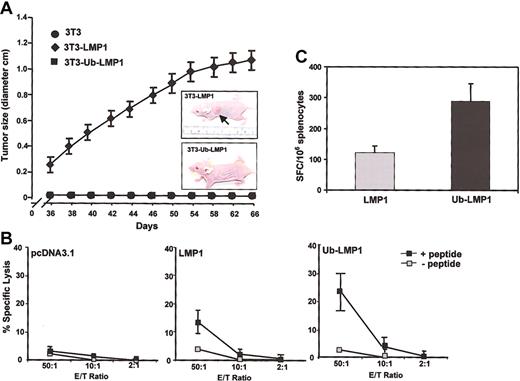
Tumorigenicity of the stably selected LMP1-GFP- and Ub-LMP1-GFP-expressing 3T3 cells was assayed by injection of 1 × 107 cells subcutaneously into 5-week-old nude mice. All of the mice (5 of 5) inoculated with the LMP1-GFP transfectants developed tumors, which first appeared at 5 to 6 weeks and grew progressively (Figure 4A). By 9 weeks, the tumors ranged in size from 0.9 to 1.2 cm, with most tumors being multilobulated. In contrast, none of the mice (0 of 5) inoculated with the Ub-LMP1-GFP transfectants developed tumors (Figure 4A). No tumors were observed at the site of injection in any of the mice inoculated with parental NIH3T3 cells. Animals with tumors were killed at 9.5 weeks. Immunoblot analysis of tumor tissue revealed detectable amounts of LMP1-GFP fusion protein in each of the tumors (data not shown).
Effect of covalent linkage of ubiquitin on the LMP1-mediated oncogenicity and LMP1-specific T-cell responses. (A) Nude mice were injected with 1 × 107 LMP1- or Ub-LMP1-expressing 3T3 cells, and tumor progression was assessed for 66 days. (B) In vivo induction of CTL responses to a model epitope fused to LMP1 following immunization with DNA expression vectors. BALB/c (H-2Kd) mice were immunized intramuscularly with DNA vectors encoding LMP1 or Ub-LMP1 fused to an H-2Kd-restricted EBV nuclear antigen 1 CTL epitope (VYGGSKTSL) at the carboxy-terminal. Splenocytes from immunized mice were stimulated with H-2Kd-restricted peptide VYGGSKTSL, and CTL activity was assessed with a standard 51Cr-release assay. The data shown are representative of 2 separate experiments. (C) Ex vivo assessment of CTL responses to a model epitope fused to LMP1 following immunization with DNA expression vectors. These responses were assessed by ELISPOT assays as described in “Materials and methods.” A minimum of 5 mice from each group were assessed for the epitope-specific T-cell reactivity. The results from panels A, B, and C are expressed as mean ± standard error (SE).
Effect of covalent linkage of ubiquitin on the LMP1-mediated oncogenicity and LMP1-specific T-cell responses. (A) Nude mice were injected with 1 × 107 LMP1- or Ub-LMP1-expressing 3T3 cells, and tumor progression was assessed for 66 days. (B) In vivo induction of CTL responses to a model epitope fused to LMP1 following immunization with DNA expression vectors. BALB/c (H-2Kd) mice were immunized intramuscularly with DNA vectors encoding LMP1 or Ub-LMP1 fused to an H-2Kd-restricted EBV nuclear antigen 1 CTL epitope (VYGGSKTSL) at the carboxy-terminal. Splenocytes from immunized mice were stimulated with H-2Kd-restricted peptide VYGGSKTSL, and CTL activity was assessed with a standard 51Cr-release assay. The data shown are representative of 2 separate experiments. (C) Ex vivo assessment of CTL responses to a model epitope fused to LMP1 following immunization with DNA expression vectors. These responses were assessed by ELISPOT assays as described in “Materials and methods.” A minimum of 5 mice from each group were assessed for the epitope-specific T-cell reactivity. The results from panels A, B, and C are expressed as mean ± standard error (SE).
Covalent linkage of ubiquitin and N-end rule targeting leads to an enhanced LMP1-specific CTL response in vivo
To assess whether the reduced stability of ubiquitinated LMP1 may enhance de novo CTL responses in vivo, 3 different groups of BALB/c mice were immunized with plasmid DNA vectors encoding either LMP1, Ub-LMP1, or the vector control pcDNA3.1. Following immunization, the CTL response against an H-2Kd-restricted CTL epitope (VYGGSKTSL) that had been fused to the carboxy-terminal end of the LMP1 and Ub-LMP1 constructs was assessed. As shown in Figure 4B, mice immunized with native forms of LMP1 demonstrated low levels of CTL activity against the epitope. In contrast, an enhanced CTL response was observed following immunization with an expression vector encoding ubiquitinated LMP1 (Figure 4B). No CTL response was detected in mice immunized with the pcDNA3.1 vector alone. Epitope-specific lysis by CTLs recovered from the ubiquitinated LMP1 mice was on average 2-fold higher than that of CTLs recovered from mice immunized with LMP1. These results were also confirmed in an ex vivo ELISPOT assay, which showed enhanced T-cell responses in mice vaccinated with Ub-LMP1 (Figure 4C). It is important to stress here that although a model epitope was used in this study to assess the immunogenicity of ubiquitinated LMP1, a formal validation of this approach would require further studies based on an LMP1-derived epitope.
