Abstract
FLT3 is a receptor tyrosine kinase involved in the proliferation and differentiation of hematopoietic stem cells. FLT3 internal tandem duplications (FLT3/ITDs) are reported in acute myeloid leukemia (AML) and predict poor clinical outcome. We found FLT3/ITDs in 11.5% of 234 children with de novo AML. FLT3/ITD-positive patients were significantly older and had higher percentages of normal cytogenetic findings or French-American-British (FAB) classification M1/M2 and lower percentages of 11q23 abnormalities or FAB M5. FLT3/ITD-positive patients had lower remission induction rates (70% vs 88%; P = .01) and lower 5-year probability rates of event-free survival (pEF) (29% vs 46%; P = .0046) and overall survival (32% vs 58%; P = .037). Patients with high ratios (higher than the median) between mutant and wild-type FLT3 had significantly worse 2-year EFS rates than FLT3/ITD-negative patients (pEFS 20% vs 61%; P = .037), whereas patients with ratios lower than the median did not (pEFS 44% vs 61%; P = .26). FLT3/ITD was the strongest independent predictor for pEFS, with an increase in relative risk for an event of 1.92 (P = .01). Using an MTT (methyl-thiazol-tetrazolium)-based assay, we studied cellular drug resistance on 15 FLT3/ITD-positive and 125 FLT3/ITD-negative AML samples, but we found no differences in cellular drug resistance that could explain the poor outcomes in FLT3/ITD-positive patients. We conclude that FLT3/ITD is less common in pediatric than in adult AML. FLT3/ITD is a strong and independent adverse prognostic factor, and high ratios between mutant and WT-FLT3 further compromise prognosis. However, poor outcomes in FLT3/ITD-positive patients could not be attributed to increased in vitro cellular drug resistance. (Blood. 2003;102:2387-2394)
Introduction
The prognosis for children with acute myeloid leukemia (AML) has improved considerably in the last few decades, with overall survival rates of 50% to 60%.1-5 However, relapse remains the most important cause of failure, occurring in 30% to 40% of children in first complete remission (CR).
The wild-type (WT) FLT3 receptor is a class 3 receptor tyrosine kinase expressed on hematopoietic progenitor cells and on most leukemias, such as AML, chronic myeloid leukemia (CML) in blast crisis, and acute lymphoblastic leukemia (ALL).6-8 Recently, an internal tandem duplication of FLT3 has been described (FLT3/ITD) that leads to ligand-independent FLT3 dimerization and constitutive activation through autophosphorylation, resulting in a proliferation and survival advantage of the cell.9-11 In humans, FLT3/ITD is found almost exclusively in AML.12-14
Recently, several large studies showed that FLT3/ITD occurs in approximately 20% to 30% of adults with AML. In most, but not all, studies it is associated with poor clinical outcome.15-18 In AML in children, the incidence of FLT3/ITD was reported to be lower than in adults, and it was also associated with poor prognosis.19-23 However, all pediatric studies published to date included only relatively small numbers of patients.19-23 Interestingly, a high mutant versus wild-type FLT3 (WT-FLT3) ratio further compromises prognosis, as has been described for adults and children with AML.23-25
Differences in prognosis may reflect differences in cellular drug resistance, which we have previously demonstrated in several other studies in childhood leukemia.26-28 Cellular drug resistance is also an independent risk-factor in childhood ALL.29 In AML in children, cellular drug resistance is related to short-term clinical response.30,31
In this study, we retrospectively investigated the incidence and prognostic impact of FLT3/ITD and the ratios between mutant and WT FLT3 in 234 children with de novo AML, included in the BFM-AML 1987, 1993, and 1998 studies or in the corresponding BFM-based Dutch acute nonlymphoblastic leukemia (ANLL) 1987 and 1994 protocols, all consisting of intensive cytarabine/anthracycline-based treatment. The present study is the largest pediatric cohort published to date. In addition, we analyzed whether increased cellular drug resistance could be a mechanism for the poor clinical outcome of FLT3/ITD-positive AML.
