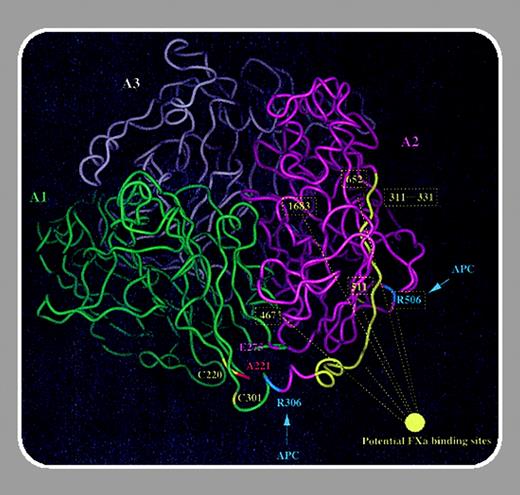
Molecular defects in factor V deficiency (parahemophilia) have been difficult to study due to the low frequency of this disorder and the expression of 2 mutant alleles in each patient. Of the approximately 20 factor V mutations characterized thus far, several appear to result primarily in impaired secretion of the molecule (Duga et al, Blood. 2003;101:173-177). In contrast, factor VNew Brunswick (Ala221Val) is associated with a qualitative factor V deficiency (Murray et al, Blood. 1995;86:1820-1827). Factor V isolated from the plasma of a patient pseudohomozygous for this mutation was activated normally by thrombin but showed reduced cofactor activity compared with control (17%). It was originally proposed that the Ala221Val mutation, located in the A1 domain, interfered with the ability of factor Va to interact with factor Xa. However, recent investigations have suggested that the factor Xa binding site is localized to the A2/A3 domains in factor Va (Kojima et al, J Biol Chem. 1998;273:14900-14905; Steen et al, J Biol Chem. 2002;277: 50022-50029; Kalafatis et al, Biochemistry. 2002;41:12715-12728).
In this issue, Steen and colleagues (page 1316) have demonstrated that the Ala221Val mutation results in an unstable factor Va molecule. Using recombinant factor V expressed in COS cells, these investigators demonstrated that the Ala221Val mutant protein was expressed, secreted, and activated by thrombin similar to control. Surprisingly, the ability of the Ala221Val mutant to serve as a cofactor in the prothrombinase complex was not compromised. The Ala221Val mutation also did not affect the rate of inactivation of factor V by activated protein C. However, the mutant factor Va (but not single-chain mutant factor V) was found to be unstable following incubation at 37°C. The instability of the Ala221Val mutant factor Va was due to dissociation of the amino terminal heavy chain and carboxyl terminal light chain, which together comprise the factor Xa binding site. These results are consistent with previously published data and provide another illustration of how loss of function mutations may have unexpected indirect mechanisms. Since the Ala221Val mutant single-chain factor V appears to be relatively stable in vitro, it remains to be determined whether this mutation also affects the stability or clearance of the single-chain pre-cursor in vivo.