Discussion
In this study we have developed a novel strategy, which both deactivates cellular signaling pathways associated with the oncogenic phenotype and simultaneously reverses poor immunogenicity of a virally encoded oncogene, LMP1. LMP1, like many other oncogenes, has been recognized as one of the most crucial latent proteins for EBV-mediated transformation of normal B cells and is uniquely able to induce malignant outgrowth and hyperplasia in transgenic mice.24 Furthermore, LMP1 is also known to exhibit pleiotropic effects on the cellular phenotype of B cells and epithelial cells25 and can act as a constitutively active receptor-like molecule independent of the binding of a ligand. The transmembrane domains mediate oligomerization of LMP1 molecules, a prerequisite for LMP1 function. Following covalent linkage of ubiquitin to LMP1, in addition to N-end rule targeting, we were able to demonstrate enhanced intracellular degradation of LMP1 and disruption of intracellular aggregation. The impact of this decreased aggregation was clearly evident from the complete blockade of LMP1-mediated activation of NF-κB and STAT. These observations are consistent with previously published reports that showed that the aggregation mediated by the transmembrane domains plays a crucial role in activating C-terminus activation region (CTAR) domains, resulting in initiation of various downstream signaling events.26-28
Another important phenotypic effect of LMP1 involves protection of cells from apoptotic death under stress conditions such as serum starvation.29 This survival signal has been proposed to play a crucial role in protecting a variety of cells, including EBV+ Hodgkin lymphoma cells, from tumor necrosis factor (TNF)-mediated apoptosis.30 The protection from apoptosis is coincident with the increased expression of antiapoptotic proteins B-cell lymphoma 2 (Bcl-2), mantle cell lymphoma 1 (Mcl-1), A20, and TNF receptor-associated factor 1 (TRAF1)29,31-33 and also involves Rel/NF-κB family members. Not surprisingly, loss of activation of NF-κB following covalent linking of ubiquitin also compromised the ability of LMP1 to prevent apoptosis of 3T3 cells under serum starvation. More importantly, while murine primary fibroblasts expressing LMP1 were uniformly tumorigenic, a complete abrogation of oncogeneic potential was achieved following ubiquitination of LMP1. To our knowledge, this is the first demonstration in which oncogenicity has been completely inhibited following specific proteasomal targeting of an oncogene. These studies demonstrate that a ubiquitinated oncogene may act as a safe vaccination alternative that can be employed for the induction of strong CTL-mediated immunity directed against transforming oncogenic products.
Prepublished online as Blood First Edition Paper, August 14, 2003; DOI 10.1182/blood-2003-03-0870.
Supported by grants from the National Health and Medical Research Council (NH&MRC) and the Queensland Cancer Fund; by a Peter Doherty Fellowship (J.T.); and by a Senior Research Fellowship from the NH&MRC (R.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Prof John Shine, Dr Ross Tellam, Dr Scott Burrows, and Dr Martina Sherritt for critically reading the manuscript.

![Figure 1. Schematic description and localization of LMP1-GFP and Ub-LMP1-GFP expression constructs. (A) Four plasmids expressing LMP1 or LMP1 as a covalent fusion with the ubiquitin gene were generated in either pcDNA3.1 (LMP1; ubiquitin-LMP1 [Ub-LMP1]), or plasmid-enhanced green fluorescent protein-N1 (pEGFP-N1) (LMP1-GFP; Ub-LMP1-GFP) as described in “Materials and methods.” The LMP1/pcDNA3.1 constructs include insertion of an H-2Kd-restricted CTL epitope (VYGGSKTSL) at the LMP1 carboxy-terminal, which allows analysis of endogenous processing of LMP1. (B) GFP fluorescence of LMP1-GFP or Ub-LMP1-GFP-expressing Hela cells. Hela cells transiently transfected with pEGFP-N1, LMP1-GFP, or Ub-LMP1-GFP were examined by means of a laser-scanning Bio-Rad (Hercules, CA) MRC600 confocal microscope. Original magnification × 63.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/13/10.1182_blood-2003-03-0870/6/m_h82435405001.jpeg?Expires=1769957113&Signature=djvcfCa8xsuyUwJihUTp~3SBx1TMPgQ1RJIuRlMFPoFMSLXJ2K~GiqBP1yVaZSKVXivMYxxovmad7uPJk7dqNM7EMp0whtgsbwW~eSb878bJOmV5PfPDPNU6gU1gVoCy5v~WBopnvZTcZNQlauPmHFsmrFS4egwulmGI6ivHoNjTBIguOUtcL6oOeOz9169h1kny80Mhu-JhM7PE5xsGw3eckzdcLTfDMtBXJwsj2mPl4ZNT7~aOqzJpuw14V80YTLmV7rPAp5hQvTw8rW3rPw5AuW9QwGIsSwrxVNFr91yCWrC41hkeMVPSqm8e4MB7AklQazgh1xEty9cCk6HzYg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)