Abstract
Inhibitors in patients with hemophilia are a rare complication of a rare disease causing pain and disability in patients and impairment to the quality of their lives. Recent advances in treatment have brought improvements, but they have done so by absorbing larger amounts of financial resources. This study involved 52 Italian patients with hemophilia with high-responding inhibitors who were longitudinally observed for 18 months to evaluate concomitantly cost of care and quality of life. Overall, 0.6 bleeding episodes per patient per month were recorded. This frequency of events was lower than that reported in other cohorts of patients with hemophilia who were not taking inhibitors. The average monthly cost of care was, in euros, €18 000 (US $18 000) per patient, mainly because of treatment products. Recombinant activated factor VII, mostly used for orthopedic surgery, represented 50% of the expenses. Quality of life, measured through validated questionnaires, was similar to that of patients with severe hemophilia without inhibitors. In particular, physical quality of life was similar to that in patients with diabetes and on dialysis, whereas mental quality of life was comparable to that in the general population. This study shows that hemophilia complicated by inhibitors, a prototype of rare disease, requires high amounts of resources for management that provides a satisfactory quality of life. (Blood. 2003;102:2358-2363)
Introduction
In persons with hemophilia, bleeding and its complications in muscles and joints often lead to pain and disability and to dramatic impairment in the overall quality of life.1 The availability of more effective treatments has generally improved the management of patients with hemophilia. However, because the cost of treatment and the complex nature of the disease warrant a multidisciplinary approach, the health care of these patients absorbs a large amount of economic and human resources and can be taken as an example of the socioeconomic impact of biotechnology in rare diseases.2-4 This situation becomes extreme when persons with hemophilia develop inhibitory antibodies that compromise the mainstay of treatment—that is factor replacement.5 The overall prevalence of inhibitors in patients with hemophilia A or B is estimated to be approximately 9% and 3%, respectively; patients with moderately severe (factor levels 1%-5%) and severe hemophilia A (less than 1%) have a greater risk for inhibitors; reported prevalences for them are between 7% and 20%.6
Special therapeutic approaches are used in patients with inhibitors to reduce antibody levels,7,8 such as the induction of immune tolerance9,10 and plasma exchange with or without immunoadsorption on protein A columns. For the actual treatment of bleeding episodes, the main therapeutic approaches are large doses of human factor VIII (FVIII), FVIII derived from porcine plasma and products that bypass the coagulation defect, such as prothrombin complex concentrates (PCCs), activated prothrombin complex concentrates (aPCCs),11-14 and recombinant activated factor VII (rFVIIa).15-19
The introduction and availability of these products in treatment have contributed to prolonging patients' life expectancy and to improving their quality of life.20 On the other hand, the management of hemophilia with inhibitors is particularly expensive, both in absolute terms and in comparison with the treatment of hemophilia without inhibitors.21 Available studies on costs and quality of life are retrospective, often carried out in single centers on very small numbers of patients, and focused mainly on drug costs.4,5 No prospective study has specifically focused on patients with hemophilia and inhibitors with the objective of assessing the global socioeconomic and cultural impact of management. To fill this gap, a prospective, longitudinal, prevalence-based, multicenter cost of care study (Cost of Care Inhibitors Study [COCIS]) was designed and carried out by the Italian Association of Hemophilia Centers. Quality of life was also evaluated in the same patients.
Patients, materials, and methods
Goals and techniques
To reach the objectives of the study, we conducted a cost-of-care analysis, a technique used to evaluate the economic burden of a disease.22 Health-related quality of life (HRQOL) was also evaluated. Because the measurement of costs depends on the point of view adopted for the analysis (eg, a hospital admission may represent a cost to the national health service or to an insurance company but not to the patient), the study was carried out from the point of view of the community, the largest entity that can have a point of view, and included the Italian third-party payer (National Health Service), patients, and their families.
