Abstract
Second-line chemotherapy followed by high-dose therapy (HDT) with autologous stem cell transplantation (ASCT) cures less than half of the patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL). Prognostic models capable of predicting outcome are essential. In 3 sequential clinical trials, conducted from January 1993 to August 2000, we treated 150 patients with relapsed or primary refractory DLBCL with ifosfamide, carboplatin, and etoposide (ICE) chemotherapy followed by HDT/ASCT for patients with chemosensitive disease. We evaluated the age-adjusted International Prognostic Index at the initiation of second-line therapy (sAAIPI) as a predictor of progression-free survival (PFS) and overall survival (OS). At a median follow-up of 4 years, the PFS and OS are 28% and 34% by intention to treat and 39% and 45% for only those patients with chemosensitive disease. Three risk groups with different PFS and OS were identified by the sAAIPI: low risk (0 factors), 70% and 74%; intermediate risk (1 factor), 39% and 49%; and high risk (2 or 3 factors), 16% and 18% (P < .001 for both PFS and OS). The sAAIPI also predicts the PFS and OS for patients with ICEchemosensitive disease: low risk, 69% and 83%; intermediate risk, 46% and 55%; and high risk, 25% and 26% (P < .001 PFS and OS). The sAAIPI predicts outcome for patients with relapsed or primary refractory DLBCL in both intent-to-treat and chemosensitive populations. This powerful prognostic instrument should be used to evaluate new treatment approaches and to compare results of different regimens.
Introduction
Initial therapy of diffuse large B-cell lymphoma (DLBCL) with anthracycline-containing combination chemotherapy regimens cures approximately 40% to 50% of patients. However, 50% to 60% of patients either will be refractory to initial therapy or will relapse from a clinical complete response.1 A prospective randomized trial has determined that consolidation therapy with high-dose chemotherapy (HDT) followed by autologous stem cell transplantation (ASCT) is the most successful therapeutic approach for patients with chemosensitive relapsed DLBCL.2 With the use of this approach, 5-year event-free-survival (EFS) of 40% to 45% can be expected for those patients undergoing ASCT. We have reported similar results for patients with primary refractory aggressive lymphoma who demonstrate chemosensitivity to second-line treatment.3
The major limitation of HDT/ASCT is that a significant percentage of patients develop disease recurrence after completion of therapy. Prognostic models capable of predicting durable responses would be extremely helpful in selecting patients for HDT. Initial studies determined that chemosensitivity to conventional dose second-line chemotherapy is a significant predictor of outcome.4,5 Additional factors with demonstrated prognostic value in this setting include masses more than 10 cm, 3 or more chemotherapy regimens prior to ASCT, elevated lactate dehydrogenase (LDH), short time to relapse, and high disease burden.6-12 However, no single prognostic model has achieved widespread acceptance in the relapsed/refractory setting, and the disparate baseline groups used to generate these models have limited their ability to be generalized. Furthermore, because chemosensitivity to conventional-dose second-line chemotherapy is an overwhelming predictor of outcome, most trials of ASCT limit enrollment to this select group of patients. This limitation makes it difficult to predict the overall benefit of HDT/ASCT for patients at the time they present with relapsed or primary refractory disease and practically impossible to identify prognostic factors that may predict overall outcome prior to administering second-line chemotherapy (and demonstrating chemosensitive disease). An ideal prognostic model should be easily applied, identify distinct risk groups, be reflective of the disease biology, and be capable of predicting durable long-term survival prior to the administration of second-line treatment and at the time of disease response.
The International Prognostic Index (IPI) is a validated scoring system predictive of survival in de novo diffuse large cell lymphoma (DLCL).13 In addition, when the IPI was retrospectively applied at relapse to 215 patients with intermediate-grade non-Hodgkin lymphoma (NHL) or high-grade NHL included in the PARMA trial, it could distinguish patients with significantly different response rates and overall survival (OS) when treated with salvage chemotherapy.14 The age-adjusted IPI (aaIPI) comprises 3 factors (performance status, LDH, and stage) that also predict survival in de novo DLCL.13 It has not been extensively evaluated as a prognostic tool in the relapsed/primary refractory setting.
