Abstract
Platelet spreading on immobilized fibrinogen (Fg) involves progression through a number of morphologic stages that, although distinctive, are not well understood mechanistically. Here we demonstrate that an association between GPIIb/IIIa and calcium- and integrin-binding protein (CIB) is required for the process of platelet spreading. Upon platelet adhesion to immobilized Fg, CIB localizes to the transiently formed filopodia and then redistributes diffusely along the membrane periphery of spread platelets. Immunoprecipitation analyses indicate that CIB and glycoprotein IIb/IIIa (GPIIb/IIIa) interact with each other as platelets adhere to immobilized Fg, and together they associate with the platelet cytoskeleton. Introduction of anti-CIB antibody or GPIIb cytoplasmic peptide into platelets blocks lamellipodia but not filopodia formation. GPIIb peptide–induced inhibition of platelet spreading is recovered by the incorporation of recombinant CIB protein, suggesting that interaction between CIB and GPIIb/IIIa is required for progression from filopodial to spread morphologies. Further, anti-CIB– or GPIIb peptide–induced inhibition of platelet spreading can be overcome by the addition of exogenous adenosine diphosphate (ADP). These data suggest that formation of the CIB-GPIIb/IIIa complex may be necessary for initiation of downstream signaling events, such as ADP secretion, that lead to platelet spreading.
Introduction
Glycoprotein IIb/IIIa (GPIIb/IIIa) is the platelet fibrinogen (Fg) receptor that mediates both platelet aggregation and spreading on vascular matrices at the site of vascular injury.1-5 In unactivated platelets, the majority of GPIIb/IIIa is in low-affinity state, unable to bind to soluble ligand; however, it is capable of directly binding immobilized Fg and von Willebrand factor.6 Binding to these ligands triggers an outside-in signaling cascade through GPIIb/IIIa that initiates platelet filopodia extension, granular secretion, spreading, and aggregation.4,7 Because each of these responses requires rearrangement of the platelet cytoskeleton, it is apparent that signaling through GPIIb/IIIa leads to the regulation of actin dynamics.8-10 However, the exact signaling mechanism that takes place upon GPIIb/IIIa binding of immobilized Fg leading to platelet spreading has been only partially elucidated.
Recently, nonreceptor tyrosine kinases such as spleen tyrosine kinase (Syk) and sarcoma virus tyrosine kinase (Src) have been shown to be involved in this signaling cascade.4,7,11,12 It is known that following platelet binding to Fg, Syk directly binds the cytoplasmic tail of the GPIIIa subunit of GPIIb/IIIa,13 and becomes activated almost immediately.13,14 Additionally, a recent study has shown that although Src is constitutively associated with GPIIb/IIIa, platelet adhesion to immobilized Fg selectively activates this kinase, possibly by the dissociation of the Src-regulatory kinase, C-terminal Src kinase (Csk), from the integrin.12 Thus, it is now believed that Src, in addition to Syk, is required for initiation of the signaling cascade that gives rise to dynamic cytoskeletal rearrangement converting discoid into shape-changed platelets.12
Upon the activation of Syk, Src, and possibly a number of other kinases as a result of GPIIb/IIIa binding to Fg, discoid platelets undergo a rapid shape change during which adherent platelets transiently exhibit filopodia-like structures. This fleeting event warrants rapid actin depolymerization and repolymerization events, resulting in a spiky morphology. Dynamic reorganization of the platelet cytoskeleton triggers a signaling cascade that results in granular secretion of platelet agonists such as adenosine diphosphate (ADP) and serotonin from platelet-dense granules and adhesive proteins such as platelet factor 4, Fg, and von Willebrand factor from α-granules.15 The signaling events required to initiate granular secretion, however, have not yet been well defined. Binding of these agonists to their receptors initiates extensive signaling events that lead to a second wave of cytoskeletal reorganization and culminate in a fully spread platelet morphology. A dynamic platelet cytoskeleton aids in propagating the signal by providing a scaffold upon which signaling molecules can localize, as evidenced by the large number of signaling molecules that translocate to the cytoskeleton of activated platelets. Focal adhesion kinase (FAK) activation has been shown to require actinpolymerization events, implicating its role in the second wave of cytoskeletal rearrangement.14,16 In fact, it has been shown that granular secretion of ADP controls activation of both FAK and phosphatidylinositol 3′-kinase (PI3-K),17,18 which is essential for platelet spreading. Upon pretreatment of platelets with ADP scavengers such as apyrase or pyruvate kinase plus phosphoenolpyruvate, platelet spreading on Fg is inhibited.17,19,20 Although these platelets fail to spread, they do, in fact, attach and progress through to a shape-changed morphology with multiple filopodia.20 Pretreatment of platelets with PI3-K inhibitors also results in the same spiky morphology.20 Thus, it is believed that extended platelet spreading on Fg requires, at least in part, granular secretion of ADP leading to the activation of PI3-K.
Although propagation of a signal through GPIIb/IIIa upon Fg binding is known to occur, the role of the cytoplasmic signaling components that act downstream of GPIIb/IIIa in this process is not well defined. Current consensus suggests that events such as platelet activation, adhesion, spreading, aggregation, and granular secretion involve the binding of cytoplasmic proteins directly to the tails of the GPIIb and GPIIIa subunits. Therefore, several proteins, including β3-endonexin,21 talin,22 Src homology and collagen protein (Shc), growth factor receptor–bound protein 2 (Grb2),23 myosin,24 integrin-linked kinase (Ilk),25 and calreticulin,26 have been proposed to play a role in GPIIb/IIIa-related downstream signaling events owing to their ability to directly bind the cytoplasmic tail of either subunit.
Previously, we have reported that CIB binds directly to the cytoplasmic domain of GPIIb27 in a calcium-dependent manner.28 Upon platelet activation by various physiological agonists, CIB translocates to the cytoskeleton in a time-dependent manner, which is contingent upon platelet aggregation and parallels the translocation of GPIIb.28 In the present study, we demonstrate for the first time that the interaction of CIB with GPIIb/IIIa is required for the process of platelet spreading on immobilized Fg. Upon ligand binding, the majority of CIB binds GPIIb/IIIa to form a complex. Inhibition of complex formation by introduction of either anti-CIB antibody or GPIIb cytoplasmic tail peptide into platelets blocks platelet spreading, but not adhesion or filopodia formation. A spread morphology can be recovered by the introduction of recombinant CIB into antibody- or GPIIb peptide–inhibited platelets. Further, the inhibitory effect of antibody or GPIIb peptide on platelet spreading can be overcome by the addition of exogenous ADP. These data suggest that formation of the CIB-GPIIb/IIIa complex may be required for the granular secretion of ADP that is crucial for platelet spreading.
