Abstract
Some patients lose chimerism following nonmyeloablative hematopoietic cell transplantation (HCT), yet, surprisingly, enjoy sustained tumor remissions. We hypothesized that host-versus-graft (HVG) alloresponses might induce antitumor effects against recipient tumors. We explored this question in mice by administering recipient leukocyte infusions (RLIs) to mixed chimeras established with nonmyeloablative conditioning. Mixed chimeras were prepared in the B10.A (H2a)→B6 (H2b) strain combination using depleting anti–T-cell monoclonal antibodies (mAbs), cyclophosphamide, and thymic irradiation. B6 myeloid leukemia cells (MMB3.19) were administered 7 days following donor lymphocyte infusion (DLI) or RLI on day 35. Conversion to full donor chimerism occurred without graft-versus-hostdisease (GVHD) following DLI, whereas RLI led to loss of chimerism. Both RLI and DLI significantly delayed tumor mortality. In another strain combination (B10.BR [H2k]→BALB/c [H2d]), RLI-induced or spontaneous loss of chimerism was associated with antitumor effects against the host-type B-cell lymphoma A20. HCT was essential for the antitumor effect of RLI. RLI induced elevated serum interferon-γ (IFN-γ) levels, and recipient-derived IFN-γ was critical for their antitumor effects. Thus, HVG reactions (spontaneous or induced by RLI) mediate antitumor effects against hematologic malignancies via a recipient-derived IFN-γ–mediated mechanism. A novel approach to achieving anti-tumor effects without the risk of GVHD is suggested.
Introduction
Hematopoietic cell transplantation (HCT) is the only curative therapy for several hematologic malignancies and might also have potential utility in the setting of solid tumors. However, HCT has yet to realize its full clinical potential because of treatment-related toxicities, including infectious complications and graft-versus-host disease (GVHD), associated with the procedure. Progress has been made toward minimizing transplantation-associated morbidity and mortality by conditioning recipients with less toxic, nonmyeloablative doses of chemotherapy and/or irradiation prior to allogeneic bone marrow transplantation.1-5 We have developed a nonmyeloablative bone marrow transplantation (BMT) regimen that uses an approach to separating GVHD and graft versus leukemia (GVL) that is based on observations in the murine model.6-8 The clinical regimen included in vivo T-cell depletion with pre and posttransplantation antithymocyte globulin (ATG), thymic irradiation, pre-transplantation cyclophosphamide, and a short course of cyclosporine, which is discontinued by 5 weeks and followed by donor lymphocyte infusion (DLI) in the absence of GVHD.4,5,9 The regimen has been well tolerated and associated with very encouraging response rates, particularly of advanced, refractory lymphomas and myeloma.4,5,9-11 Although all patients receiving this regimen have developed initial mixed hematopoietic chimerism, a fraction (about 30%) of them lose this chimerism, usually within the first 100 days, apparently because of an immunologic rejection process.4,9,12 Sustained antitumor responses have occurred with highest frequency in patients who achieve full donor chimerism following DLI.10 Remarkably, however, a fraction (about 20%) of the patients who lose donor chimerism enjoy sustained remissions of advanced hematologic malignancies, particularly lymphomas and multiple myeloma.13-17 These results led us to hypothesize that host antidonor immune responses might, under some conditions, induce regression of recipient tumors. The current studies were designed to test this hypothesis in a murine model. The results demonstrate, in 2 of 2 hematologic malignancy models studied, that host antidonor immune responses can lead to significant antitumor responses against recipient-type leukemias and lymphomas and that recipient-derived interferon-γ (IFN-γ) plays a major role in this phenomenon.
Materials and methods
Animals
Female B10.A (H2a), B10.BR (H2k), and C.129S7(B6)-Ifngtm1Ts (common name, BALB/c-Ifngtm1Ts) (H2d) (BALB/c IFN-γ deficient) mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and used at 8 to 12 weeks of age. Female C57BL/6 (B6) (H2b) and BALB/c wild-type (WT) (H2d) mice were purchased from the Frederick Cancer Research Facility, National Cancer Institute (Frederick, MD) and used at 8 to 12 weeks of age. All mice were housed in autoclaved microisolator environments, and all manipulations were performed in a laminar flow hood.
Cell lines
MMB3.19 is a c-myc retrovirus-transformed myeloid cell line of B6 origin expressing both class I and class II major histocompatibility complex (MHC) antigens.18 The cells were kindly provided to us by Dr Robert Korngold (Jefferson Medical University, Philadelphia, PA). MMB.3.19 cells (3 × 106) were administered intraperitoneally 7 days following recipient leukocyte infusion (RLI) or DLI. A20 is a B-cell leukemia/lymphoma of BALB/c origin that occurred spontaneously in a 15-month-old mouse.19 A20 has been shown to be uniformly lethal within 30 days at a dose of 5 × 105 cells administered intravenously.20 A20 was administered at a dose of 5 × 105 cells 7 days after RLI (day 56 after BMT). Both cell lines were maintained in culture for a maximum of 1 week in RPMI 1640 (Mediatech Cellgro, Herndon, VA) supplemented with 10% fetal calf serum (FCS) (Sigma, St Louis, MO), nonessential amino acids (Gibco, Gaithersburg, MD), L-glutamine, sodium pyruvate, penicillin/streptomycin (Gibco), 0.01 M HEPES (N-(2-hydroxyethyl)piperazine-N′-ethanesulfonic acid) buffer (Fisher Biotech, Fair Lawn, NJ), and 10 μM 2-mercaptoethanol (Sigma) at 37°C in 7% CO2.
Bone marrow transplantation
Mixed chimerism was induced in female B6 (H2b), BALB/c IFN-γ– deficient, or BALB/c WT (H2d) mice by a nonmyeloablative regimen as described previously7 using in vivo T-cell–depleting anti-CD4 (GK1.5) (1.76 mg/mouse) and anti-CD8 mAb (2.43) (1.4 mg/mouse) on day –5, cyclophosphamide (CTX) 200 mg/kg on day –1, and 7 Gy thymic irradiation from a 60Co source on day 0. Donor bone marrow cells (BMCs) were harvested from B10.A (H2a) or B10.BR (H2k) mice, and single cell suspensions were prepared as described previously.7 Four to 6 hours after completion of irradiation, recipients were injected intravenously through the tail vein with 20 × 106 unmanipulated donor BMCs.