We developed the ICE (ifosfamide, carboplatin, etoposide) chemotherapy regimen to deliver effective pretransplantation cytoreduction with minimal nonhematologic toxicity and effective stem cell mobilization.8 In our initial intent-to-treat trial of 51 patients with DLBCL or peripheral T-cell lymphoma, the second-line IPI (sIPI) prospectively evaluated at the time of relapse or primary refractory disease predicted failure-free survival (FFS) (sIPI 1/2 versus 3/4 = 45% versus 9%, P < .001).15 Furthermore, we have demonstrated that the second-line AAIPI (sAAIPI) is predictive of survival for patients with primary refractory aggressive lymphoma.3 We now report the analysis of 150 patients with DLBCL treated with uniform second-line ICE chemotherapy followed by HDT/ASCT for patients with chemosensitive disease, demonstrating that the sAAIPI is easily applied and is an excellent predictor of progression-free survival (PFS) and OS in both intent-to-treat and chemosensitive patients.
Patients and methods
Patients
Two hundred twenty-two patients eligible for transplantation and who gave consent were enrolled on 3 consecutive institutional review board (IRB)–approved protocols for relapsed or primary refractory aggressive lymphoma at Memorial Sloan-Kettering Cancer Center (MSKCC) between 12 October 1993 and 1 August 2000. All procedures were in accordance with the Helsinki protocol. One hundred fifty of these patients had DLBCL, and their results are reported in this analysis. Forty-five of these patients were included in a previous analysis that described the ICE-based regimen.15
Eligibility for second-line therapy
All patients were staged according to the Cotswold modification of the Ann Arbor system.16 Histologic review of the original and pre-ICE biopsy specimens was performed by MSKCC hematopathologists. Histopathology was initially classified according to the International Working Formulation,17 with subsequent retrospective classification according to the World Health Organization/Revised European-American Lymphoma (WHO/REAL) system.18 Patients with transformed lymphoma (low-grade lymphoma at initial diagnosis with intermediate grade or immunoblastic lymphoma at relapse) or discordant histology (presenting originally with both low-grade and either intermediate-grade or immunoblastic lymphoma) were eligible. All patients had biopsy confirmation of relapsed or primary refractory diffuse large B-cell lymphoma prior to the initiation of ICE-based chemotherapy and had previously received only one anthracycline-based chemotherapy program for DLBCL. Additional eligibility criteria required patients to have normal baseline cardiac function (left ventricular ejection fraction > 50%), bidimensionally measurable disease, and a serum creatinine of 132.6 μmol/L (1.5 mg/dL) or less (or creatinine clearance of 1 mL/second or more [60 mL/minute]).
Eligibility for high-dose therapy and autologous stem cell transplantation
Patients were eligible for HDT/ASCT if a bone marrow biopsy revealed adequate cellularity and no involvement with large cell lymphoma at the conclusion of ICE-based chemotherapy. Patients with small cleaved lymphoid cells in the bone marrow were eligible. Patients had to have adequate pulmonary function defined by a DLCO (diffusing capacity of lung for carbon monoxide) more than 50% of predicted and liver function defined as a serum bilirubin 34.2 μmol/L or less (≤ 2 mg/dL). Furthermore, patients had to have achieved a partial or complete response to ICE-based chemotherapy and have a minimum of 2 × 106 CD34+ cells per kilogram body weight collected by leukapheresis.
A modification of the International Working Group response criteria19 incorporating functional imaging was used. A complete response was defined as no evidence of disease by computerized tomography (CT) scan and a normal functional imaging study (gallium or positron emission tomography [PET]) as documented by restaging approximately 2 weeks after the completion of ICE. A conditional complete response was defined as no clinical signs or symptoms of lymphoma, but residual radiographic abnormalities less than 2 cm that were inaccessible to biopsy and showed at least a 75% regression in size from the original radiographic study with negative functional imaging (gallium or PET). A partial response was defined as more than a 50% decrease in the sum of the products of the diameters of each measurable lesion.