Study cohort
After informed consent was obtained, patients were consecutively enrolled during 1998 and 1999 in 11 hemophilia centers throughout Italy. They had to satisfy the following inclusion criteria: age older than 14 years; moderate or severe hemophilia A or B; and presence of inhibitors, irrespective of their levels. Clotting factor deficiency was considered severe (less than 1%) or moderate (1%-5%), according to factor VIII (FVIII) or FIX plasma levels. Patients with inhibitors were considered low (less than 5 Bethesda units [BU]) responders or high (more than 5 BU) responders according to the historical peak inhibitor level. Patients were excluded from the study if they had autoantibodies and if inhibitors disappeared after an initial onset. Approval for the study was granted by the insitutional review board of the coordinating center, IRCCS Maggiore Hospital.
Observation period
Patients enrolled were observed globally for 18 months. They were asked to provide information on resource use and HRQOL in the 6 months preceding the enrollment; subsequently, they were prospectively followed up for 12 months. For the purpose of this study, data were collected 3 times during the study period—at enrollment and 6 and 12 months later.
Data collection
To evaluate the cost of care and the HRQOL, patients were interviewed by means of a specially designed semistructured questionnaire administered to them by a physician at each hemophilia center. At the time of the enrollment visit, information was obtained on demographic characteristics, number and sites of bleeding episodes, replacement therapy, surgical procedures, concomitant diseases, physicians' visits, hospitalizations, and, in general, all events leading to hemophilia-related absorption of health care resources during the 6 months before enrollment. This information was also collected prospectively during the follow-up period of 12 months. Orthopedic status was evaluated by calculating the World Federation of Hemophilia Orthopedic Joint Score23 at enrollment and during the follow-up visits. Specifically, joint swelling, chronic synovitis, muscular atrophy and crepitus on motion during movement, disability and flexion contractures, axial deformity in knee or ankle, functional deficit, and pain were recorded. The global orthopedic joint score per body side ranges between 0 (best orthopedic condition) and 15 (worst).
Cost-of-care analysis
Medical costs were quantified considering the point of view of the third-party payer, the National Health Service (NHS), which in Italy, is in charge of financing and providing health care services to patients with hemophilia. Direct medical costs borne by the NHS were calculated by multiplying resources absorbed by their unit cost. They included the cost of therapy with coagulation factors, hospitalizations because of bleeding, laboratory and other diagnostic examinations, surgery, rehabilitation procedures, physicians' visits, and any other possible cost. Diagnosis-related group (DRG)24 tariffs were applied to estimate the cost of hospitalizations. When hospitalizations resulted from bleeding episodes or other hemophilia-related reasons, the tariff corresponding to DRG 397 was applied. Hospitalizations for joint replacement were computed applying the tariff for DRG 209. Even though DRG tariffs normally include the costs for replacement therapy during hospitalization, these costs were considered separately in our analysis because this method of reimbursement for health care services is not adequate for hemophilia care. Accordingly, in some but not in all Italian regions, a separate budget is used for costs related to replacement therapy. Tariffs were applied also to compute other costs for hemophilia care, specifically physiotherapy and medical visits at the hemophilia centers.25 Costs for minor surgery, conducted on an outpatient or day surgery basis (eg, dental or eye surgery), were also included in the analysis. All costs are expressed in euros (€): at the time of analysis, €1 was roughly equivalent to $1 US. Unit costs are reported in Appendix 2.