Materials and methods
Antibodies and reagents
Monoclonal anti-CIB antibodies, UN7.79 or UN2, were produced as previously described.27 SEW-8, an antirabbit polyclonal antibody against GPIIb, was a generous gift from P. J. Newman (Blood Center, Milwaukee, WI). HB67, a control immunoglobulin G1 (IgG1) antibody (cIgG), was obtained from the American Type Culture Collection (Manassas, VA). Fluorescein isothiocyanate (FITC)–conjugated donkey antirabbit IgG and Texas Red–conjugated donkey antimouse IgG were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). FITC-conjugated phalloidin was purchased from Molecular Probes (Eugene, OR).
Heparin, prostaglandin 1 (PGE1), apyrase (grade I), ADP, dimethylsulfoxide (DMSO), leupeptin, deoxycholate, aprotinin, phenylmethylsulfonyl fluoride (PMSF), antagonists of ADP receptors (2-methylthio-adenosine monophosphate [2MeSAMP] for P2Y12, and MRS 2179 for P2Y1), and bovine serum albumin (BSA) were purchased from Sigma (St Louis, MO). Purified human Fg was obtained from Enzyme Research Laboratories (South Bend, IN).
Recombinant CIB protein was prepared as previously described.27,28 GPIIb peptide (LVLAMWKVGFFKRNRPPLEEDDEEGQ) corresponding to the C-terminus of the GPIIb subunit was synthesized and purified by high-pressure liquid chromatography (HPLC) by Joel Schneider (University of Delaware, Newark, DE). Scrambled peptide against the GPIIb cytoplasmic tail was a generous gift from Leslie Parise (University of North Carolina, Chapel Hill).
Blood collection
Whole blood was drawn into 6:1 (vol/vol) citric acid/citrate/dextrose (ACD) (pH 4.5) or in 9:1 (vol/vol) 3.8% sodium citrate by venipuncture from healthy, drug-free volunteers older than 18 years of age under informed consent. Approval was obtained from the University of Delaware institutional review board for these studies according to the Declaration of Helsinki. Platelet-rich plasma (PRP) and washed platelets were obtained as previously described27 and used within 3 hours of isolation. To obtain discoid platelets, PRP was diluted with an equal volume of 8% paraformaldehyde in phosphate-buffered saline (PBS) (pH 7.2) as a fixative for 10 minutes at room temperature. All experiments using platelets were performed at least 3 times with platelets from several donors. Platelets were counted with a Beckman-Coulter Z2 (Miami, FL) counter.
Immunofluorescence
Immunofluorescence studies were conducted according to procedures described previously.29 Platelets exposed to BSA- or Fg-precoated glass cover-slide chambers were fixed, permeabilized, and incubated with indicated primary antibodies, followed by appropriate secondary antibodies (tetromethylrhodamine isothiocyanate [TRITC]–conjugated donkey anti-mouse, or FITC-conjugated donkey antirabbit) or FITC-phalloidin to stain F-actin. Stained samples were analyzed by means of a Zeiss LSM510 (Carl Zeiss, Thornwood, NY) confocal microscope, and images were processed in Adobe Photoshop (Adobe, San Jose, CA).
Immunoprecipitation and immunoblotting
Immunoprecipitation studies were performed with platelets (4.5 × 108/mL) as previously described.12 In brief, platelets were lysed with Triton X-100, and 500 μL precleared lysates containing equal amounts of protein per sample (500 to 800 μg across experiments) were incubated with UN7.79 overnight at 4°C. Simultaneously, the Triton X-100–insoluble cytoskeletal pellet from these samples was washed and gently resuspended in radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl; 1% Nonidet P40 [NP40], 0.05% deoxycholate, and 0.1% sodium dodecyl sulfate [SDS] in 50 mM Tris [tris(hydroxymethyl)aminomethane], pH 8.0) until the pellet was dissolved. The supernatant was used for immunoprecipitation with UN7.79 as above. The immunocomplexes were captured and washed 3 times with lysis buffer in the absence of protease inhibitors. Samples were then processed for immunoblotting as previously described.27 Band intensity was analyzed by means of the Gel Doc 2000 (Bio-Rad Laboratories, Richmond, CA). Statistical analyses were performed by means of the paired Student t test.
Introduction of peptides and proteins into platelets
To load specific antibodies, peptides, or proteins into platelets prior to adhesion to Fg-coated glass slides, DMSO was used as previously described with minor modifications.30 Aliquots of PRP (6 μL) or washed platelet suspensions (6 μL) containing approximately 1.8 × 106 platelets were diluted in Tyrode buffer (87 μL), and pulsed with 1 μL DMSO in the presence of indicated proteins or peptides, or a combination of the two, every 20 seconds for 2 minutes at room temperature to a final DMSO concentration of 6%. The mixture was incubated for 10 minutes at room temperature and then diluted 5-fold by plating on a cover-glass slide chamber containing 500 μL Tyrode buffer. Platelets were observed occasionally under a phase-contrast microscope to ensure spreading. After 45 minutes to 1 hour of incubation, platelets were fixed and processed for immunofluorescence as described.
Spreading restoration assay
An aliquot of 15 μL platelet suspension containing 1.5 × 107 platelets was added onto an Fg-precoated cover-slide chamber containing 250 μL Tyrode buffer. Control platelets were allowed to spread for 45 minutes at 37°C. In one set of experiments, platelets were allowed to spread on Fg in the presence of 1 μM 2MeSAMP, of 1 μM MRS 2179 to inhibit ADP receptors, or of 1 U/mL apyrase to scavenge secreted ADP. In another set of experiments, platelets were allowed to spread for 45 minutes on Fg and then treated with apyrase for 20 minutes. In certain experiments, platelets were first allowed to spread on Fg in the presence of apyrase for 45 minutes and then washed 2 times with Tyrode buffer. ADP (0 to 20 μM) was then added, and platelets were incubated further for 20 minutes. UN7.79- or GPIIb peptide–loaded platelets were allowed to attach on Fg for 45 minutes, treated with 0 to 20 μM ADP, and then allowed to recover for 20 minutes. Although a range of ADP concentrations was tested, some spreading was restored at concentrations as low as 0.2 μM. After these indicated treatments, platelets were fixed and processed for immunofluorescence as described above.