ICE second-line chemotherapy and stem cell mobilization
Three cycles of ICE chemotherapy were administered followed by autologous stem cell collection as previously reported.8
Transplantation-conditioning regimens
One hundred two patients underwent ASCT. Conditioning regimens were determined based on prior radiotherapy and protocol design at the time of transplantation. Fifty-eight patients (57%) received a chemotherapy-only conditioning regimen, whereas 44 patients (43%) received a total body irradiation (TBI)–based conditioning regimen.
Involved field radiotherapy (boost radiotherapy) was administered to 61 patients prior to the high-dose conditioning regimen in patients whose residual disease after ICE-based therapy was limited to 2 or less anatomical regions. After restaging, patients eligible for HDT/ASCT were evaluated for radiotherapy. The following guidelines were used: TBI was only used if patients were younger than 60 years and previously unirradiated, and it was administered in an accelerated fractionation schedule, with a total dose of 12 Gy delivered as 1.5-Gy fractions twice daily for 4 days. Involved field radiotherapy (IFRT) was added to sites of disease measuring 5 cm or more before ICE was started or to sites with residual nodal masses more than 2 cm in size before HDT, if prior dose-limiting radiotherapy had not been administered to those sites. IFRT was given in 1.5-Gy fractions twice daily. For patients receiving TBI, IFRT dose was limited to 18 Gy, with a total dose to the site of 30 Gy after TBI. Patients receiving IFRT and not TBI were treated in 1.5-Gy fractions twice daily to a total dose of 30 Gy. IFRT was administered as an outpatient, with a minimal interval between the 2 daily fractions of 7 hours.
Second-line IPI (sIPI) and age-adjusted IPI (sAAIPI)
The sIPI, assessed prior to the initiation of ICE chemotherapy, comprises 5 risk factors: age older than 60 years, extranodal sites more than 1, LDH more than upper limit of normal, stage III or IV disease, and Karnofsky performance status (KPS) less than 80%. The sAAIPI, assessed prior to the initiation of ICE chemotherapy, comprises 3 of the IPI risk factors: LDH, stage, and KPS. These factors are identical to the age-adjusted prognostic index described by Shipp et al13 in patients with de novo diffuse large cell lymphoma. sAAIPI groups are low risk (L) with zero factors, low-intermediate (LI) risk with 1 factor, high-intermediate risk (HI) with 2 factors, and high risk (H) with all 3 factors present.
Response criteria
CT scans of the chest, abdomen, and pelvis were performed prior to the initiation of ICE and at the completion of ICE to evaluate the extent of disease and response to chemotherapy. Functional imaging (gallium or PET) and bone marrow biopsy were done prior to the initiation of ICE and repeated after ICE if the studies were initially positive. Patients must have no detectable disease by functional imaging to be considered a complete remission (CR).
Statistics: hypothesis and endpoints
The hypothesis of this study was that the sAAIPI would be predictive of survival. The primary endpoints were OS and PFS, defined as the time from initiation of ICE-based second-line therapy until last follow-up or death, and as the time from initiation of ICE-based therapy until the time of disease progression, respectively.
Statistical analysis
Survival analyses were performed using the methods of Kaplan and Meier.20 The log-rank test21 was used to compare the survival distributions for the sAAIPI groups and to compare the survival for relapsed verus refractory status. On the basis of Kaplan-Meier curves for all 4 sAAIPI groups, the high-intermediate and high risk sAAIPI categories are not distinct and may be considered in aggregate, forming 3 risk categories: low risk (L), intermediate risk (LI), and high risk (HI/H). The sAAIPI was applied to all patients, having demonstrated prognostic ability in both the younger than 60 years and 60 years or older age groups in de novo DLCL.13
The following factors, assessed at the time of study entry (pre-ICE chemotherapy), were evaluated as possible prognostic indicators for overall survival: age (< 60 versus ≥ 60 years), Karnofsky performance status (< 80% versus ≥ 80%), LDH (normal versus elevated), number of extra nodal sites (ENS) involved (1 or none versus > 1), and stage (I/II versus III/IV), sIPI (1 versus 2 versus 3 versus 4), and sAAIPI (L versus LI versus HI versus H). Univariate analysis was performed using the log-rank test.21 Factors that were potential predictive of OS (P < .05) were entered into a multivariate analysis using the Cox proportion hazards model.22 All statistical analysis was performed using SPSS version 10.0 (SPSS, Chicago, IL).