Quality of life
To evaluate HRQOL, widely used self-administered questionnaires such as the EuroQoL (EQ-5D) and the Medical Outcome Survey Short Form-36 (SF-36) were adopted. EQ-5D is applicable to a wide range of medical conditions and treatments and generates a health profile (EQ profile) consisting of 5 domains (mobility, self-care, anxiety/depression, usual activities, and pain/discomfort) and 3 levels (“no problem,” “some or moderate problems,” “extreme problems/impossible to do”). A visual analog scale (EQ-VAS) scores the overall HRQOL from 0 (the worst imaginable health status) to 100 (the best imaginable health status).26,27 Results from the EQ profile can be converted to utility score, suitable for economic evaluations, by means of an algorithm that uses population-based (social) values.28,29 Because specific conversion values for the Italian population are not available yet, to convert our EQ profile results in EQ utility index, the algorithm was implemented with values from 2 European regions (United Kingdom and Catalonia).30
Designed for use in clinical practice and research, health policy evaluations, and general population surveys, the SF-36 questionnaire is considered a standard instrument for patient-based health care outcome assessment.31,32 It has been recently validated in Italy33 and been used in several studies ranging from epidemiological to clinical trials. Applicable to adults and adolescents, SF-36 assesses 8 dimensions of HRQOL that relate to the physical and mental components of health perception. Physical functioning, role-physical, and bodily pain are more related to the physical component; social functioning, role-emotional, and mental health are more related to the mental component; and energy/vitality and general health relate to both components. These 8 dimensions can be grouped in 2 summary scores (physical component summary [PCS] and mental component summary [MCS]).31,32 These global measures were estimated using standard US algorithms.34 To test the internal consistency of SF-36 in inhibitor patients, the Cronbach α was computed, with values greater than 0.70 considered satisfactory.35
Statistical analysis
For cost-of-care analysis, we used means as central tendency parameters, generally expressed as mean cost per patient per month, because this parameter can be easily used to make projections on different populations and is of easy use for policy makers. Because of the highly skewed distribution of cost variables, we report, as a variability measure, the distribution of costs per patient per month, instead of standard deviations. Costs were stratified according to their category, specifically costs of treatment with clotting factors, hospitalizations, surgery, and other sources of resource adsorption. Descriptive statistics were applied also to define HRQOL and health status measurement variables. All analyses were performed using SPSS version 11.0 software (SPSS, Chicago, IL).
Results
Clinical evaluation
The main demographic and clinical characteristics are shown in Table 1. Fifty-two hemophilia patients with inhibitors, ranging from 15 to 64 years of age, were originally enrolled in the study. Of that group, 10 were fully evaluated at baseline but could no longer be followed up by 1 center for administrative reasons: 42 patients were followed-up for 6 months and 41 for 12 months, totaling 810 person-months. All patients had hemophilia A, and most of them had severe FVIII defects, were positive for hepatitis C virus (HCV), and had a high degree of disability because of hemophilia. A small proportion of patients was infected with HIV. Even though at the time of enrollment half the patients had high levels of inhibitors (median, 16.5 BU; range, 6-1300 BU) and half had low levels of inhibitors (median, 2.0 BU; range, 1-5 BU), on the basis of the maximum level recorded historically all patients but one were classified as high responders. For orthopedic status, the total score and the sum of left- and right-side scores ranged widely, with a median of 9, without differences between the left and right contributions and over the follow-up period. Approximately one fourth of the patients had synovitis.
Overall, 488 bleeding episodes were reported during the 18-month study period, corresponding to an average of 0.6 events per patient per month (Table 2). Of the patients enrolled, 81% had at least one bleeding event: 72% had hemarthrosis, and 44% had hematomas. As to other events that required treatment with clotting factors, 11 surgical procedures were carried out during the study period (18 months): 5 knee replacements, 1 hip replacement, 4 dental surgeries, and 1 cataract surgery. Four patients were involved in accidents, of which at least one was attributable to joint disability (femur fracture resulting in a fall in a patient with severe gait problems).
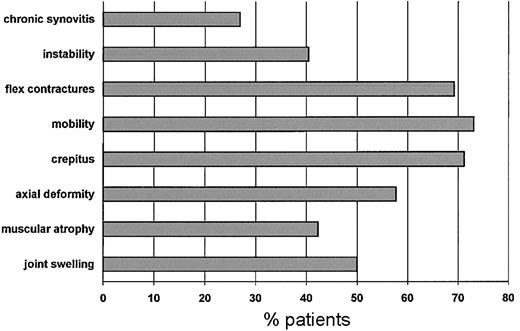
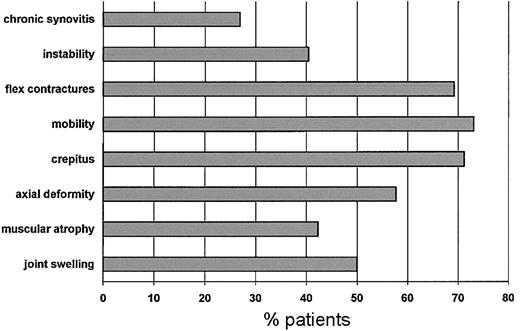
Figure 1 shows the frequency of patients who had impaired orthopedic functioning at enrollment for each of the items included in the orthopedic joint score. The most frequent problems were flexion contractures, reduced joint mobility, and crepitus on motion. During the study period only one patient scored 0 in all dimensions. These data were reproducible at assessments performed 6 and 12 months later.