Results
CIB redistributes as platelets spread on immobilized Fg
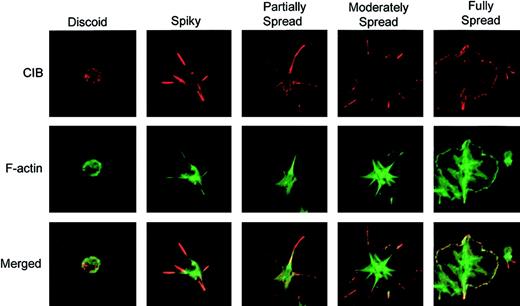
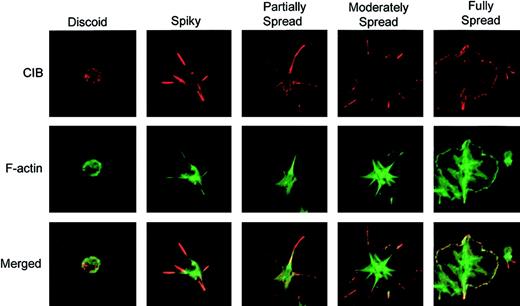
To investigate the role of CIB in platelet spreading, platelets were allowed to attach to immobilized Fg for various amounts of time up to 45 minutes, and then immunostained to observe CIB distribution. CIB was found to localize diffusely along the membrane of discoid platelets (Figure 1; discoid). However, as adhesion to Fg occurred, distinct filopodial extensions were formed, and the majority of CIB was accumulated at these filopodial extensions (Figure 1; spiky). As adhesion continued, filopodia evolved into lamellipodia, resulting in partially spread platelets, and CIB became redistributed from the filopodia to the edges of lamellipodia (Figure 1; partially spread). When platelets were allowed to spread maximally, CIB was localized along the membrane (Figure 1; fully spread). These observations demonstrate that CIB changes its cellular distribution upon platelet attachment to Fg, and is localized to defined areas as platelets undergo different stages of spreading. Interestingly, during these stages of platelet spreading, CIB was found to colocalize with cortical F-actin (Figure 1; merged).
Change in CIB localization as platelets undergo morphologic changes on immobilized Fg. Immunofluorescence staining of washed platelets that were allowed to spread on Fg for various periods of time. Platelets were then fixed and immunostained for CIB (red; upper row) and F-actin (green; middle row). The bottom row shows the merged confocal image. Images are representative of 4 different progressive stages of platelet spreading on Fg. Original magnification, × 1600.
Change in CIB localization as platelets undergo morphologic changes on immobilized Fg. Immunofluorescence staining of washed platelets that were allowed to spread on Fg for various periods of time. Platelets were then fixed and immunostained for CIB (red; upper row) and F-actin (green; middle row). The bottom row shows the merged confocal image. Images are representative of 4 different progressive stages of platelet spreading on Fg. Original magnification, × 1600.
CIB associates with GPIIb/IIIa during platelet adhesion
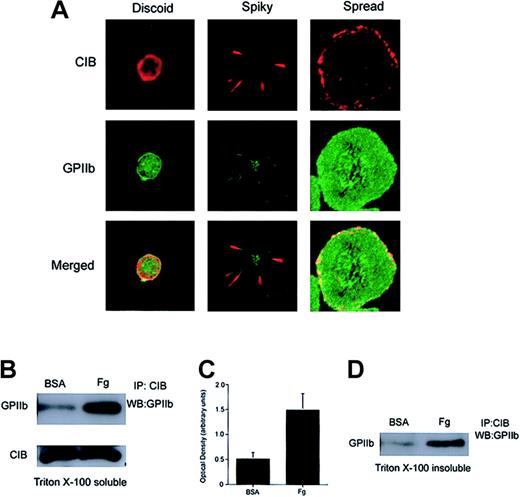
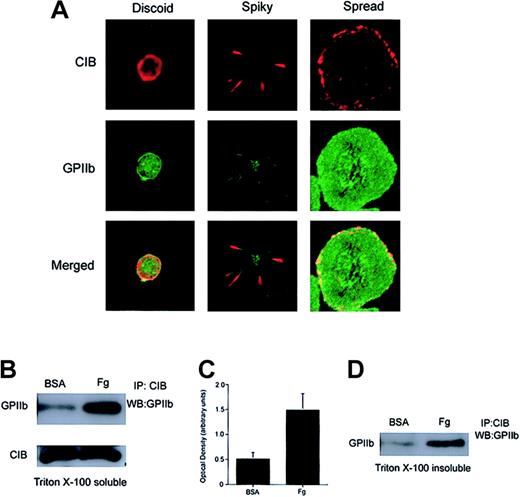
Because CIB is a GPIIb cytoplasmic domain–interacting protein27 and has subsequently been shown to be involved in GPIIb/IIIa activation,31 it is conceivable that CIB binds GPIIb/IIIa at the onset of platelet activation, and that both proteins are then coordinately involved in the mechanisms leading to platelet spreading on Fg. To determine the relationship between CIB and GPIIb/IIIa during outside-in signaling, we first studied the spatial distribution of the 2 proteins with respect to each other during the event of platelet spreading. We found that both CIB and GPIIb/IIIa were originally present at the membrane of discoid platelets (Figure 2A; discoid). As platelets become spiky with numerous filopodia, CIB and GPIIb colocalize at the filopodia (Figure 2A; spiky). Note that GPIIb is also diffusely present in trace amounts on the rest of the platelet membrane (Figure 2A; spiky). However, after full platelet spreading, CIB redistributes diffusely along the membrane periphery, where it distinctly colocalizes with GPIIb/IIIa (Figure 2A; spread).
Association of CIB with GPIIb upon platelet adhesion to immobilized Fg. (A) Confocal images of washed platelets that were fixed at various stages of platelet spreading on Fg. Platelets were then stained for CIB (red) and GPIIb (green). The bottom row shows the merged images. Original magnification, × 1600. (B) Western blot analysis of CIB immunoprecipitates from detergent extracts of platelets plated on BSA or Fg. The majority of CIB interacts with GPIIb only after platelet adhesion to Fg. (C) Densitometric analysis of panel B, indicating that significant amounts of CIB and GPIIb/IIIa associate when platelets spread on Fg (P < .05). Error bars indicate mean ± sem of at least 3 independent experiments. (D) Western blot analysis of CIB immunoprecipitates from the RIPA buffer–solubilized Triton X-100–insoluble pellet of platelets plated on BSA or Fg. CIB and GPIIb/IIIa are associated with each other upon Fg binding, and this association is not disrupted upon translocation to the platelet cytoskeleton.
Association of CIB with GPIIb upon platelet adhesion to immobilized Fg. (A) Confocal images of washed platelets that were fixed at various stages of platelet spreading on Fg. Platelets were then stained for CIB (red) and GPIIb (green). The bottom row shows the merged images. Original magnification, × 1600. (B) Western blot analysis of CIB immunoprecipitates from detergent extracts of platelets plated on BSA or Fg. The majority of CIB interacts with GPIIb only after platelet adhesion to Fg. (C) Densitometric analysis of panel B, indicating that significant amounts of CIB and GPIIb/IIIa associate when platelets spread on Fg (P < .05). Error bars indicate mean ± sem of at least 3 independent experiments. (D) Western blot analysis of CIB immunoprecipitates from the RIPA buffer–solubilized Triton X-100–insoluble pellet of platelets plated on BSA or Fg. CIB and GPIIb/IIIa are associated with each other upon Fg binding, and this association is not disrupted upon translocation to the platelet cytoskeleton.