Recipient lymphocyte infusions and donor lymphocyte infusions
Recipient (B6, BALB/c IFN-γ deficient, or BALB/c WT) or donor (B10.A) spleens were harvested and gently teased in ammonium chloride potassium (ACK)–lysing buffer (Biowhittaker, Walkersville, MD). Single cell suspensions were filtered through nylon mesh and administered intravenously at a dose of 30 × 106 per recipient, 1 week after evidence of initial T-cell recovery was seen in white blood cells (WBCs), on day 35 or 49 after BMT. Animals with similar chimerism levels were randomly assigned to treatment groups according to WBC chimerism analysis and randomly assigned between cages to avoid cage-related bias.
Assessment of engraftment and chimerism and flow cytometric analysis
Chimerism in various WBC lineages was analyzed by 2-color flow cytometry (FCM) as described previously.7 Briefly, peripheral blood was collected into heparinized Eppendorf tubes and subjected to deionized water lysis. Cells were washed and stained in fluorescence-activated cell sorter (FACS) buffer (Hanks balanced salt solution containing 0.1% bovine serum albumin [BSA] and 0.1% NaN3). To reduce nonspecific antibody binding, 10 μL culture supernatant containing mAb 2.4G2 (anti-Fcγ-RII receptor, CDw32)21 was added to all tubes. In the B10.A to B6 combination, the recipients' cells were detected by KH-95-Bio (H2Db) (Pharmingen, San Diego, CA) and revealed by phycoerythrin (PE)–labeled streptavidin (Pharmingen). In addition, the following antibodies were used for chimerism analysis in various cell lineages: anti-CD4–fluorescein isothiocyanate (FITC), anti-CD8β–FITC, anti-B220–FITC (all purchased from Pharmingen), and anti-Mac-1–FITC (CalTag, San Francisco, CA). Nonreactive control mAb HOPC-1–FITC or HOPC-1–Bio (mouse immunoglobulin G2a [IgG2a] prepared in our laboratory) was used as a negative control. In the B10.BR to BALB/c combination, recipients' cells were labeled with 34-2-12–FITC (H2d) (prepared in our laboratory), and chimerism was analyzed in various cell lineages with the following antibodies: anti-CD4–PE, anti-CD8β–PE, anti-B220–PE, and anti-Mac-1–PE (CalTag). Nonreactive mAb HOPC-1 FITC and Rat-IgG2a–PE were used as negative controls.
The expression of IFN-γ receptor on A20 cells was measured by staining cells with biotinylated rat antimouse CD119 (GR 20; Pharmingen) developed with PE-streptavidin. Biotinylated mouse IgG2a mAb (HOPC-1) was used as nonreactive negative control antibody.
Exclusion of dead cells was performed by propidium iodide (PI) staining and gating on PI-negative cells. Usually, 10 000 viable cells were acquired for each tube on a FACS Calibur flow cytometer (Becton Dickinson, Mountain View, CA) and analyzed using CellQuest software (Becton Dickinson, San Jose, CA). The different peripheral blood leukocyte populations were distinguished by their forward scatter (FSC) and side scatter (SSC) properties: FSC low and SSC low (lymphocytes), SSC high (granulocytes), and FSC high and SSC low (monocytes). Using the recipient marker KH95-Bio in the B10.A to B6 combination or 34-2-12–FITC in the B10.BR to BALB/c combination, the relative percentage of donor cells in a chimera was calculated using the following formula: 100% × [1 – [(recipient phenotype percentage positive – isotype control)]/[(recipient phenotype positive – isotype control) + (recipient phenotype percent negative – isotype control)]].
ELISA for the determination of serum IFN-γ levels
Serum was collected 7 days after RLI injection in different groups of treated mice. IFN-γ concentrations were measured using a specific enzyme-linked immunosorbent assay (ELISA) kit for mouse IFN-γ purchased from R&D Systems (Minneapolis, MN) according to the manufacturer's instructions.
Proliferation assay
A20 cells and WEHI-279 cells (1 × 104/well) were incubated in triplicate in 96-well plates with various concentrations of mouse recombinant IFN-γ (rIFN-γ) (Pharmingen) in complete medium. Cultures were pulsed with 1 μCi (0.037 MBq) 3H-thymidine (Amersham Pharmacia Biotech, Piscataway, NJ) 24, 48, 72, and 96 hours after incubation and harvested 16 hours later. 3H-thymidine uptake was counted on a Betaplate β counter (Wallac, Gaithersburg, MD). Data are represented as the mean ± SD (cpm) of the triplicate samples. WEHI-279, a murine lymphoma cell line that is sensitive to an antiproliferative effect of IFN-γ, was used as an assay control.
Measurement of apoptosis
We measured apoptosis by flow cytometry. Briefly, A20 cells (2 × 105/well) were incubated in 48-well plates with various concentrations of mouse rIFN-γ (Pharmingen) in complete medium. Cells were harvested after 24, 48, and 72 hours of incubation and washed and incubated with allophycocyanin-conjugated Annexin-V (CalTag) in a calcium-containing buffer (FACS buffer with 2.5 mM CaCl2). After 30 minutes of incubation at 4°C, the samples were washed and analyzed on a FACS Calibur flow cytometer immediately following the addition of 0.5 μg 7-aminoactinomycin D (7-AAD; Sigma). The data were analyzed using WinMDI software (TSRI, La Jolla, CA).
Statistical analysis
Survival data were analyzed using Prism software (GraphPad, San Diego, CA) and the log-rank test. Differences between group means were tested using the Student t test by Microsoft Excel software (Redmond, WA). P < .05 was considered to be significant.