Patients, materials, and methods
Patient samples
We tested bone marrow or peripheral blood samples from children from birth to 18 years of age. Samples were taken only after informed consent had been obtained and with VU University Medical Center institutional review board (IRB) approval. Two collaborative groups participated in this study: the AML-Berlin-Frankfurt-Münster Study Group (AML-BFM; Münster, Germany) and the Dutch Childhood Leukemia Study Group (DCLSG; The Hague, the Netherlands). Each study group performed central review of the diagnosis and classification and clinical follow-up of the patients. For drug-resistance testing, samples were sent to the Research Laboratory of Pediatric Oncology in Amsterdam. These samples were assessed for FLT3/ITD in collaboration with one of the coauthors of this study (S.M.) for patients included in the BFM-AML 1987 and 1993 studies and the corresponding Dutch protocols. A second cohort of patients, included in the BFM-AML 1998 protocol, was analyzed for FLT3/ITD by another coauthor (F.G.).
All patients were reclassified according to the BFM-AML risk group classification criteria. Patients at standard risk (SR) were defined as having favorable morphology (French-American-British [FAB] classification M2 with Auer rods, M3, or M4Eo) and blast cell reduction in the bone marrow on day 15 to less than 5% (not obligatory for FAB M3). All other patients were considered at high risk HR.32
Treatment protocols
Patients were treated according to several different treatment protocols, including the German AML-BFM 1987, 1993, and 1998 studies and the Dutch protocols DCLSG ANLL 1987 and 1994, which were based on their corresponding BFM counterparts.1,2,32-34 Because the treatments according to these protocols were similar, patients were analyzed together for the prognostic significance of FLT3/ITD.
In brief, the AML BFM 1987 protocol started with an 8-day induction course, followed by a 6-week consolidation block. Subsequently, 2 intensification courses were given. Intrathecal chemotherapy, cranial irradiation, or both were given as CNS prophylaxis (irradiation in approximately half the patients, randomly assigned).35 This was followed by maintenance therapy, up to a total treatment duration of 18 months. Sibling donor allogeneic stem cell transplantation (SCT) was advised for patients at high risk in first CR.
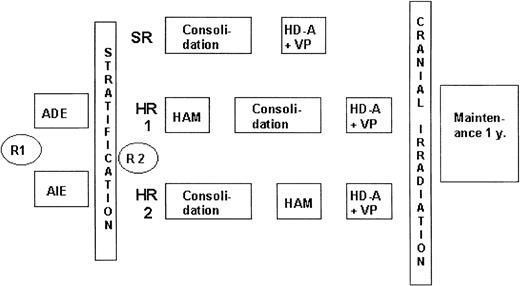
In the AML BFM 1993 study (Figure 1), patients were stratified according to risk groups. At diagnosis, patients were randomly assigned to daunorubicin (ADE) or idarubicin (AIE) induction therapy, which did not result in differences in long-term outcome.1 For HR patients, one of the intensification blocks was changed to high-dose cytarabine with mitoxantrone (HAM), which improved treatment outcome compared with the AML BFM 1987 protocol.2 Sibling SCT was advised for HR patients in first CR. Patients at standard risk did not receive HAM, and outcomes were identical to those in the AML BFM 1987 study.
AML-BFM 93 treatment protocol. In the AML BFM 93 study, patients were stratified according to risk groups (SR or HR) after induction therapy. At diagnosis, patients were randomly assigned between induction therapy with daunorubicin (ADE) and idarubicin (AIE). SR patients were then treated with a 6-week consolidation block followed by an intensification course. For HR patients, 2 intensification blocks were given. The first (HAM) was given either before or after the 6-week consolidation block; the second (HD-A+VP) was given as the last intensification block. Sibling SCT was advised for HR patients in first CR. R1 indicates first randomization; R2, second randomization; y, year; ADE, Ara-C (cytarabine), daunorubicin, etoposide; AIE, Ara-C, idarubicin, etoposide; HAM, high-dose Ara-C and mitoxantrone; HD-A, high-dose Ara-C; and VP, etoposide.
AML-BFM 93 treatment protocol. In the AML BFM 93 study, patients were stratified according to risk groups (SR or HR) after induction therapy. At diagnosis, patients were randomly assigned between induction therapy with daunorubicin (ADE) and idarubicin (AIE). SR patients were then treated with a 6-week consolidation block followed by an intensification course. For HR patients, 2 intensification blocks were given. The first (HAM) was given either before or after the 6-week consolidation block; the second (HD-A+VP) was given as the last intensification block. Sibling SCT was advised for HR patients in first CR. R1 indicates first randomization; R2, second randomization; y, year; ADE, Ara-C (cytarabine), daunorubicin, etoposide; AIE, Ara-C, idarubicin, etoposide; HAM, high-dose Ara-C and mitoxantrone; HD-A, high-dose Ara-C; and VP, etoposide.