To more precisely determine the association of CIB with GPIIb/IIIa, we allowed washed platelets to maximally adhere to Fg-coated dishes. BSA-coated dishes were used as a control. Extracts of platelets from these dishes were then subjected to CIB immunoprecipitation, after which the immunoprecipitate was probed with anti-GPIIb antibody. We found that CIB coimmunoprecipitated a substantial amount of GPIIb from extracts of platelets spread on Fg (Figure 2B). Note that a small amount of GPIIb was immunoprecipitated from platelets incubated on BSA. This small amount of GPIIb/CIB association could arise from unintentional activation during washing. This difference, however, is not due to a difference in the amount of CIB immunoprecipitation, as CIB levels were similar in both samples (Figure 2B). In fact, it appears as though the majority of CIB that was immunoprecipitated interacts with GPIIb when platelets are exposed to Fg as compared with BSA. Densitometric analysis indicated that significant amounts (P < .05) of CIB and GPIIb associate with each other after platelet adhesion to Fg as compared with BSA (Figure 2C). These results are consistent with the colocalization data suggesting that CIB predominantly associates with GPIIb in spread platelets.
We have previously shown that CIB is entirely present in the Triton X-100–soluble fraction of unactivated platelets.28 Upon platelet aggregation, however, CIB translocates to the cytoskeletal fraction (Triton X-100–insoluble fraction), which parallels the translocation of GPIIb/IIIa.28 To determine if CIB and GPIIb remain associated after translocation to the cytoskeleton, we performed coimmunoprecipitation experiments from cytoskeletal fractions (Triton X-100–insoluble fractions) of platelets fully spread on immobilized Fg. We found that an increased amount of GPIIb was present in the CIB immunoprecipitate of platelets spread on Fg as compared with the cytoskeleton of platelets exposed to BSA (Figure 2D). Unintentional activation of platelets during preparation may account for the small amount of CIB-GPIIb complex observed in fractions of platelets exposed to BSA. Taken together, these results suggest that upon binding to Fg, GPIIb/IIIa associates with CIB and a portion of this complex associates with the cytoskeleton.
CIB is required for platelet spreading on immobilized Fg
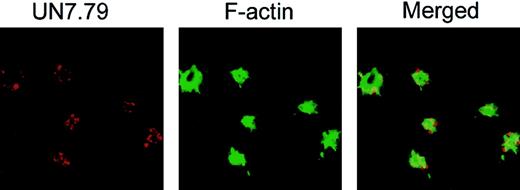
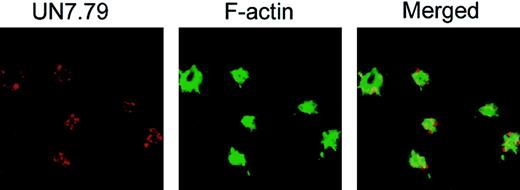
To investigate whether CIB is in fact required for the formation of these dynamic morphologic structures, we sought to determine whether inhibition of CIB by anti-CIB antibody, UN7.79, would affect any of the observed morphologic changes. To achieve this, we first had to develop a method to successfully introduce antibodies inside unactivated platelets before allowing them to spread on Fg. We tested 3 separate techniques previously reported to be successful for protein introduction into platelets (Triton X-100 and DMSO)30,32 and cells (Chariot reagent [Active Motif, Carlsbad, CA]).33 We found that the DMSO method, with certain modifications, was most consistent. Successful antibody introduction inside platelets was determined by introducing UN7.79, which was visualized with TRITC-labeled antimouse secondary antibody under confocal microscopy. FITC-labeled phalloidin staining was used to stain F-actin to determine that the observed TRITC-labeled staining was associated with platelets (Figure 3). Upon optimization of antibody concentration and the time of incubation after treatment, we were able to successfully incorporate a detectable amount of antibody into more than 75% of platelets that attached to Fg. We also observed that although filopodia are present in these platelets, CIB staining was not detected at the filopodia as observed in Figure 1. This could be because the introduction of UN7.79 sequestered CIB and blocked its translocation to the filopodia.
Successful incorporation of antibody into platelets by DMSO treatment. Confocal images of washed platelets loaded with UN7.79 using DMSO as described in “Materials and methods.” Platelets were allowed to spread for 45 minutes, then fixed and stained with secondary antibody to detect UN7.79 (red) or phalloidin to detect F-actin (green). Although filopodia are visible, CIB cannot be detected at the tip of the filopodia owing to antibody inhibition of CIB relocation. Original magnification, × 600.
Successful incorporation of antibody into platelets by DMSO treatment. Confocal images of washed platelets loaded with UN7.79 using DMSO as described in “Materials and methods.” Platelets were allowed to spread for 45 minutes, then fixed and stained with secondary antibody to detect UN7.79 (red) or phalloidin to detect F-actin (green). Although filopodia are visible, CIB cannot be detected at the tip of the filopodia owing to antibody inhibition of CIB relocation. Original magnification, × 600.