Results
RLI leads to loss of chimerism in the B10.A→B6 mixed chimerism model
Stable mixed chimerism in both myeloid and lymphoid lineages was induced in B6 (H2b) mice by administration of in vivo T-cell–depleting anti-CD4 and anti-CD8 mAbs on day –5, CTX 200 mg/kg on day –1, and 7 Gy thymic irradiation on day 0 followed by transplantation with 20 × 106 unmanipulated BMCs from fully MHC-mismatched B10.A (H2a) donors, as previously described.7 Mice showed evidence of initial T-cell recovery in the peripheral blood by day 28 after BMT as indicated by the presence within the lymphocyte population of more than 10% CD4+ T cells and more than 0.5% CD8+ T cells (not shown). In our experience, peripheral WBC T-cell recovery correlates with clearance of T-cell–depleting antibodies administered in the conditioning regimen. On day 35 after BMT, recipients were given RLI in the form of 30 × 106 B6 spleen cells. As shown in Figure 1A, this led to a complete loss of chimerism within about 2 weeks of RLI administration. Additional mice received DLI on day 35, and these mice converted to full donor chimerism (Figure 1A). These animals (as well as control chimeras and RLI recipients) showed no evidence of GVHD and survived more than 150 days (data not shown), consistent with previous results.7
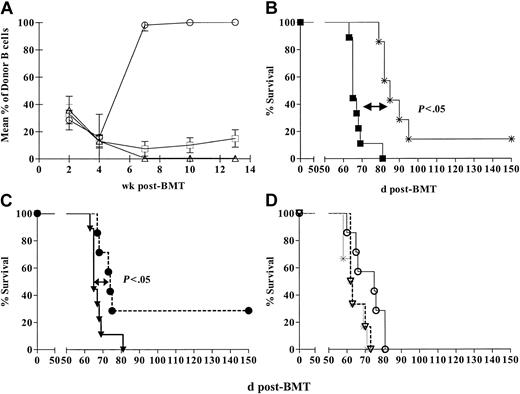
Antitumor effect of DLI and RLI against a myeloid leukemia, MMB3.19. (A) Mean (± SEM) B-cell chimerism at various times in control mixed chimeras (□; n = 7) and chimeras receiving recipient leukocyte infusion (RLI) (▵;n = 7) and donor lymphocyte infusion (DLI) (○; n = 9) on day 35. Similar results were seen for all WBC lineages examined (T cells, granulocytes, monocytes). (B) Mixed chimeras received MMB3.19 at a dose of 1 × 106 intraperitoneally on day 42 with no additional treatment (▪; n = 9) or 1 week following DLI (3 × 107 B10.A spleen cells intravenously) on day 35 (*; n = 7). (C) Mixed chimeras received MMB3.19 at a dose of 1 × 106 cells intraperitoneally on day 42 with no additional treatment (▪; n = 9) or 1 week following RLI (3 × 107 B6 spleen cells intravenously) (•; n = 7). (D) Survival of conditioned, non-BMT control mice that received RLI (3 × 107 B6 splenocytes intravenously) on day 35 and MMB3.19 (1 × 106 cells) on day 42 after BMT (▿; n = 6) is compared with that of simultaneously prepared mixed allogeneic chimeras also receiving MMB3.19, with (○;n = 7) or without (*;n = 6) RLI.
Antitumor effect of DLI and RLI against a myeloid leukemia, MMB3.19. (A) Mean (± SEM) B-cell chimerism at various times in control mixed chimeras (□; n = 7) and chimeras receiving recipient leukocyte infusion (RLI) (▵;n = 7) and donor lymphocyte infusion (DLI) (○; n = 9) on day 35. Similar results were seen for all WBC lineages examined (T cells, granulocytes, monocytes). (B) Mixed chimeras received MMB3.19 at a dose of 1 × 106 intraperitoneally on day 42 with no additional treatment (▪; n = 9) or 1 week following DLI (3 × 107 B10.A spleen cells intravenously) on day 35 (*; n = 7). (C) Mixed chimeras received MMB3.19 at a dose of 1 × 106 cells intraperitoneally on day 42 with no additional treatment (▪; n = 9) or 1 week following RLI (3 × 107 B6 spleen cells intravenously) (•; n = 7). (D) Survival of conditioned, non-BMT control mice that received RLI (3 × 107 B6 splenocytes intravenously) on day 35 and MMB3.19 (1 × 106 cells) on day 42 after BMT (▿; n = 6) is compared with that of simultaneously prepared mixed allogeneic chimeras also receiving MMB3.19, with (○;n = 7) or without (*;n = 6) RLI.
Both RLI and DLI delay the mortality induced by the B6 MMB3.19 leukemia
To determine whether or not RLI could mediate antitumor effects against a host-type leukemia, a group of mixed chimeras in the previously described experiments received RLI consisting of 3 × 107 B6 spleen cells on day 35, followed by 1 × 106 MMB3.19 B6 myeloid leukemia cells intraperitoneally on day 42 after BMT. Control mixed chimeras received tumor without RLI. Because we have previously shown that DLI can mediate antitumor effects against another host-type leukemia in B10.A→B6 chimeras prepared with this nonmyeloablative regimen,22 we also evaluated the effect of DLI given on day 35 on the growth of MMB3.19, to compare the magnitude of putative GVL effects induced by RLI and DLI. As shown in Figure 1B-C, MMB3.19-induced mortality in the control group of mixed chimeras (n = 9) led to a median survival time (MST) of 23 days after tumor administration (ie, 65 days after BMT). Mixed chimeras receiving both tumor and DLI (n = 7) showed a significantly prolonged MST of 43 days after tumor administration (85 days after BMT) (P < .05 versus tumor without DLI), and 1 of 7 mice survived more than 150 days (Figure 1B). As in nonleukemic controls, DLI administration led to conversion to full donor chimerism in leukemic mice without GVHD (not shown). Thus, DLI-induced conversion of mixed to full donor chimerism was associated with GVL effects against this myeloid tumor.
In the same experiment, RLI to mixed chimeras (n = 7) also significantly delayed tumor mortality. Two of 7 mice survived more than 150 days, and MST was prolonged to 32 days after tumor administration (74 days after BMT) (P < .05 versus tumor alone). As in nonleukemic controls, RLI administration led to loss of donor chimerism in leukemic mice. No significant difference was detected in the survival prolongation achieved with DLI compared with RLI in mixed chimeras. Thus, loss of donor chimerism in association with RLI was associated with significant antitumor responses.