Results
Patient characteristics
One hundred fifty (n = 150) patients with relapsed or primary refractory DLBCL were analyzed on the basis of intent to proceed to ASCT. Pre-ICE therapy characteristics of all patients are listed in Table 1. The median age at the initiation of chemotherapy was 49 years (range, 16-68 years). All patients had previously failed an anthracycline-containing first-line regimen; 55% had relapsed disease, and 45% had primary refractory DLBCL. The median follow-up of surviving patients is 4 years (range off therapy, 8-98 months). Twenty-two percent of patients had bone marrow involvement prior to ICE therapy, and 78% had advanced (stage III or IV) disease. Serum lactate dehydrogenase was elevated in 58% of patients.
All 150 patients received ICE second-line chemotherapy. One hundred eight patients (72%) responded to ICE-based second-line chemotherapy: 42 complete responses (28%) and 66 partial responses (44%). Sixty-one patients received involved field radiotherapy (dose, 1500-4500 cGy) to residual disease as described in “Transplantation-conditioning regimens.” The characteristics of patients with chemosensitive disease are summarized in Table 2. Ninety-eight (91%) of the 108 patients with chemosensitive disease underwent ASCT.
The reasons for not proceeding to ASCT include rapid disease progression in the 2- to 4-week interval between post-ICE restaging and planned ASCT in the 7 patients with partial response (PR) to ICE, and patient preference in 3 patients who achieved a CR to ICE. Of these patients, 8 have died of disease, whereas 2 with relapsed DLBCL (both with CR to ICE) remain with no evidence of disease. Four patients with chemoresistant disease to ICE underwent ASCT (total ASCT = 102). Of these 4 patients, 2 received additional chemotherapy after ICE treatment that resulted in chemosensitive disease, 1 patient received a syngeneic transplant, and the fourth underwent transplantation at an outside institution. None of these 4 patients had long-term survival. All patients are included in the analysis.
Treatment outcome
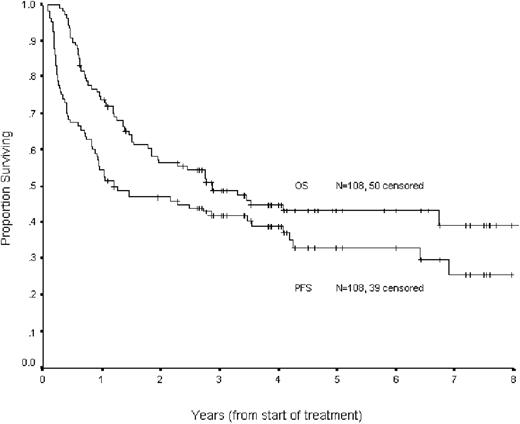
The Kaplan-Meier estimate of the proportion of patients remaining progression free at 4 years following the initiation of the second-line ICE therapy is 28%, with an estimated overall survival at 4 years of 34% (Figure 1). The estimated PFS and OS for patients with chemosensitive disease was 39% and 45%, respectively (Figure 2).
Progression free and overall survival for all 150 patients (intention to treat) receiving ICE chemotherapy.
Progression free and overall survival for all 150 patients (intention to treat) receiving ICE chemotherapy.
Progression free and overall survival for patients with chemosensitive disease (CR or PR to ICE).
Progression free and overall survival for patients with chemosensitive disease (CR or PR to ICE).