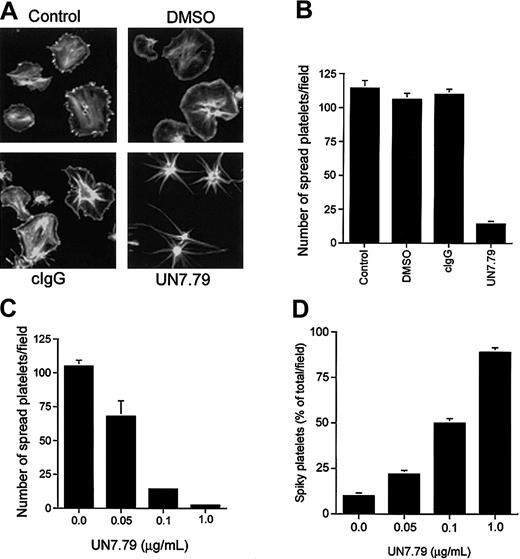
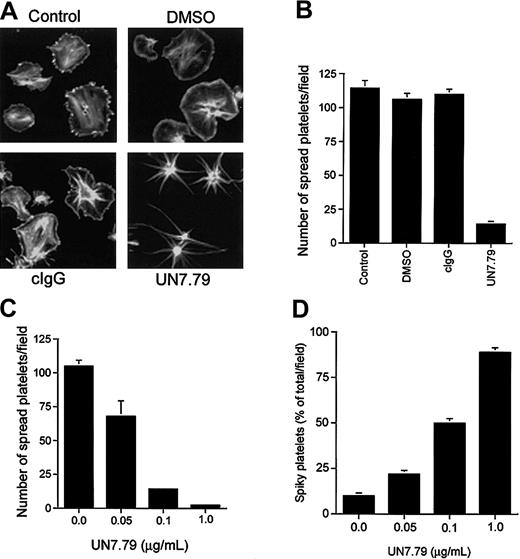
Having developed a means to successfully introduce intact antibody inside resting platelets, we next determined the effect of UN7.79 on platelet spreading on Fg. Generally, untreated platelets spread fully to a pancake-like morphology on Fg within 45 minutes of attachment (Figure 4A; control). Platelets treated with DMSO (vehicle) alone also progressed through characteristic spreading stages (Figure 4A; DMSO). As an additional control, we incorporated an irrelevant antimouse antibody (cIgG) before exposure to Fg, which also had no effect on platelet spreading (Figure 4A; cIgG). However, when UN7.79 was introduced prior to plating on Fg, platelets failed to spread to the typical pancake-like morphology, even after 1 hour of attachment (Figure 4A; UN7.79). In fact, these platelets progressed only from a discoid to a spherical morphology with numerous filopodia, suggesting that CIB may be required for lamellipodia formation leading to platelet spreading. A polyclonal antibody generated against CIB had the same effect (data not shown). Quantitation of the effect of UN7.79 on platelet spreading revealed that about 95% of the attached platelets failed to fully spread as compared with control, DMSO-treated, or cIgG-loaded platelets (Figure 4B). In addition, UN7.79 dose-dependently inhibited a fully spread platelet morphology, as the number of spread platelets amounts to about 5% of attached platelets at the highest tested concentration of UN7.79 (Figure 4C). Conversely, the number of platelets arrested at a filopodial, spiky morphology, increased in a UN7.79 dose–dependent manner (Figure 4D). Taken together, these data suggest that CIB may be required for the transition from spiky to spread platelet morphologies.
Inhibition of platelet spreading by incorporation of UN7.79. (A) Confocal images of washed platelets allowed to spread on Fg for 45 minutes and stained for F-actin. Untreated platelets (control), platelets treated with DMSO alone (DMSO), and platelets incorporated with a control IgG (cIgG) spread fully. Platelets loaded with UN7.79 showed long filopodia. Original magnification, × 600. (B) Quantitation of the number of fully spread platelets in panel A. (C) Dose-dependent inhibition of the number of fully spread platelets by UN7.79 incorporation. (D) Corresponding dose-dependent increase of the number of spiky platelets by UN7.79 incorporation. Error bars indicate mean ± SEM of at least 3 independent experiments.
Inhibition of platelet spreading by incorporation of UN7.79. (A) Confocal images of washed platelets allowed to spread on Fg for 45 minutes and stained for F-actin. Untreated platelets (control), platelets treated with DMSO alone (DMSO), and platelets incorporated with a control IgG (cIgG) spread fully. Platelets loaded with UN7.79 showed long filopodia. Original magnification, × 600. (B) Quantitation of the number of fully spread platelets in panel A. (C) Dose-dependent inhibition of the number of fully spread platelets by UN7.79 incorporation. (D) Corresponding dose-dependent increase of the number of spiky platelets by UN7.79 incorporation. Error bars indicate mean ± SEM of at least 3 independent experiments.
Both CIB and GPIIb/IIIa are required to achieve a fully spread morphology
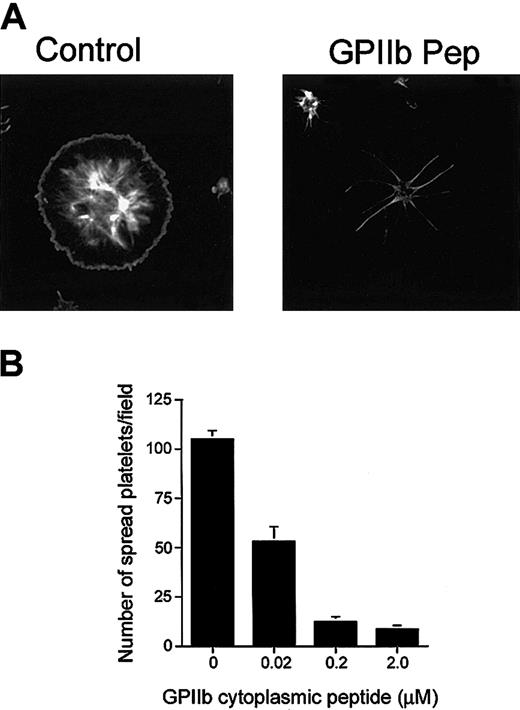
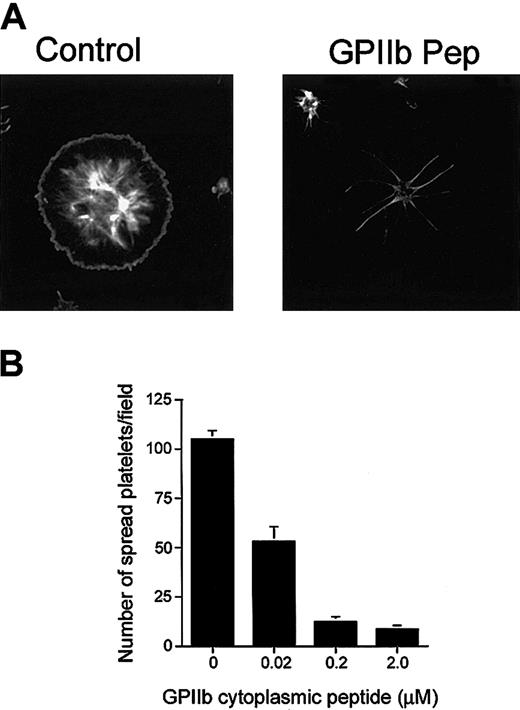
Because CIB interacts with GPIIb/IIIa during outside-in signaling, it is possible that formation of this complex is required for the process of platelet spreading. With this reasoning, it should therefore be possible to inhibit platelet spreading on Fg by introducing excess amounts of GPIIb cytoplasmic domain peptide into platelets to competitively inhibit the interaction of CIB with GPIIb/IIIa. When synthetic peptide corresponding to the GPIIb cytoplasmic domain was introduced into platelets by DMSO treatment, we found that platelet spreading was blocked before lamellipodia formation (Figure 5A), a morphology similar to that observed when UN7.79 was used (Figure 4A). At the same time, however, platelet adhesion to Fg was not affected. A scrambled GPIIb cytoplasmic domain peptide had no effect on platelet spreading (data not shown). The inhibition of platelet spreading was observed to be dose dependent, as the number of spread platelets decreased as the concentration of GPIIb peptide introduced increased (Figure 5B). Accordingly, the reverse was also found to be true, in that the number of spiky platelets increased upon a GPIIb peptide concentration increase (data not shown). These results can be interpreted to mean that introduction of GPIIb peptide sequesters available CIB, keeping it from forming the CIB-GPIIb/IIIa complex, and thereby suggesting that formation of the CIB-GPIIb/IIIa complex is necessary for the downstream signaling needed for progression of platelet spreading beyond the spiky filopodial stage.