Protocol AML BFM 1998 consists of induction therapy with the idarubicin block, followed by HAM. In the consolidation phase, patients were randomly assigned to receive the 6-week consolidation block followed by 1 intensification block versus 3 intensive courses of chemotherapy.
Study DCLSG ANLL 1987 was similar to AML BFM 1987, but no prophylactic cranial irradiation or maintenance therapy was given. In protocol DCLSG ANLL 1994, all patients received the idarubicin arm in induction and the HAM block for consolidation. No maintenance therapy was given. Patients underwent transplantation with either an HLA-identical sibling donor graft or an autograft.
Analysis of the FLT3/ITD
A first cohort of patients was tested by Meshinchi et al,19 who developed a genomic polymerase chain reaction (PCR) for the detection of FLT3/ITD. DNA was obtained from bone marrow or peripheral blood cytospin slides (prepared after density gradient centrifugation using Ficoll Isopaque) that had been stored in liquid nitrogen. After thawing, extraction of genomic DNA was performed by adding 10 μL water to the leukemic cells. The DNA was PCR amplified (35 cycles) as described before.15,19 Patients who were FLT3/ITD positive were further examined using gene scan analysis to determine the ratio between mutant and WT-FLT3. The 2 primers used were 5FLT3/ITD, 5′-6FAMGCA ATT TAG GTA TGA AAG CCA GC-3′, and 3FLT3/ITD, 5′-CCT TCA GCA TTT TGA CGG CAA CC-3′ (Applied Biosystems, Foster City, CA). PCR was carried out in duplicate in a final volume of 50 μL containing genomic DNA (10 and 25 ng), KCl (50 mM), Tris-HCl (20 mM, pH 8.4), MgCl2 (3 mM), primers (10 μM each), nucleotides (0.2 mM each), and platinum Taq DNA polymerase (2.5 U; Invitrogen, Carlsbad, CA). Samples were processed through 35 cycles at 94°C for 5 minutes, 94°C for 30 seconds, 66°C for 1 minute, and 72°C for 2 minutes, with a final step for 7 minutes at 72°C. PCR products were diluted 1:100 with filtered Nanopure water and analyzed. This 1-μL sample was diluted in a 10-μL cocktail of formamide and size standard (0.05-μL size standard/sample) and run on the 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA) with the POP-4 Polymer. Data were analyzed using gene scan software.
A second cohort of patients was tested by F.K., L.M., and M.P. Total RNA was extracted from 1 × 107 cells using the RNEASY Minikit (Qiagen, Hilden, Germany). For the amplification of the ITD, the protocol of Nakao et al14 with the reported primers R5 and R6 was used. Samples were rated ITD positive if the ratio between FLT3/ITD and WT-FLT3 was 0.03 or greater.25
To study possible differences between genomic and reverse transcription-PCR (RT-PCR) detection methods, 10 samples were examined (blinded) to determine the FLT3/ITD status. No discrepancy was detected between the 2 methods, as has also been reported by Schnittger et al.18
Drug-resistance testing
Cellular drug-resistance studies were performed by C.M.Z. et al. Mononuclear cells were isolated by density gradient centrifugation, and, if necessary, blast cell enrichment was performed as described before.27 To measure cellular resistance, cells were seeded in 96-well microculture plates and cultured for 4 days. A panel of 14 different drugs was tested, including those frequently used in AML treatment.27 Control cell survival (CCS) was assessed by culturing cells without adding drugs. After 4 days, MTT was added, which could be converted by viable cells to a colored formazan product and measured spectrophotometrically at 562 nm. Optical density (OD) was linearly related to the number of viable cells. Results were considered evaluable only if control wells contained 70% or more leukemic cells and if the mean control OD exceeded 0.05 arbitrary units. The LC50 value, which is the drug concentration that kills 50% of the leukemic cells, was used as a measure of resistance. Part of the data on drug resistance in AML have been published elsewhere, but not in relation to FLT3/ITD.27,36
Statistical analysis
To assess outcome the following parameters were used: CR, event-free survival (EFS), disease-free survival (DFS), overall survival (OS), and cumulative incidence of relapses. CR was defined as less than 5% leukemic cells in a bone marrow aspirate, no evidence of leukemia at any other site, and hematologic recovery according to the Cancer and Leukemia Group B (CALGB) criteria.37 Early death was defined as death before or during the first 6 weeks of treatment. EFS was defined as the time from diagnosis until the date of the first adverse event (relapse, death owing to any cause, or second malignancy) or, if no events occurred, until the date of last follow-up. Failure was deemed to have occurred if CR was not attained at time 0. DFS was defined as EFS for patients in remission only. OS was defined as the time from diagnosis until death owing to any cause or last follow-up. Probabilities of EFS (pEFS) and OS (pOS) were estimated using the Kaplan-Meier method with standard errors according to Greenwood and were compared using the log-rank test. Cumulative incidence functions of relapse were constructed by the method of Kalbfleisch and Prentice for patients who achieved CR and were compared using the Gray test. Prognostic factors were examined by multivariate Cox regression analysis.