BMT is required for the antitumor effect of RLI
The surprising observation that RLI led to a significant antitumor effect might be a consequence of the host antigraft alloresponse or might, alternatively, reflect enhanced immune reconstitution because of infusion of 3 × 107 healthy host-type lymphocytes on day 42. To distinguish between these possibilities, we assessed the requirement for initial BMT in order for RLI to mediate an antitumor effect. Groups of B6 mice received conditioning with anti-CD4 and anti-CD8 mAbs on day –5, CTX 200 mg/kg on day –1, 7 Gy thymic irradiation on day 0 and either did or did not receive allogeneic B10.A unmanipulated BMT on day 0. On day 35 after BMT, some groups received RLI consisting of 3 × 107 spleen cells. Some of these animals and non-RLI recipients received 1 × 106 MMB3.19 cells intraperitoneally on day 42 after BMT.
The results are shown in Figure 1D. Mixed chimeras that received the tumor without RLI (n = 6) had an MST of 18.5 days after tumor administration (62.5 days after BMT). In the group of mixed chimeras that received RLI and tumor (n = 7), MST was prolonged to 33 days after tumor administration (75 days after BMT) (P < .05 versus BMT and tumor without RLI), confirming the antitumor effect of RLI. The group that received conditioning (without BMT) followed by RLI and MMB3.19 (n = 6) also showed rapid tumor mortality (MST, 20.5 days), similar to mice receiving no RLI (not significant [NS] versus mixed chimeras without RLI). Thus, in the absence of chimerism, RLI did not mediate a measurable antitumor effect. These results demonstrate that the antidonor alloresponse was essential for the antitumor effect of RLI. All control animals receiving no further treatment after BMT or RLI remained healthy and survived more than 150 days (data not shown). Administration of RLI to mixed chimeras again led to complete loss of donor chimerism in all lineages (data not shown), whereas control mixed chimeras showed sustained chimerism.
Antitumor effect of RLI against a B-cell leukemia/lymphoma, A20
We next evaluated the capacity of RLI to mediate antitumor responses against an additional tumor, the BALB/c (H2d) A20 B-cell leukemia/lymphoma.19 Initial multilineage mixed chimerism was achieved in all BALB/c mice conditioned with depleting anti-CD4 and anti-CD8 mAbs on day –5, CTX 200 mg/kg on day –1, and 7 Gy thymic irradiation on day 0 prior to transplantation of 20 × 106 unmanipulated BMCs from B10.BR (H2k) donors, consistent with previous results.7 Control groups received no conditioning or BMT, or received conditioning alone. In this model, no evidence of peripheral blood T-cell recovery was achieved on day 28 after BMT, as shown by the absence in the lymphocyte population of detectable CD8+ T cells and the presence of less than 1% CD4+ T cells. Because this finding suggested that clearance of the T-cell–depleting antibodies was incomplete on day 28, the RLI and tumor injection were delayed in this second model. Evidence of WBC T-cell recovery, was apparent by day 42, and some groups were given RLI consisting of 3 × 107 BALB/c spleen cells on day 49 after BMT. Some of these groups then received 5 × 105 A20 cells intravenously on day 56 after BMT.
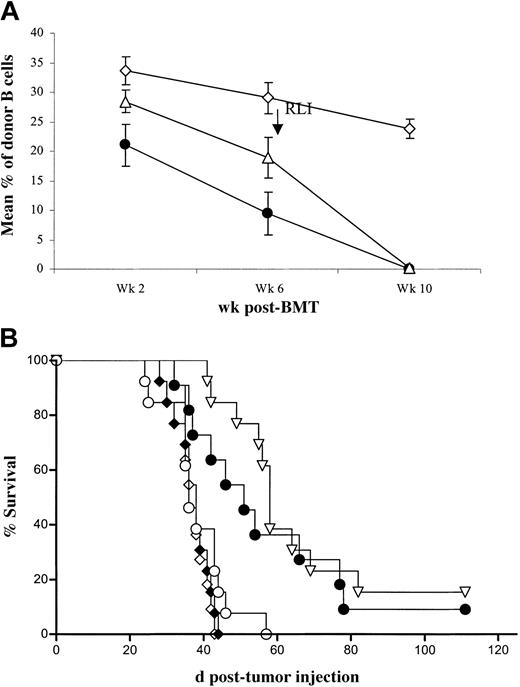
Results of 2 similar experiments are combined in Figure 2. Among BMT mice that did not receive RLI, some had already lost chimerism by 6 weeks prior to BMT, and we also examined tumor mortality in this subgroup of non-RLI recipients. By 6 weeks after BMT, 22 of 28 non-RLI mice were chimeric, and, by 10 weeks, 16 of them remained chimeric (not shown). All animals that received RLI were chimeric at 6 weeks, prior to RLI administration at week 7. RLI led to loss of chimerism by week 10 in 19 of 19 animals (Figure 2A). Among the nontumor controls, 2 of 22 mice that received BM transplants died of unknown causes (not shown). Survival of A20 tumor recipients is shown in Figure 2B. In mice receiving conditioning alone followed by A20 at week 8 (n = 13), MST was 36 days after tumor administration (92 days after BMT). The group receiving conditioning (without BMT), RLI, and A20 (n = 13) showed a similar MST of 36 days, and all mice succumbed by day 44 after tumor administration (NS versus conditioned mice receiving A20), confirming that RLI without BMT does not lead to antitumor effects. Among mice receiving conditioning, BMT, and A20, in which chimerism was detectable at 10 weeks after BMT (n = 11), MST was 38 days, and all mice succumbed by day 43 after tumor administration. In contrast, the group receiving conditioning, BMT, RLI, and A20 (n = 13), all of which were chimeric at the time of RLI, showed prolonged survival (MST, 58 days [P < .05] versus stable chimeras receiving tumor without RLI). Among BMT mice that lost chimerism spontaneously prior to 10 weeks after BMT (without RLI, n = 12), survival was also prolonged (MST, 51 days after tumor administration [P < .05] versus stable chimeras receiving tumor without RLI and NS versus chimeras receiving tumor and RLI).