Inhibition of platelet spreading by incorporation of GPIIb peptide. (A) Confocal images of washed platelets allowed to spread on Fg for 45 minutes and stained for F-actin. Platelets treated with DMSO alone (control) spread fully. Platelets loaded with GPIIb cytoplasmic domain peptide (GPIIb Pep) showed long filopodia. Original magnification, × 600. (B) Dose-dependent inhibition of the number of fully spread platelets by GPIIb cytoplasmic peptide incorporation. Error bars indicate mean ± sem of at least 3 independent experiments.
Inhibition of platelet spreading by incorporation of GPIIb peptide. (A) Confocal images of washed platelets allowed to spread on Fg for 45 minutes and stained for F-actin. Platelets treated with DMSO alone (control) spread fully. Platelets loaded with GPIIb cytoplasmic domain peptide (GPIIb Pep) showed long filopodia. Original magnification, × 600. (B) Dose-dependent inhibition of the number of fully spread platelets by GPIIb cytoplasmic peptide incorporation. Error bars indicate mean ± sem of at least 3 independent experiments.
Introduction of recombinant CIB protein into platelets overrides GPIIb cytoplasmic peptide–induced blockade of platelet spreading
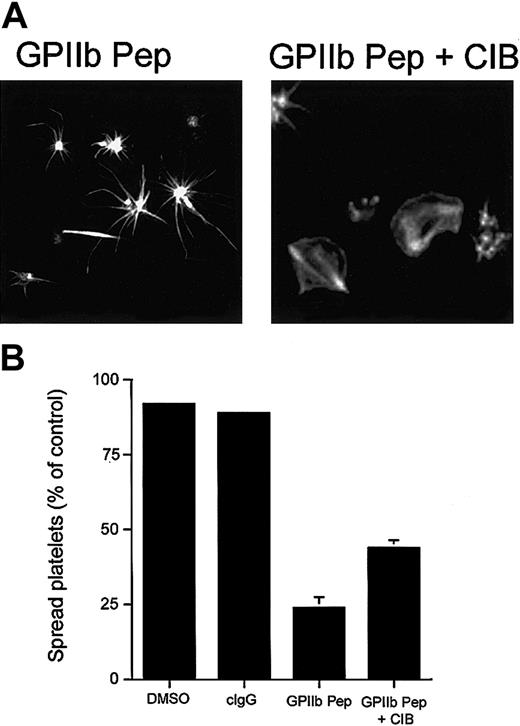
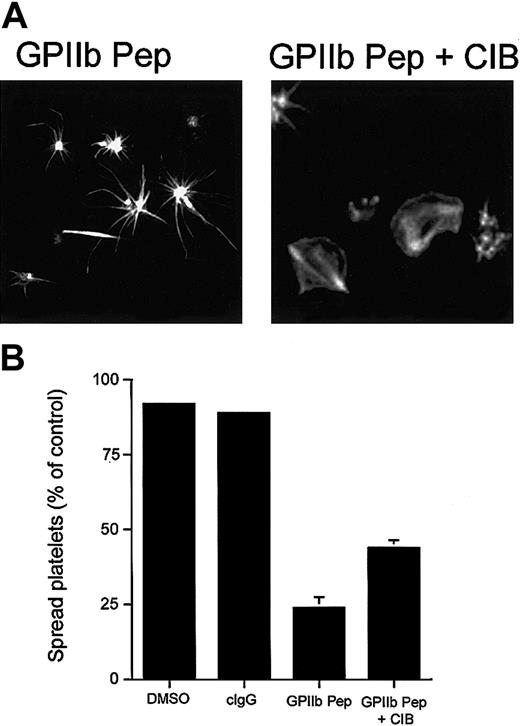
To further establish the role of the CIB-GPIIb/IIIa complex in platelet spreading, we performed a gain-of-function experiment using recombinant CIB protein. We reasoned that a spread-platelet morphology should be able to be rescued by introducing molar excess of recombinant CIB into platelets along with GPIIb cytoplasmic domain peptide. As expected, introduction of recombinant CIB alone did not affect platelet spreading (data not shown), and as before, introduction of GPIIb peptide into platelets arrested them in a spiky morphology (Figure 6A; GPIIb Pep). However, after introduction of recombinant CIB protein along with GPIIb peptide, platelets were able to spread on Fg (Figure 6A; GPIIb Pep + CIB). Although spreading was not recovered in 100% of platelets, the inclusion of recombinant CIB did enhance the number of spread platelets 2-fold as compared with the number of spread platelets in its absence (Figure 6B).
Effect of incorporation of CIB protein and GPIIb peptide. Incorporation of CIB protein along with GPIIb peptide allows platelets to spread. (A) Confocal images of washed platelets allowed to spread on Fg for 45 minutes and stained for F-actin. Platelets loaded with GPIIb cytoplasmic peptide (GPIIb Pep) showed long filopodia. Platelets loaded with GPIIb peptide and recombinant CIB protein (GPIIb Pep + CIB) recovered to a spread platelet morphology. Original magnification, × 600. (B) Quantitation of the percentage of spread platelets in panel A. Error bars indicate mean ± sem of at least 3 independent experiments.
Effect of incorporation of CIB protein and GPIIb peptide. Incorporation of CIB protein along with GPIIb peptide allows platelets to spread. (A) Confocal images of washed platelets allowed to spread on Fg for 45 minutes and stained for F-actin. Platelets loaded with GPIIb cytoplasmic peptide (GPIIb Pep) showed long filopodia. Platelets loaded with GPIIb peptide and recombinant CIB protein (GPIIb Pep + CIB) recovered to a spread platelet morphology. Original magnification, × 600. (B) Quantitation of the percentage of spread platelets in panel A. Error bars indicate mean ± sem of at least 3 independent experiments.