Frequency distribution of patients with impaired orthopedic function for each dimension at enrollment.
Frequency distribution of patients with impaired orthopedic function for each dimension at enrollment.
Use of resources
The product most widely used for treatment during the observation period was recombinant FVIIa (rFVIIa) (13 mg per patient per month) used by 71% of patients, followed by aPCC (3000 IU per patient per month), used by almost half the patients (46.7%). Thirty-one percent of them received high doses of human FVIII, plasma-derived (6300 IU per patient per month; 25%) or recombinant (2140 IU per patient per month; 5.8%) to treat or prevent bleeding episodes or to induce immunotolerance.
Table 3 shows the annual per capita consumption of health care resources other than concentrates, for hemorrhages or other reasons attributable to hemophilia. Overall, 30 hospital admissions were recorded, corresponding to 0.4 admissions per patient-year. As to the reasons for admission, joint and muscle bleeding was the most frequent cause (37%), and 20% of admissions were for joint surgery. An average of 6 in-hospital days per patient-year were recorded, of which more than 43% were for joint surgery, whereas 21.6% were attributable to joint or muscle bleeding. One third of the in-hospital stays were for patients in the intensive care unit. Almost one medical visit per patient-year was recorded, mostly at the hemophilia center. Finally, patients had a mean of 10 physiotherapy sessions per year.
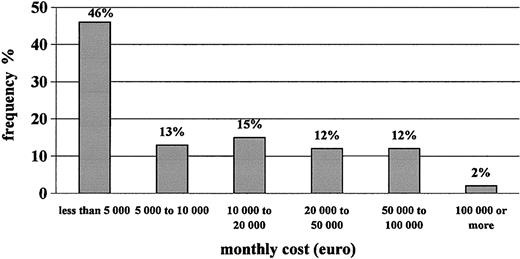
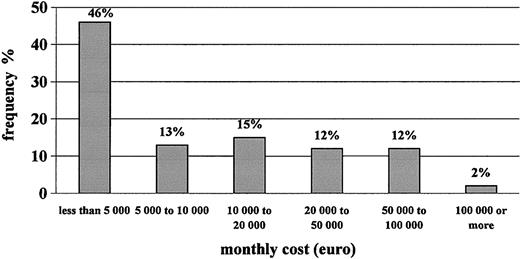
Table 4 shows that the monthly cost of care to the NHS for hemophilia patients with inhibitors is approximately €18 000 per patient-month. Replacement therapy accounts for 99% of total medical costs, with rFVIIa representing approximately half of them, followed by plasma-derived FVIII, rFVIII, and aPCC. The frequency distribution of patients according to cost per month for therapy with clotting factors is shown in Figure 2. Approximately 60% of patients generated monthly costs of less than €10 000, whereas a small portion of them, less than 15% of our sample, generated monthly costs higher than €50 000. The major component of cost, rFVIIa, was used mostly for surgery (almost half the total consumption). The average cost per surgical intervention with rFVIIa was €256 000.
Frequency distribution of patients according to monthly cost of clotting factors used.
Frequency distribution of patients according to monthly cost of clotting factors used.