RLI mediates an antitumor effect against a B-cell lymphoma, A20. BALB/c (H2d) recipient mice were conditioned with anti-CD4– and anti-CD8–depleting mAbs intraperitoneally on day –5, CTX 200 mg/kg intraperitoneally on day –1, and 7 Gy thymic irradiation on day 0 prior to transplantation of 20 × 106 B10.BR BMCs intravenously. The indicated groups received 3 × 107 recipient spleen cells (RLI) intravenously on day +49 after BMT and/or 5 × 105 A20 BALB/c B lymphoma cells intravenously on day +56 after BMT. (A) Mean (± SEM) of B-cell chimerism at various times in mice receiving conditioning and BMT, including chimeras with spontaneous loss of chimerism between 6 and 10 weeks after BMT, without RLI (•; n = 12); chimeras with persistent chimerism at 10 weeks (no RLI) (⋄; n = 11); and chimeras receiving RLI on day 49 (▵; n = 13). Results of 2 similar experiments are combined. (B) Survival of the A20 tumor groups receiving conditioning alone (○; n = 13), conditioning and RLI without BMT (♦; n = 13), conditioning and BMT without RLI with spontaneous loss of chimerism by week 10 (•;n = 12), conditioning and BMT without RLI with persistent chimerism at Week 10 (⋄; n = 11), and conditioning, BMT and RLI (▿; n = 13). Results of 2 similar experiments are combined.
RLI mediates an antitumor effect against a B-cell lymphoma, A20. BALB/c (H2d) recipient mice were conditioned with anti-CD4– and anti-CD8–depleting mAbs intraperitoneally on day –5, CTX 200 mg/kg intraperitoneally on day –1, and 7 Gy thymic irradiation on day 0 prior to transplantation of 20 × 106 B10.BR BMCs intravenously. The indicated groups received 3 × 107 recipient spleen cells (RLI) intravenously on day +49 after BMT and/or 5 × 105 A20 BALB/c B lymphoma cells intravenously on day +56 after BMT. (A) Mean (± SEM) of B-cell chimerism at various times in mice receiving conditioning and BMT, including chimeras with spontaneous loss of chimerism between 6 and 10 weeks after BMT, without RLI (•; n = 12); chimeras with persistent chimerism at 10 weeks (no RLI) (⋄; n = 11); and chimeras receiving RLI on day 49 (▵; n = 13). Results of 2 similar experiments are combined. (B) Survival of the A20 tumor groups receiving conditioning alone (○; n = 13), conditioning and RLI without BMT (♦; n = 13), conditioning and BMT without RLI with spontaneous loss of chimerism by week 10 (•;n = 12), conditioning and BMT without RLI with persistent chimerism at Week 10 (⋄; n = 11), and conditioning, BMT and RLI (▿; n = 13). Results of 2 similar experiments are combined.
These results confirm in another tumor model that RLI mediates antitumor effects and that the host-versus-graft (HVG) alloresponse is essential for this antitumor effect. Additionally, they show that spontaneous loss of chimerism is associated with a similar antitumor effect.
BALB/c-derived IFN-γ plays a major role in the antitumor effect of RLI against A20 but is not required for loss of chimerism
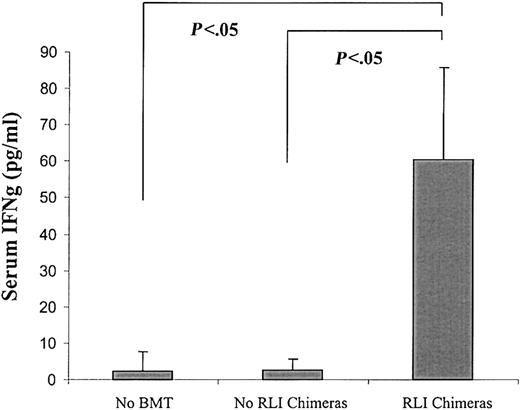
Because IFN-γ has been shown to participate in antitumor effects against a variety of tumors,23-25 including A20 in another model,26 we speculated that the HVG alloresponse associated with RLI might lead to IFN-γ production and that this cytokine might participate in the antitumor effect of RLI. We, therefore, measured serum IFN-γ levels simultaneously with WBC chimerism 7 days following RLI administration (day 56). Serum IFN-γ levels were significantly elevated in mice that received BMT and RLI and lost chimerism by this time (n = 7) (Figure 3) compared with those in mice that received conditioning without BMT or RLI (n = 5, P < .05) or to those that received BMT without RLI and that showed mixed chimerism at this time (n = 9, P < .05). Serum IFN-γ levels were not increased above background in 2 mixed chimeras that had not completely lost donor chimerism by day 7 after RLI (not shown).
Elevated serum IFN-γ levels in association with RLI in mixed allogeneic chimeras. BALB/c recipient mice were conditioned with anti-CD4– and anti-CD8–depleting mAbs on day –5, CTX 200 mg/kg intraperitoneally on day –1, and 7 Gy thymic irradiation on day 0 prior to transplantation of 20 × 106 B10.BR BMCs. The indicated group received 3 × 107 recipient spleen cells (RLI) from BALB/c mice on day +49 after BMT. Mean (± SEM) of serum IFN-γ levels measured 7 days after RLI are shown. Mice received conditioning without BMT or RLI (no BMT; n = 5), BMT without RLI (no RLI chimeras; n = 9), or received BMT and RLI and lost chimerism by 1 week after RLI (RLI chimeras; n = 7).
Elevated serum IFN-γ levels in association with RLI in mixed allogeneic chimeras. BALB/c recipient mice were conditioned with anti-CD4– and anti-CD8–depleting mAbs on day –5, CTX 200 mg/kg intraperitoneally on day –1, and 7 Gy thymic irradiation on day 0 prior to transplantation of 20 × 106 B10.BR BMCs. The indicated group received 3 × 107 recipient spleen cells (RLI) from BALB/c mice on day +49 after BMT. Mean (± SEM) of serum IFN-γ levels measured 7 days after RLI are shown. Mice received conditioning without BMT or RLI (no BMT; n = 5), BMT without RLI (no RLI chimeras; n = 9), or received BMT and RLI and lost chimerism by 1 week after RLI (RLI chimeras; n = 7).