For patients achieving a CR to ICE-based chemotherapy compared with those attaining only a partial response, there was a trend toward improved PFS (47.5% versus. 34%, P = .11) and a statistically significant improved OS (57.4% versus 37%, P = .05). Patients who fail ICE-based chemotherapy have a poor prognosis, with a median OS of 4 months.
sIPI and sAAIPI as predictors of outcome
When the components of the sIPI (age > 60 years; KPS < 80%; LDH > upper limit of normal; sites of extranodal disease ≥ 2; Ann Arbor stage III/IV) were assessed in univariate analysis by log rank, age was not predictive of PFS or OS (P = .48 and P = .32), whereas ENS (P = .005 and P = .001), PS (P < .0001 for both), LDH (P < .001 for both), and stage (P = .001 and P < .001) predicted both PFS and OS. When entered into a Cox regression model for multivariate analysis, 3 factors, PS, LDH, and stage, remain predictive by intent to treat. These significant components were identical to those in the sAAIPI, which was subsequently used to stratify patients into risk groups.
The sAAIPI applied at the time of relapsed or primary refractory disease and prior to second-line chemotherapy predicts both PFS and OS. However, the outcomes for patients with high-intermediate and high-risk disease (2 or 3 adverse factors) were not statistically distinct (OS P = .13; PFS P = .12) and have been grouped together, identifying 3 risk groups: low risk (0 factors), intermediate risk (1 factor), and high risk (2 or 3 factors) (Table 3). The 4-year PFS and OS for these risk groups were as follows: low risk, PFS = 70%, OS = 74%; intermediate risk, PFS = 39%, OS = 49%; high risk, PFS = 16%, OS = 18% (Figure 3A-B). Forty-two patients failed ICE chemotherapy; not surprisingly, the majority of these patients (81%) had high-risk disease. In comparison to the ICE failure rate of 38% in high-risk patients, patients with low- and intermediate-risk disease had ICE failure rates of 10% and 15%, respectively; nonetheless, all ICE failures had an equally poor prognosis (Table 3).
PFS (A) and OS (B) intention-to-treat analysis, stratified by sAAIPI risk group: low, intermediate, and high. (A) Kaplan-Meier curves of PFS and (B) OS in 150 patients with DLBCL analyzed by intention to treat and stratified by sAAIPI risk groups: low risk (0 factors), intermediate risk (1 factor), high risk (2-3 factors). sAAIPI risk factors comprise LDH greater than normal value, stage of III/IV, and KPS less than 80%.
PFS (A) and OS (B) intention-to-treat analysis, stratified by sAAIPI risk group: low, intermediate, and high. (A) Kaplan-Meier curves of PFS and (B) OS in 150 patients with DLBCL analyzed by intention to treat and stratified by sAAIPI risk groups: low risk (0 factors), intermediate risk (1 factor), high risk (2-3 factors). sAAIPI risk factors comprise LDH greater than normal value, stage of III/IV, and KPS less than 80%.
sAAIPI in chemosensitive patients as a predictor of survival
When applied to patients with chemosensitive disease, sAAIPI continues to separate the patients into statistically distinct groups with PFS (P = < .001) and OS (P = < .001) at 4-year median follow-up of low = 69% and 83%, intermediate = 46% and 55%, and high = 25% and 26% (Figure 4A-B). A greater proportion of patients with low-risk disease achieved CR to ICE (67%) compared with intermediate-(32%) and high-risk (39%) groups (Table 4).
PFS (A) and OS (B) for chemosensitive patients, stratified by sAAIPI risk group: low, intermediate, and high. (A) Kaplan-Meier curves of PFS and (B) OS in 108 chemosensitive patients with DLBCL, stratified by sAAIPI risk groups: low risk (0 factors), intermediate risk (1 factor), high risk (2-3 factors). sAAIPI risk factors comprise LDH greater than normal value, stage of III/IV, and KPS less than 80%.
PFS (A) and OS (B) for chemosensitive patients, stratified by sAAIPI risk group: low, intermediate, and high. (A) Kaplan-Meier curves of PFS and (B) OS in 108 chemosensitive patients with DLBCL, stratified by sAAIPI risk groups: low risk (0 factors), intermediate risk (1 factor), high risk (2-3 factors). sAAIPI risk factors comprise LDH greater than normal value, stage of III/IV, and KPS less than 80%.