Quality of life
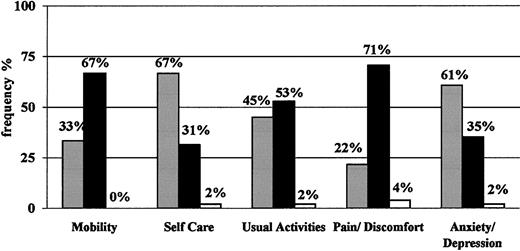
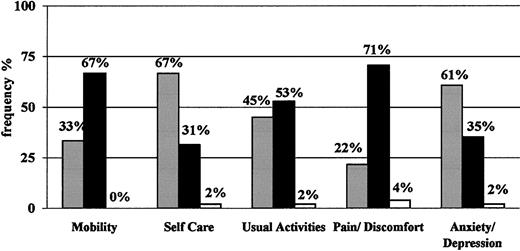
On average, the level of HRQOL shown by the sample of subjects enrolled was stable over time (data not shown). For simplicity, therefore, only results at the baseline visit are reported. Figure 3 shows the EQ-5D profile: approximately two thirds of patients reported “some/moderate problems” in the physical sphere, specifically for mobility, and pain/discomfort. Approximately half of them had some problems in performing usual activities, whereas only one third reported “some/moderate problems” in self-care and anxiety/depression. No more than 2 (4%) patients reported “extreme problems” in one or more dimensions. The mean utility values indicated by these patients with the EQ-5D instrument were 0.66 (SD, 0.25; median, 0.69; range, -0.02 to 1.00; United Kingdom conversion values) and 0.69 (SD, 0.25; median, 0.71; range, -0.03 to 1.00; Catalonia conversion values) (see “Patients, materials, and methods”). These results are consistent with those obtained with the visual analogue scale, which showed a mean of 65.5 (SD, 16.5), a median of 66, and a range from 30 (4% of patients) to 95 (4% of patients).
EQ-5D profile. Expressed in percentages of patients classified as having no problems (▪), some or moderate problems (•), or extreme problems/impossible to do (□) at the baseline visit for each dimension.
EQ-5D profile. Expressed in percentages of patients classified as having no problems (▪), some or moderate problems (•), or extreme problems/impossible to do (□) at the baseline visit for each dimension.
Table 5 shows that SF-36 gave lower scores in the dimensions related more to the physical components of health (physical functioning, role-physical, and bodily pain) and higher scores in dimensions related more to the mental component (social functioning, role-emotional, mental health). For dimensions equally related to physical and mental health components, the general health score was relatively low, whereas the vitality score was higher. The global physical and mental measures scored, respectively, means of 36.9 and 50.2. Internal consistency of SF-36 was excellent, with Cronbach α values ranging from 0.8 to 0.9 for every dimension of the questionnaire.
Discussion
This is a longitudinal, prospective, prevalence-based multicenter study that enrolled approximately one third of the adult population of hemophiliac patients with inhibitors in Italy. When put in the context of a rare complication of a rare disease (it can be estimated that there are 3 patients with inhibitors per 1 million population36 ), the sample size of 52 patients should be considered representative. Surprisingly, patients, almost all of whom were high responders, reported a low incidence of bleeding episodes, less than 1 per patient-month, with 19% of them reporting no bleeding at all. Possible explanations for this are reluctance to seek treatment that in the past was poorly effective or particularly prudent behavior in daily activities because of the fear of bleeding or of severe impairment of motion.
Our study provides evidence of the strikingly high amount of financial and medical resources absorbed for the care of hemophiliac patients with inhibitors.5,21 The average cost to care for a patient with inhibitor is estimated to be €18 000 per month. That is likely one of the highest burdens for a disease. This cost becomes less impressive when one calculates that, according to these figures and to the prevalence of hemophiliac patients with inhibitors in Italy, the cost of care met by each Italian citizen for all these patients is approximately €0.7 per year. Clotting factors amount to more than 99% of medical costs, half of which are attributable to the use of rFVIIa. Almost half of rFVIIa, and, hence, almost one fourth of the total costs were used for surgical interventions, mainly joint replacements. It should be noted that during the 18-month period, 6 of 52 patients underwent surgery for orthopedic consequences of hemophilia, corresponding to an incidence rate of 7.4 per 1000 person-months. As a consequence, the distribution of the cost of care for hemophiliac patients with inhibitors is extremely skewed (Figure 2), with a major component attributable to patients who underwent joint replacement. The incidence rate of knee replacement seems particularly high and is likely to be a short-term observation, perhaps attributable to the need for surgery accumulating in past years because of previous difficulties in performing these interventions. If this explanation is valid, the estimates from our study are representative of short- and middle-term costs but are likely to become comparatively smaller in the long-term. Medical costs other than those for replacement therapy, even though their monetary values to the NHS were minimal, represented a relatively high burden, not in financial but in human resources terms, given the high frequency of hospital admissions and physiotherapy sessions.