We next analyzed the role of IFN-γ in the antitumor effect of RLI. BALB/c WT and BALB/c IFN-γ–deficient mice received the earlier described nonmyeloablative conditioning regimen with or without BMT from B10.BR (H2k) donors. Control groups received conditioning alone. On day 49 after BMT, some BALB/c WT chimeras received RLI consisting of 3 × 107 BALB/c WT spleen cells, and some BALB/c IFN-γ–deficient recipients were given RLI consisting of 3 × 107 BALB/c IFN-γ–deficient spleen cells. A20 cells (5 × 105) were administered intravenously on day 56 after BMT.
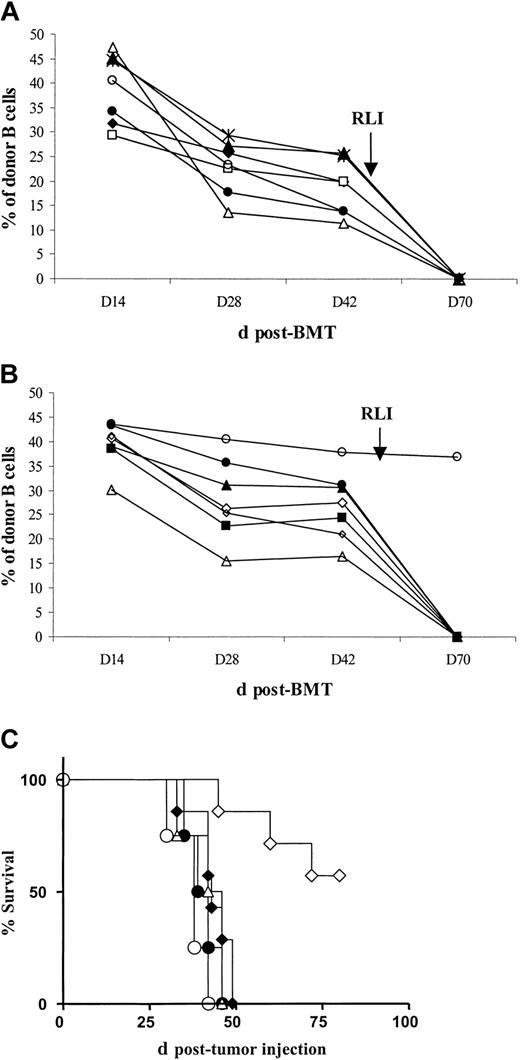
Mixed chimerism was achieved in both BALB/c and BALB/c IFN-γ–deficient mice. Almost all animals that were chimeric at week 6 and received RLI at week 7 from either WT or IFN-γ– deficient BALB/c mice lost chimerism by day 70 (Figure 4A-B). In contrast, chimeric mice that did not receive RLI maintained stable donor chimerism (not shown). Tumor survival curves are shown in Figure 4C. In mice receiving conditioning alone (n = 4), MST was 38 days after tumor administration. Among BALB/c WT and IFN-γ–deficient mice receiving conditioning, BMT and A20 without RLI (n = 4 per group), MST was 44 and 40.5 days after tumor administration, respectively (NS). As expected, BALB/c WT chimeras receiving conditioning, BMT, RLI from BALB/c WT mice, and A20 (n = 7) showed prolonged survival (MST not reached; P < .0007 versus no BMT controls; P < .007 versus mice receiving tumor without WT RLI). In contrast, BALB/c IFN-γ– deficient chimeras receiving conditioning, BMT, RLI from BALB/c IFN-γ–deficient mice, and A20 (n = 7) did not show prolonged survival compared with controls (MST, 43 days after tumor administration; NS versus chimeras receiving tumor without RLI). These results demonstrated that recipient and/or RLI-derived IFN-γ plays a critical role in the antitumor effect of RLI.
BALB/c-derived IFN-γ plays a critical role in the antitumor effect of RLI against A20 but is not required for loss of chimerism. BALB/c WT and BALB/c IFN-γ knock-out (KO) recipient mice were conditioned with anti-CD4– and anti-CD8–depleting mAbs on day –5, CTX 200 mg/kg intraperitoneally on day – 1, and 7 Gy thymic irradiation on day 0 prior to transplantation of 20 × 106 B10.BR BMCs intravenously. Some groups received 3 × 107 recipient spleen cells (RLI) from BALB/c WT or BALB/c IFN-γ KO mice intravenously on day +49 after BMT and/or 5 × 105 A20 BALB/c B lymphoma cells intravenously on day +56 after BMT. (A) Levels of peripheral blood B-cell chimerism before and after RLI in BALB/c WT mice receiving conditioning, BMT and WT RLI. B-cell chimerism is representative of chimerism in all lineages. Each line represents an individual animal. (B) Levels of peripheral blood B-cell chimerism before and after RLI in BALB/c IFN-γ KO mice receiving conditioning, BMT, and IFN-γ KO RLI. B-cell chimerism is representative of chimerism in all lineages. Each line represents an individual animal. (C) Survival of A20 tumor recipients. BALB/c WT mice had been treated with conditioning alone (○; n = 4); conditioning and BMT without RLI (▵; n = 4); or conditioning, BMT, and WT RLI (⋄; n = 7). BALB/c IFN-γ KO mice received conditioning and BMT without RLI (•;n = 4) or conditioning, BMT, and IFN-γ KO RLI (♦;n = 7).