Association of CIB with GPIIb/IIIa may be necessary for granular secretion of ADP, leading to platelet spreading
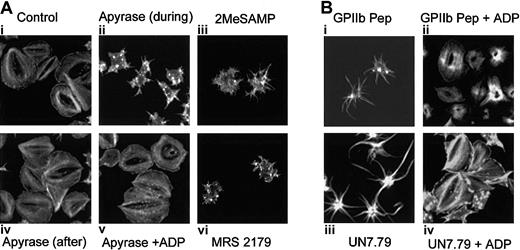
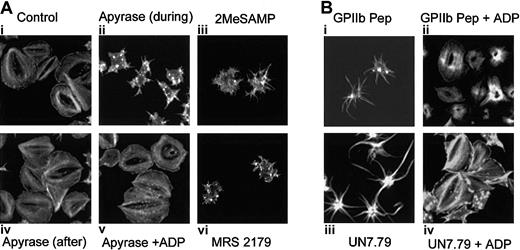
It has been shown that platelet granular secretion of ADP is responsible for platelet spreading.34 In fact, platelets in which ADP secretion is blocked exhibit a spiky morphology,17,19,20 similar to the morphology we observe when formation of the CIB-GPIIb/IIIa complex is inhibited. We thus hypothesized that binding of CIB to GPIIb/IIIa may play a role in platelet secretion of ADP. To better understand the mechanism by which the CIB-GPIIb/IIIa complex induces platelet spreading, we analyzed the effect of secreted ADP on the spreading process by allowing platelets to spread on Fg in the presence of the ADP scavenger, apyrase. As compared with control (Figure 7Ai), the presence of apyrase during adhesion to Fg blocked a fully spread platelet morphology (Figure 7Aii), consistent with previous reports.17,19 Interestingly, although these platelets showed distinct filopodia, they did not appear as spiky as those observed when UN7.79 or GPIIb peptide was introduced. This could be due to the fact that apyrase will scavenge only ADP, leaving numerous other secreted agonists unaffected. Addition of apyrase to platelets already spread on Fg had no effect on their spread morphology (Figure 7Aiv). However, when platelets were allowed to adhere to Fg in the presence of apyrase, then washed to remove apyrase and treated with exogenous ADP, a spread morphology was rescued (Figure 7Av), suggesting that secreted ADP may be responsible for platelet spreading. To more convincingly demonstrate this point, known ADP receptor antagonists were used. Platelets failed to fully spread in the presence of P2Y1 and P2Y12 receptor antagonists (Figure 7Aiii and 7Avi, respectively), and exhibited a morphology similar to that observed after apyrase treatment.
Relationship of the CIB-GPIIb/IIIa complex to platelet granular secretion of ADP. Formation of the CIB-GPIIb/IIIa complex may be necessary for platelet granular secretion of ADP. (A) Confocal images of washed platelets allowed to spread on Fg and stained for F-actin. Ai: Untreated platelets are fully spread. Aii: Apyrase is present during platelet adhesion. Aiv: Apyrase was added to spread platelets. Av: Platelets were allowed to spread in the presence of apyrase. After 45 minutes, platelets were washed free of apyrase and exogenous ADP was added. ADP receptor P2Y12 antagonist, 2MeSAMP (Aiii) or P2Y1 antagonist MRS 2179 (Avi), is present during platelet adhesion to Fg. (B) Confocal images of washed platelets allowed to spread on Fg and stained for F-actin. Bi: Platelets loaded with GPIIb cytoplasmic peptide (GPIIb Pep) showed filopodia. Bii: Exogenous ADP was added to GPIIb Pep-loaded platelets. Biii: Platelets loaded with UN7.79 showed long filopodia. Biv: Exogenous ADP was added to UN7.79-loaded platelets. Original magnification, × 600.
Relationship of the CIB-GPIIb/IIIa complex to platelet granular secretion of ADP. Formation of the CIB-GPIIb/IIIa complex may be necessary for platelet granular secretion of ADP. (A) Confocal images of washed platelets allowed to spread on Fg and stained for F-actin. Ai: Untreated platelets are fully spread. Aii: Apyrase is present during platelet adhesion. Aiv: Apyrase was added to spread platelets. Av: Platelets were allowed to spread in the presence of apyrase. After 45 minutes, platelets were washed free of apyrase and exogenous ADP was added. ADP receptor P2Y12 antagonist, 2MeSAMP (Aiii) or P2Y1 antagonist MRS 2179 (Avi), is present during platelet adhesion to Fg. (B) Confocal images of washed platelets allowed to spread on Fg and stained for F-actin. Bi: Platelets loaded with GPIIb cytoplasmic peptide (GPIIb Pep) showed filopodia. Bii: Exogenous ADP was added to GPIIb Pep-loaded platelets. Biii: Platelets loaded with UN7.79 showed long filopodia. Biv: Exogenous ADP was added to UN7.79-loaded platelets. Original magnification, × 600.
We next asked whether the addition of exogenous ADP to platelets that were arrested in a filopodial morphology by the introduction of UN7.79 or GPIIb peptide could rescue a fully spread morphology on Fg. As before, GPIIb peptide–incorporated platelets showed spiky filopodia but failed to spread (Figure 7Bi), but addition of exogenous ADP to these platelets allowed them to spread completely (Figure 7Bii). Similarly, platelets arrested in a filopodial stage by incorporation of UN7.79 (Figure 7Biii) readily acquired a fully spread morphology after ADP treatment (Figure 7Biii). It should be noted that in some cases, platelets achieve a clam-like, rather than a pancake-like, morphology. However, in a given experiment, the recovered platelet morphology resembled that of control platelets; thus, we classified both morphologies as spread. The differences between these appearances could simply be due to F-actin organization at the time of fixation and staining. Taken together, these data suggest that the association of CIB with GPIIb/IIIa during outside-in signaling may lead to granular secretion of ADP that is required for complete spreading of platelets.
Discussion
Of the data presented here that we found most intriguing was the dynamic cellular redistribution of CIB during platelet spreading on immobilized Fg. This specific distribution pattern suggests that CIB may be involved in regulating multiple events that occur during this process. This may be particularly true considering the ability of CIB to interact with a number of diverse signaling molecules.35-42 Additionally, since CIB is a Ca2+-binding protein having similarity to calmodulin and calcineurin B,27 it is therefore possible that CIB may interact with different signaling proteins in a Ca2+-dependent manner.