Our longitudinal study provides indirect evidence of higher costs of care for hemophiliac patients with inhibitors compared with those without inhibitors4 and confirms findings from the retrospective studies of Goudemand and Honda et al.5,20 Goudemand compared therapeutic costs in a cohort of French patients with severe hemophilia A or B, with and without inhibitors.5 Before the introduction of rFVIIa, the average annual costs for patients with high titers of inhibitors were more than €56 000 per patient; this was 1.3 times more than costs for patients without inhibitors. After the introduction of therapy with rFVIIa, the costs to treat high responders increased to almost €190 000 per patient per year, 3 times more than the costs to treat patients without inhibitors. These data, corresponding to almost €16 000 per month, are comparable to those obtained in our study.
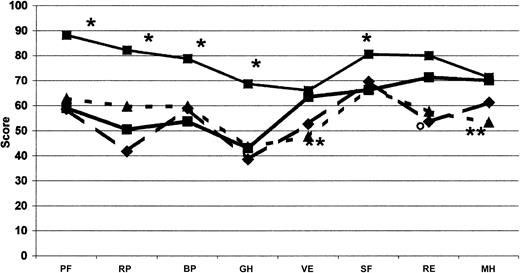
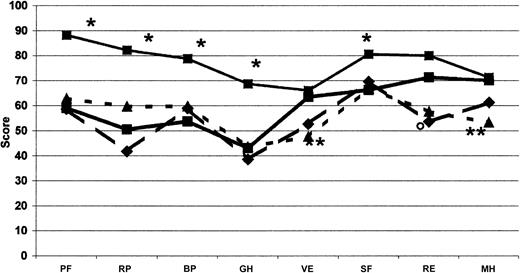
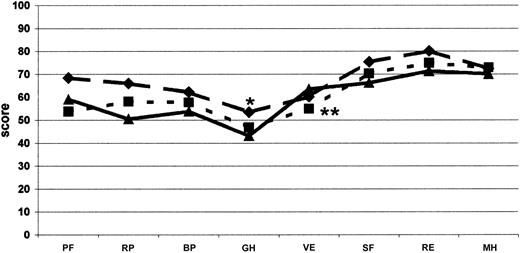
We found it essential to frame these huge costs in the context of quality of life, which was evaluated concomitantly with costs in this cohort of Italian patients with inhibitors. The most impaired areas of HRQOL are those related to physical domains. When compared with men from the general Italian population37 and with other groups of patients—those with severe hemophilia,38 European patients with moderate and severe hemophilia on on-demand treatment,39 Italian patients with diabetes and on dialysis37 —it appears that the expensive care of inhibitor patients led to a satisfactory level of HRQOL. Even though our patients showed an important impairment of physical health perception compared with the Italian general population37 (Table 5; Figure 4), the mental domains, excluding social activities, did not show substantial differences. On the other hand, no difference was found between our patients and other hemophilia patients without inhibitors38,39 with regard to domains related more to the physical and mental components of health perception. Our patients had slightly higher values for vitality and lower for general health (Table 5; Figure 5). Moreover, mental health perception, as summarized by the MCS score, was similar between our patients and patients with severe hemophilia without inhibitors,38 whereas the PCS score, pertaining to the physical component of health perception, was higher (Table 5). Our patients perception of HRQOL was substantially comparable to that of Italian patients with diabetes and on dialysis,37 apart from vitality and mental health for both conditions and role-emotional for patients on dialysis, which were better in hemophilia patients with inhibitors (Table 5; Figure 4). HRQOL, evaluated by means of the EQ-5D instrument, was comparable in our inhibitor patients and in patients with severe hemophilia without inhibitors evaluated by Miners et al.38 Moreover, the utility index and the VAS score calculated in our inhibitor patients were similar to those reported by Miners et al,38 whose median utility index was reported to be 0.66 (range, -0.48 to 1.00) and whose median VAS score was 70 (range, 20-98). On the whole, comparing hemophilia patients with inhibitors with other patients, the global quality of life did not differ from that reported for other hemophilia patients without inhibitors38,39 (Figure 5) or from that reported for patients with such severe chronic diseases as diabetes and dialysis-dependent chronic renal failure37 (Figure 4).