BALB/c-derived IFN-γ plays a critical role in the antitumor effect of RLI against A20 but is not required for loss of chimerism. BALB/c WT and BALB/c IFN-γ knock-out (KO) recipient mice were conditioned with anti-CD4– and anti-CD8–depleting mAbs on day –5, CTX 200 mg/kg intraperitoneally on day – 1, and 7 Gy thymic irradiation on day 0 prior to transplantation of 20 × 106 B10.BR BMCs intravenously. Some groups received 3 × 107 recipient spleen cells (RLI) from BALB/c WT or BALB/c IFN-γ KO mice intravenously on day +49 after BMT and/or 5 × 105 A20 BALB/c B lymphoma cells intravenously on day +56 after BMT. (A) Levels of peripheral blood B-cell chimerism before and after RLI in BALB/c WT mice receiving conditioning, BMT and WT RLI. B-cell chimerism is representative of chimerism in all lineages. Each line represents an individual animal. (B) Levels of peripheral blood B-cell chimerism before and after RLI in BALB/c IFN-γ KO mice receiving conditioning, BMT, and IFN-γ KO RLI. B-cell chimerism is representative of chimerism in all lineages. Each line represents an individual animal. (C) Survival of A20 tumor recipients. BALB/c WT mice had been treated with conditioning alone (○; n = 4); conditioning and BMT without RLI (▵; n = 4); or conditioning, BMT, and WT RLI (⋄; n = 7). BALB/c IFN-γ KO mice received conditioning and BMT without RLI (•;n = 4) or conditioning, BMT, and IFN-γ KO RLI (♦;n = 7).
Minimal direct effect of IFN-γ on proliferation and failure to induce apoptosis of A20 cells in vitro
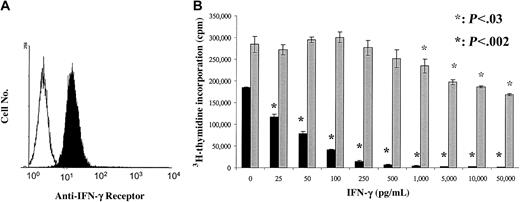
In view of the observed role of IFN-γ in the antitumor effect of RLI, we performed in vitro studies to determine whether or not this cytokine has direct inhibitory effects on the growth of A20. Consistent with this possibility, as shown in Figure 5A, A20 cells express the receptor for IFN-γ. To evaluate the effect of mouse rIFN-γ on the proliferation of A20 cells in vitro, A20 cells were incubated with increasing concentrations of mouse rIFN-γ for 48 hours, and cell proliferation was measured by tritiated thymidine incorporation. Results are shown in Figure 5B. As expected, IFN-γ significantly inhibited the proliferation of IFN-γ–sensitive WEHI-279 cells at a concentration as low as 25 pg/mL. In contrast, IFN-γ did not affect the proliferation of A20 cells at concentrations below 1000 pg/mL. IFN-γ concentrations of 1000 to 50 000 pg/mL significantly decreased the proliferation of the tumor cells by up to 40%.
Minimal antiproliferative effect of IFN-γ on A20 cells. (A) A FACS profile showing expression of IFN-γ receptor on A20 cells. A20 cells were stained with anti-CD119 (IFN-γ receptor α chain) (filled histogram) and with isotype control (open histogram). (B) Proliferation of A20 and WEHI-279 cells cultured in complete medium with increasing concentrations of mouse rIFN-γ. Cells were pulsed after 48 hours of culture with 3H-thymidine and harvested 16 hours later. Data are presented as the mean ± SD (cpm) of triplicate cultures in each culture condition. Results from 1 representative experiment of 3 are shown.
Minimal antiproliferative effect of IFN-γ on A20 cells. (A) A FACS profile showing expression of IFN-γ receptor on A20 cells. A20 cells were stained with anti-CD119 (IFN-γ receptor α chain) (filled histogram) and with isotype control (open histogram). (B) Proliferation of A20 and WEHI-279 cells cultured in complete medium with increasing concentrations of mouse rIFN-γ. Cells were pulsed after 48 hours of culture with 3H-thymidine and harvested 16 hours later. Data are presented as the mean ± SD (cpm) of triplicate cultures in each culture condition. Results from 1 representative experiment of 3 are shown.
We also assessed whether or not IFN-γ might directly induce the apoptosis of A20 cells. A20 cells were incubated with varying concentrations of mouse rIFN-γ for 24 to 72 hours. Apoptosis was determined by flow cytometry using double staining with APC-labeled Annexin-V and 7-AAD. Despite a slight increase in the percentages of apoptotic (Annexin-V+, 7-AADlow) and dead (Annexin-V+, 7-AADhigh) cells over time, no significant difference was detected between A20 cells incubated with or without increasing concentrations of IFN-γ up to 10 000 pg/mL (Table 1).
Discussion
The studies reported here confirm the hypothesis that an HVG immune response can lead to measurable antitumor effects against a host-type tumor. This ability of RLI to mediate antitumor effects was demonstrated for both a myeloid and a lymphoid malignancy. The antitumor effect of RLI was dependent on the antidonor alloresponse, because similar infusions of recipient lymphocytes to mice that had received conditioning without bone marrow transplantation, and, therefore, could not mediate an alloresponse, was not associated with prolonged tumor survival in either model. To our knowledge, this is the first direct demonstration that an alloresponse directed against the hematopoietic cell donor can lead to antitumor effects against a recipient tumor. We hypothesized that this might occur on the basis of clinical observations in patients who received nonmyeloablative allogeneic BM transplants with a similar regimen. About 30% of these patients undergo immunemediated rejection of the donor graft,9,12 and, among these, a fraction (about 20%) have enjoyed dramatic, sometimes sustained, responses of advanced, chemorefractory lymphomas and multiple myelomas.13-17 Studies are under way to clarify the mechanism of this effect in patients. A similar phenomenon has been reported in patients with myelodysplastic syndromes who lose chimerism after haploidentical hematopoietic cell transplantation following conditioning with a different nonmyeloablative regimen.27 Of note, spontaneous loss of chimerism was also associated with an antitumor effect against A20 lymphoma in the murine model described here. If these clinical results are indeed related to the phenomenon described here, then a new, low-risk approach to achieving antitumor responses may be suggested. Initial mixed chimerism can be achieved with relatively low toxicity or risk of GVHD, even in the haploidentical setting.16 Administration of nontolerant recipient lymphocytes harvested prior to conditioning might, by augmenting the rejection process, lead to an antitumor response, with no risk of GVHD. This effect could potentially be further augmented by presensitizing the recipient T cells to donor alloantigens prior to infusion. It is also conceivable that this approach could be associated with antitumor effects against solid tumors, and we are in the process of evaluating this possibility in the mouse model.