Among patients with chemosensitive disease, an analysis was performed to determine the effect of conditioning regimen, TBI, and involved field radiotherapy on OS and PFS. Multivariate analysis for OS/PFS using a Cox proportion hazards model demonstrated that only the sAAIPI was a significant predictor of survival.
Relapsed versus primary refractory status and treatment outcome
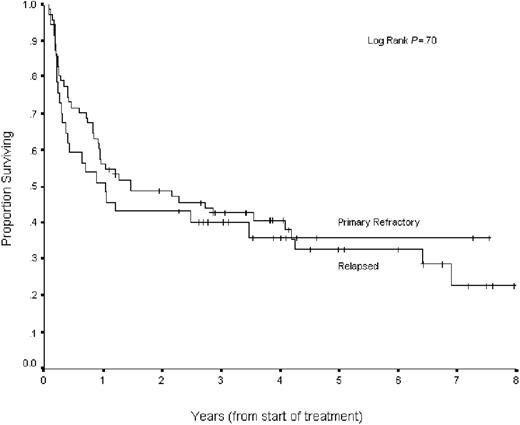
Patients with relapsed disease had a response rate to ICE chemotherapy of 85.5% (CR = 36.1%, PR = 49.4%), whereas patients with primary refractory disease had a response rate of 55% (CR = 18%, PR = 37%) (overall response rate [ORR] relapsed versus primary refractory, P < .001). By intent-to-treat analysis, patients with relapsed disease had improved PFS and OS compared with primary refractory patients (35% and 40% versus 20% and 27%, respectively; both P = .005). When analyzed by chemosensitive disease, patients with chemosensitive relapsed and chemosensitive primary refractory disease had similar outcomes with ASCT (PFS, 41% versus 36%, P = .7; OS, 45% versus. 46%, P = .99) (Figure 5), confirming our prior report.3 In chemosensitive-relapsed patients, there was a trend for improved OS with CR versus PR (P = .07) and no significant difference for chemosensitive primary refractory patients (P = .44).
PFS for chemosensitive patients with relapsed versus primary refractory disease. Kaplan-Meier curves of PFS for 150 patients with DLBCL, stratified by relapsed (n = 83) versus primary refractory (n = 67) status at time of second-line therapy.
PFS for chemosensitive patients with relapsed versus primary refractory disease. Kaplan-Meier curves of PFS for 150 patients with DLBCL, stratified by relapsed (n = 83) versus primary refractory (n = 67) status at time of second-line therapy.
A survival analysis stratified by disease status (relapsed versus refractory) was performed. The sAAIPI risk groups remain predictive for PFS in both relapsed (log-rank P = .002) and primary refractory patients (log-rank P = .001), although there are very few primary refractory patients with low-risk disease (only 2 of 67). When restricted to chemosensitive relapsed and primary refractory patients, the sAAIPI predicts PFS (log-rank P = .02 and P = .05) and OS (log-rank P = .002 and P = .03).
Discussion
Second-line chemotherapy regimens have proven curative in less than 15% of patients with aggressive NHL who fail upfront cyclophosphamide, doxorubicin, Oncovin, prednisone (CHOP)–based chemotherapy.23-27 The PARMA study established HDT with ASCT as the standard treatment for patients with chemosensitive relapsed aggressive NHL, improving OS and EFS compared with standard-dose second-line chemotherapy.2 We and others have demonstrated the efficacy of this approach in the primary refractory setting.3,28 Consequently, HDT followed by ASCT has been adopted as the standard second-line approach for patients with relapsed or primary refractory chemosensitive aggressive NHL with 5-year EFS of 40% to 45% expected.
Given these results, a substantial population of patients will have chemotherapy-refractory disease (30%-50%) and more than half of all patients receiving transplants will fail to have long-term disease-free survival. Prognostic models capable of predicting durable responses in this group are imperative if we are to identify at-risk populations and design new therapy. Initial studies determined that chemosensitivity to conventional-dose second-line chemotherapy was the most important predictor of outcome4,5 ; thus, most subsequent ASCT trials enrolled only this select group of patients. As a consequence, the ability to predict the overall benefit of HDT/ASCT for a given patient before second-line chemotherapy administration is difficult, and previously identified prognostic factors predicting outcome are generally applicable only for chemosensitive patients.