CIB was originally isolated by the yeast 2-hybrid system with use of the cytoplasmic domain of GPIIb as bait.27 Subsequently it has been shown that CIB specifically interacts with the GPIIb/IIIa complex.27,43 However, the physiological relevance of this interaction has not been well understood. It was recently reported that CIB is capable of activating GPIIb/IIIa to bind soluble Fg with the use of an in vitro binding assay.31 This report also showed that introduction of a peptide corresponding to the C-terminus of CIB into platelets can block ADP-induced platelet activation. The author thus concluded that binding of CIB to the cytoplasmic domain of GPIIb is sufficient for the inside-out signaling leading to the activation of GPIIb/IIIa.31 However, human erythroleukemic (HEL) cells, which express both CIB and GPIIb/IIIa endogenously, show no activation of GPIIb/IIIa.44,45 Similarly, overexpression of CIB in Chinese hamster ovary (CHO) cells expressing GPIIb/IIIa has no effect on inside-out signaling (U.P.N. et al, unpublished data, January 2002). This does not mean that CIB is not involved in inside-out signaling, but it may require additional platelet-specific factors to do so. On the other hand, it has also been shown that CIB interacts only with activated GPIIb/IIIa,43 and that CIB translocates to the cytoskeleton upon platelet activation and parallels the translocation of GPIIb/IIIa.28 Additionally, the translocation of CIB and GPIIb/IIIa is dependent upon Fg binding to activated GPIIb/IIIa.28 These data thus support the converse idea that CIB may interact with GPIIb/IIIa during outside-in signaling. Immunoprecipitation experiments performed at different stages of platelet activation would be helpful to delineate the stages of platelet activation at which CIB and GPIIb/IIIa interact; however these experiments have been unsuccessful thus far in platelets.28 Here we report for the first time the immunoprecipitation of CIB with GPIIb/IIIa from platelets. We find that the association of CIB with GPIIb/IIIa is dependent upon platelet adhesion to immobilized Fg and that the complex remains bound upon translocation to the cytoskeleton. Thus, our results are consistent with idea that CIB may be involved in outside-in signaling.
To conclusively determine the function of the CIB-GPIIb/IIIa complex in outside-in signaling, we felt it necessary to introduce complex disrupting agents inside platelets. Since platelets are small, anucleated cells that can be neither transfected nor injected, it is difficult to study protein overexpression or antibody loading in these cells by conventional methods. Previously, DMSO has been used to load platelets with aequorin,30 and recently, Triton X-100 has been successfully used to introduce anti–actin-related protein 2 (anti-Arp2) antibody into platelets.32 In our hands, although Triton X-100 allowed for successful introduction of UN7.79, there was substantial loss of platelets as a result of this treatment. We found the DMSO method to be much more reliable and reproducible with no detectable platelet loss. This method was particularly useful in the introduction of various sizes of molecules, ranging from small peptides (approximately 2 kDa; GPIIb cytoplasmic domain peptide) to small proteins (approximately 20 kDa; CIB) and large proteins (approximately 150 kDa; UN7.79).
The changes in CIB localization as a result of adhesion to immobilized Fg suggest that signaling through GPIIb/IIIa is responsible for this effect. Because binding of CIB to GPIIb requires Ca2+, CIB may interact with GPIIb as a result of the Ca2+ rise that occurs during outside-in signaling.28,31,43 Additionally, our results suggest that interaction of CIB with the GPIIb cytoplasmic domain is important in the progression of platelet spreading from filopodia to lamellipodia. We cannot, however, rule out the possibility that UN7.79 or GPIIb peptide may also interfere with the interaction of CIB with other signaling molecules at this time. Nonetheless, the fact that CIB binds GPIIb/IIIa during spreading and remains bound while the 2 translocate to cytoskeleton, as indicated by coimmunoprecipitation experiments, suggests that this association is of primary importance for platelet spreading.
Our results using apyrase or ADP receptor antagonists suggest that secreted ADP is necessary for platelet spreading and that introduction of agents that block CIB-GPIIb/IIIa complex formation blocks this spreading. These findings further imply that CIB binding to the GPIIb cytoplasmic domain may be upstream of platelet granular secretion. This notion is consistent with the report in which platelets with a naturally occurring genetic mutation that results in GPIIIa cytoplasmic domain deletion adhere to immobilized Fg and secrete granular contents, but do not spread upon activation by LSARLAF peptide.46 The results in that report suggest that the GPIIb cytoplasmic domain is sufficient to propagate signaling events that lead to granular secretion, whereas the GPIIIa cytoplasmic domain is needed for events that take place independently of, or after, secretion, leading to platelet spreading.46 However, it is also just as likely that the secretion is unrelated to the interaction of CIB with GPIIb/IIIa, occurring as a parallel event, and that blockade of either one can affect platelet spreading. In fact, such coordinated signaling has been shown to occur in platelets. Recently, it has been shown that coordinated signaling from GPIIb/IIIa (outside-in) and ADP receptors is necessary for thromboxane A2 release.47 Future study of the effect of CIB-GPIIb/IIIa complex blockade on platelet secretion will be useful for further understanding of this mechanism.
In addition to GPIIb, CIB has also been shown to interact with polo-like kinase (Plk) family members, Plk2/serum-inducible kinase (Snk) and Plk3/fibroblast growth factor–inducible kinase (Fnk).37 It has been shown that expression of Plk3 is up-regulated during adhesion of monocytes to fibronectin through outside-in signaling.38 However the importance of this interaction in the process of platelet spreading remains to be determined.
Recently, CIB has also been shown to interact with Rac3, but not Rac1.40 This interaction has been shown to promote fibroblast adhesion and spreading.40 The interaction of CIB with Rac3 is of particular relevance to platelet spreading since Cdc42/Rac1–dependent activation of the p21-activated kinase (PAK) has been shown to regulate platelet shape change resulting from inside-out signaling induced by thrombin.48 Because Rac1 does not interact with CIB, it appears unlikely that CIB is involved in the Cdc42/Rac1–dependent activation of PAK. However, the role of Rac3 in outside-in signaling, if any, remains to be determined.
Taken together, our results enhance the present understanding of the process of platelet spreading. Previous studies show that GPIIb/IIIa binding to immobilized Fg results in the initiation of outside-in signaling that involves dissociation of Csk from the GPIIIa cytoplasmic domain and the activation of Syk by Src.12 This leads to intracellular Ca2+ rise, cytoskeletal rearrangement, and granular secretion. Our results are consistent with this idea and further extend the signaling steps to include the Ca2+-dependent binding of CIB to the GPIIb cytoplasmic domain,28 which may be required for granular secretion of ADP. Binding of secreted ADP to its receptor then induces further signaling events that may include activation of FAK, PAK, and/or PI3-K. These signaling events then cause the cytoskeletal rearrangement needed for lamellipodia formation and platelet spreading.
Prepublished online as Blood First Edition Paper, April 24, 2003; DOI 10.1182/blood-2003-02-0591.
Supported by grants (to U.P.N.) from the National Institutes of Health (HL57630) and the National Center for Research Resources (1P20RR155801).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Dr K. Czymmek for his excellent help with confocal microscopy, C. Blamey for providing recombinant CIB, and K. Eckfeld for help in preparing the manuscript.