The mechanism of the rather surprising RLI-mediated antitumor effect remains to be fully elucidated. We hypothesized that the powerful HVG response mediated by RLI might be associated with massive, systemic cytokine release, and that these cytokines could be directly toxic to the lymphoid and myeloid tumors evaluated here. The rapid loss of chimerism induced by RLI is evidence of a strong in vivo alloresponse. We have previously demonstrated, in another mixed chimerism model, that the loss of chimerism induced by RLI is associated with initial splenomegaly and suppression of anti–third-party cytotoxic T lymphocyte (CTL) responses,6 similar to what had previously been considered to be GVH-associated immunodeficiency.28,29 Our study showed that this phenomenon, in which cytokines such as IFN-γ,30,31 IFN-α/β, transforming growth factor (TGF-β), macrophage colony-stimulating factor (M-CSF), tumor necrosis factor (TNF-α), and others32,33 have been implicated, can be just as readily induced by an HVG as by a GVH reaction.6 Among these cytokines, IFN-γ has been reported to mediate antitumor effects.34-39
Consistent with the earlier hypothesis, serum IFN-γ levels were significantly elevated in animals that lost chimerism rapidly following RLI. Moreover, the complete absence of an antitumor effect of RLI in IFN-γ–deficient mice receiving IFN-γ–deficient RLI demonstrates a critical role of IFN-γ in the antitumor response induced by RLI. Interestingly, despite the absence of IFN-γ, these mice lost donor chimerism, whereas chimeric IFN-γ–deficient mice not receiving RLI showed stable levels of donor chimerism by 10 weeks. Thus, the loss of chimerism in IFN-γ–deficient recipients of IFN-γ–deficient RLI shows clearly that host-derived IFN-γ is not needed for this effect.
In vitro studies suggest that IFN-γ mediates an antitumor effect against A20 via indirect, rather than direct, mechanisms. IFN-γ did not demonstrate a direct proapoptotic effect on A20, and only very high concentrations of IFN-γ partially inhibited the proliferation of the tumor. This modest antiproliferative effect was evident only at 48 hours and not at other time points (24, 96 hours also evaluated [data not shown]). Most importantly, IFN-γ did not affect the proliferation of A20 cells at concentrations similar to those detected in sera of RLI recipients that lost chimerism by the time of tumor injection (7 days after RLI, ie, < 250 pg/mL).
IFN-γ has been reported to induce the expression of MHC class I and Fas on tumor cells and thereby increase their immunogenicity and susceptibility to CTL killing.40,41 Preliminary studies indicate that IFN-γ increases the expression of Fas on A20 cells (not shown), consistent with this latter hypothesis. Studies are in progress to investigate the possible collaboration between IFN-γ and alloreactive cellular effectors in the antitumor effect of RLI. One possible mechanism for the antitumor effect of RLI-induced alloreactivity is that activated recipient CTLs recognizing donor alloantigens kill tumor cells by a bystander mechanism. Such a mechanism, involving Fas-mediated killing of the tumor, has been implicated in the antitumor effect of nonspecific, allogeneic, and autologous anti–third-party CTLs against human chronic lymphocytic leukemia (CLL) in vitro and in an immunodeficient mouse model.42,43
Another possible pathway by which RLI might mediate antitumor effects could be an adjuvant effect on recipient antigen-presenting cells (APCs) that process and present donor alloantigens as peptides in the context of host MHC molecules. These same APCs, which would be activated by T cells recognizing donor alloantigens through the indirect pathway, might also process and present tumor antigens in the context of host MHC molecules to T cells. The APC activation by alloreactive T cells would help to induce an effective tumor antigen–specific response. It is noteworthy that no MHC antigens were shared by donor and recipient in these studies, ruling out a similar adjuvant effect because of direct allorecognition of donor APCs by T cells in the RLI. We are currently testing the hypothesis that sharing of MHC antigens by donor and host, as in the haploidentical or MHC-identical, minor histocompatibility antigen-mismatched setting, might lead to even stronger antitumor effects of RLI, because of the ability of the same donor APCs to present donor alloantigens recognized through the more potent direct pathway of allorecognition, as well as tumor antigens in the context of host MHC molecules.
We have recently shown that lymphohematopoietic GVH reactions induced by DLI can mediate GVL effects without causing GVHD in a T-cell leukemia/lymphoma model, EL4.8,22 The current results extend this observation to a myeloid leukemia model in mixed chimeras established with nonmyeloablative conditioning. It is noteworthy that the magnitude of antitumor effects against this tumor was not strikingly different between the DLI and RLI recipients. However, additional studies, including titrations of tumor numbers, evaluation of truly lymphohematopoietic tumors (the myeloid tumor studied, MMB.319, grew mainly in a nonlymphohematopoietic site, the peritoneal cavity), and evaluation of nonlymphohematopoietic tumors will be essential to compare the relative potencies and optimal situations in which DLI or RLI might be superior approaches to achieving antitumor effects.
In summary, we report here a novel strategy for achieving antitumor effects, involving the induction of mixed hematopoietic chimerism with nonmyeloablative conditioning, followed by intentional elimination of chimeric cells with a recipient lymphocyte infusion. Elucidation of the mechanisms of this phenomenon may provide insights into the paradoxical antitumor effects seen in some patients who lose chimerism following nonmyeloablative BMT and suggest a new, low-risk approach to achieving antitumor effects following nonmyeloablative hematopoietic cell transplantation.
Prepublished online as Blood First Edition Paper, June 5, 2003; DOI 10.1182/blood-2002-12-3949.
Supported by National Institutes of Health (grant RO1 CA79989), La Fondation de France and La Federation Nationale des Centres de Lutte Contre le Cancer (M-T.R.), and Deutsche Krebshilfe, Mildred-Scheel-Stiftung (Y-M.K.).
M-T.R. and Y-M.K. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Mr Orlando Moreno for expert animal care, Drs Ephraim Fuchs and Ronjon Chakraverty for helpful review of the manuscript, and Ms Robin Laber for expert assistance in manuscript preparation.