A number of prior studies have sought to identify prognostic factors at the time of relapsed/primary refractory disease. Vose et al6 reported a model defining good prognosis at the time of ASCT as patients without a mass of 10 cm or greater and having 1 or less of the following factors: 3 or more chemotherapy regimens prior to ASCT, elevated LDH, and chemotherapy resistance. This good prognosis group had a 3-year failure-free survival (FFS) of 45%, as opposed to a FFS of 10% for the poor-risk group. This model was useful in shaping the role of HDT/ASCT; however, currently HDT/ASCT is generally applied for patients in first relapse and rarely for patients with chemoresistant lymphoma. We and others have demonstrated that achieving a CR to conventional-dose second-line therapy confers an improved survival with ASCT as compared with patients achieving only a PR.7,8 Other studies have demonstrated that normal LDH levels, time to relapse, and low disease burden were associated with prolonged survival.9-12
Blay et al14 performed a retrospective analysis of the 215 patients with relapsed NHL included in the initial PARMA study, evaluating the prognostic significance of the IPI at relapse.14 The age-adjusted IPI was calculated for all 215 patients, but ultimately only 106 evaluable chemosensitive patients were randomly assigned between dexamethasone/high-dose cytarabine/cisplatin (DHAP) and ASCT (54 patients received ASCT, 52 patients received DHAP). The aaIPI predicted OS in the DHAP group, but not in the ASCT group. In a further stratified analysis, OS and PFS were superior for patients with aaIPI 1 or more in the ASCT group. However, only 16 patients in the ASCT group had high-intermediate or high-risk disease by the aaIPI, which may have limited the power of the analysis and consequently the predictive value of the aaIPI. Furthermore, a significant number of the patients in the PARMA study had histologies different from DLCL, and patients with bone marrow involvement were excluded. In contradistinction, our study has more robust patient numbers with uniform histology and greater distribution among aaIPI risk groups.
Guglielmi et al29 performed a retrospective analysis of 474 patients with relapsed DLCL from 47 centers in Italy.29 A variety of salvage regimens were used, and ultimately only 20% (95 patients) underwent HDT with ASCT. Relapse less than 12 months from initial diagnosis and the AAIPI components taken individually (advanced stage, elevated LDH, and poor PS) were independent prognostic factors for OS and PFS. Incorporated into a 4-factor risk score, this model was predictive of OS and PFS for both patients with chemosensitive relapse and for those patients who underwent HDT with ASCT.
In this study, we evaluated 150 patients with DLBCL enrolled on 3 sequential intent-to-treat studies and treated with ICE chemotherapy followed by HDT/ASCT for patients with chemosensitive disease. All patients were evaluated for OS and PFS from the initiation of second-line therapy, enabling analysis of prognostic factors and endpoints that are not contingent on the response to second-line therapy. Through these studies, we have confirmed that (1) the sAAIPI calculated at the initiation of second-line therapy for relapsed/primary refractory disease is predictive of OS and PFS when analyzed by both intention to treat and for chemosensitive patients, (2) a CR to second-line conventional dose ICE chemotherapy predicts an improved overall survival compared with patients achieving only a PR, (3) patients with relapsed disease have a higher response rate to second line ICE chemotherapy compared with patients with primary refractory disease, and (4) patients with chemosensitive primary refractory disease have an outcome equivalent to patients with relapsed disease.