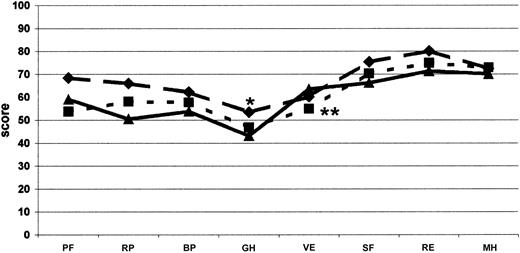
Comparison of SF-36 profile. Measured at the baseline for each dimension with other profiles in healthy men from the general Italian population (•, thin solid line; n = 999), patients with diabetes (▴, dotted line; n = 98), and patients on dialysis (♦, dashed line; n = 87).38 •, thick line indicates COCIS (n = 50). *Significant difference (P < .05), as computed by Student t test, between our hemophilia patients with inhibitors and the Italian male population. **Italian patients with diabetes and on dialysis. ○ indicates Italian patients with diabetes; PF, physical functioning; BP, bodily pain; RP, role-physical; GH, general health; VT, energy/vitality; RE, role-emotional; SF, social functioning; and MH, mental health.
Comparison of SF-36 profile. Measured at the baseline for each dimension with other profiles in healthy men from the general Italian population (•, thin solid line; n = 999), patients with diabetes (▴, dotted line; n = 98), and patients on dialysis (♦, dashed line; n = 87).38 •, thick line indicates COCIS (n = 50). *Significant difference (P < .05), as computed by Student t test, between our hemophilia patients with inhibitors and the Italian male population. **Italian patients with diabetes and on dialysis. ○ indicates Italian patients with diabetes; PF, physical functioning; BP, bodily pain; RP, role-physical; GH, general health; VT, energy/vitality; RE, role-emotional; SF, social functioning; and MH, mental health.
Comparison of SF-36 profile. Measured at the baseline visit for each dimension and compared with other studies on hemophilia. The continuous line (▴; n = 50) shows the results from this study; the dotted line (♦; n = 590), the data from Royal et al39 on adult moderate and severe hemophiliacs without inhibitors on on-demand treatment; and the broken line (•; n = 65), the data from Miners et al38 on patients with severe hemophilia without inhibitors. Significant difference (P < .05), as computed by Student t test (*Miners et al38 ; **Royal et al39 ). Abbreviations as used in Figure 4.
Comparison of SF-36 profile. Measured at the baseline visit for each dimension and compared with other studies on hemophilia. The continuous line (▴; n = 50) shows the results from this study; the dotted line (♦; n = 590), the data from Royal et al39 on adult moderate and severe hemophiliacs without inhibitors on on-demand treatment; and the broken line (•; n = 65), the data from Miners et al38 on patients with severe hemophilia without inhibitors. Significant difference (P < .05), as computed by Student t test (*Miners et al38 ; **Royal et al39 ). Abbreviations as used in Figure 4.
In conclusion, hemophilia with inhibitors represents an example of a rare disease that requires high amounts of resources, but it also demonstrates that effective care provides a satisfactory quality of life. The costs, which look exorbitant in absolute terms, are small when ascribed to individual citizens. Moreover, it is likely that therapeutic strategies aimed at preventing severe arthropathy, and consequently the need for surgery, are likely to dramatically affect health care costs, disability, and a patient's perception of health.
Appendix 1: study group members
Franco Baudo (Niguarda Hospital, Milan); Mauro Berrettini (Ospedale Civile, Orvieto); Gianpiera Bertolino (IRCCS Policlinico, Pavia); Luisa Bizzoni (La Sapienza University, Rome); Giovanni Longo (Careggi Hospital, Firenze); Roberto Musso (Garibaldi Hospital, Catania); Monica Rivi, Elena Santagostino (IRCCS Maggiore Hospital, Milan); Angiola Rocino (S. Benedetto Hospital, Naples); Antonio F. Scaraggi (Policlinic Hospital, Bari); Giuseppe Tagariello (Ospedale Civile, Castelfranco Veneto); and Annarita Tagliaferri (Policlinic Hospital, Parma).
Prepublished online as Blood First Edition Paper, June 19, 2003; DOI 10.1182/blood-2003-03-0941.
Supported by an unrestricted research grant from Novo Nordisk Farmaceutici.
The list of COCIS investigators appears in “Appendix 1.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.