In this group of patients, the Kaplan-Meier estimates of PFS and OS for the intent-to-treat group at 4 years are 28% and 34%, respectively. Furthermore, the sAAIPI divided patients into 3 well-defined risk groups: low risk (0 factor), intermediate risk (1 factor), and high risk (2 or 3 factors). When analyzed by risk group, the PFS for the low-, intermediate-, and high-risk groups was 70%, 39%, and 16%, respectively (P < .0001). Importantly, the sAAIPI continues to predict outcome among the chemosensitive patients. Thus, establishing chemosensitivity does not abrogate the prognostic value of the sAAIPI. Stated simply, patients with high-risk sAAIPI who have chemosensitive disease and then proceed to ASCT continue to have poorer outcomes, implying that the sAAIPI reflects inherent biologic differences among the risk groups. In ongoing molecular studies, we hope to identify the biologic basis for these differences, similar to those already performed in previously untreated DLBCL.30,31
Patients with low-risk disease have an excellent response to second-line ICE-based chemotherapy and HDT/ASCT. For this population, ICE second-line therapy followed by HDT/ASCT can be expected to result in 4-year OS/PFS rates of 74% and 70%. For those patients with intermediate risk, this approach results in OS/PFS rates of 49% and 39%. Achieving a CR rather than a PR predicts an improved survival, although this may reflect patient or disease-related features, rather than the amount of disease present at the time of ASCT or the degree of chemotherapy responsiveness. Thus, a logical method of improving these results would be increasing the proportion of patients achieving a CR to second-line chemotherapy. The addition of rituximab to ICE (R-ICE) may confer such an advantage, as we observed an ORR and CR rate to R-ICE of 81% and 55%, respectively, which compares favorably with the historical ORR and CR rates of 73% (P = .503) and 28% (P = .006) in patients with similar prognostic factors treated with ICE alone.32 Median follow-up on that study is too short to evaluate whether increasing the CR rate will confer a survival advantage, as the biologic differences that predict for CR versus PR may also be predictive of outcome after ASCT.
Unfortunately, patients with high-risk disease (sAAIPI = HI/H) represent the largest group of relapsed/primary refractory patients in this series, and they fair less well with an ICE-HDT/ASCT approach. The intent-to-treat OS and PFS of 18% and 16%, respectively, compare favorably with the reported OS/PFS (5%-10%) for patients with the poorest risk in previous models.6,29 Approximately 25% of chemosensitive patients in the high-risk group can expect long-term survival. However, these results are still disappointing, and novel treatment strategies are necessary for this group of patients. The ORR of 62% with ICE therapy in this group is inferior to the 85% to 90% ORR in the low- and intermediate-risk groups (Table 3). Efforts to increase the chemotherapy responsiveness of this group are necessary.
The strengths of this study are that all patients had a uniform biopsy-proven histology, received a uniform second-line chemotherapy regimen, and had prognostic factors evaluated before the initiation of therapy in an intention-to-treat analysis. When taken in conjunction with prior studies evaluating the sAAIPI,14,29 one can conclude that the sAAIPI is an easily determined clinical index with significant prognostic import for patients presenting with relapsed or primary refractory disease. The sAAIPI continues to predict outcome once chemosensitivity is established and can be used to design risk-stratified transplantation regimens according to the patient's pretreatment prognosis. The ability of the sAAIPI to identify poor-risk patients before the initiation of therapy may allow them to benefit from novel treatment approaches designed to improve their curability. High-risk patients should be offered clinical trials that seek to improve these results by strategies such as incorporating novel agents earlier in the treatment paradigm, augmenting second-line regimens (eg, with immunotherapy or biologic modifiers), or allogeneic stem cell transplantation. In the future, the sAAIPI will be prospectively validated in a large-scale international randomized study comparing the R-ICE regimen against R-DHAP (rituximab, dexamethasone, high-dose cytarabine, and cisplatin) prior to a standardized chemotherapy-based ASCT. Posttransplantation rituximab consolidation will be administered. This design will evaluate the applicability of the sAAIPI to alternative second-line regimens and limit subsequent variability by using a uniform HDT/ASCT regimen.
Prepublished online as Blood First Edition Paper, April 3, 2003; DOI 10.1182/blood-2002-12-3837.
Supported by the Mortimer J Lacher Fund, The Singer Family Lymphoma Research Fund, The Priovolos Family Lymphoma Research Fund, The Lombardi Fund, and the National Institute of Health (grant P01 CA05826).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the house staff members, nurse practitioners, and nurses at MSKCC for their dedication and excellent patient care.