For statistical comparisons of categorical variables, χ2 analysis was used. To assess differences in the distribution of LC50 values the nonparametric Mann-Whitney U test was used. P ≤ .05 was considered statistically significant (2-tailed test).
Results
Patient characteristics
We attempted to assess the FLT3/ITD status of 273 children with newly diagnosed AML included in the aforementioned protocols between February 1990 and August 2001. For 8 patients this could not be determined because of low DNA recovery from cytospin slides. Excluded were patients with mixed lineage leukemia (n = 2) and MDS (n = 3). One patient was not treated with curative attempt and was excluded as well. Samples from 13 children with Down syndrome (DS) and AML and from 12 children with secondary AML were excluded from the prognostic analysis, but the samples were assessed for FLT3/ITD. Therefore, we successfully evaluated 234 patients for the prognostic significance of FLT3/ITD, which is the study population described here. This included 165 (18%) samples from the 934 de novo childhood AML patients included in the AML-BFM SG trials and 69 (40%) samples from 171 patients included in the DCLSG trials during that time. Eighty patients were classified as SR and 154 as HR, according to the AML-BFM risk group criteria.32 Patient characteristics are given in Table 1. The study population included 20 infants (defined as younger than 1 year).
We analyzed the potential of selection bias for patients included in this study from AML-BFM study group trials. There were no significant differences in AML-BFM risk group distribution (SR, 36% vs 30%; P = .20), cytogenetics (good risk cytogenetics, 21% vs 28%; P = .14), sex (52% boys vs 56% boys; P = .30), or age (median, 8.9 vs 8.2 years; P = .08) between the 165 AML-BFM study group patients included in this study and the 769 not included in this study. However, included patients had higher white blood cell (WBC) counts at diagnosis (30 vs 17 × 109/L; P = .0001). This did not result in significant differences in clinical outcome between the 2 groups regarding 5-year pEFS (49% vs 51%; P = .76) and pOS (62% vs 60%; P = .60).
Of the study population of 234 samples, drug-resistance testing was performed in 173 samples. In 39 (22.5%) samples, the MTT assay was not successful; hence, we present drug-resistance data from 134 children with AML. Patient characteristics are given in Table 1. Patients with successful drug-resistance assays had significantly higher WBC counts at diagnosis (median, 39.9 × 109/L vs 13.8 × 109/L; P = < .001) and were older than children without successful drug-resistance assay (median, 8.7 vs 3.9 years; P = .009). There was no significant difference for sex (P = .80).
Analysis of FLT3/ITD
Of the 234 evaluable patients, 27 (11.5%) patients were positive for FLT3/ITD—9 standard and 18 high-risk patients. Patients with FLT3/ITD were significantly older than children without this mutation (median, 13.4 vs 8.8 years; P < .001). No FLT3/ITD was found in the 20 infants younger than 1 year included in this study (P = .09 for infants vs older children). In children aged 1 to 9 years, the frequency of FLT3/ITD was 5.7%, whereas in children 10 years and older, the frequency rose to 19.4% (P = .002). There was no significant difference in WBC count (median, 50.3 vs 30.5 × 109/L; P = .26), when WBC count was analyzed as a continuous variable. When we categorized the patients as those with WBC counts less than 50 × 109/L and those with WBC counts 50 × 109/L or higher, again we found no significant differences between patients with and without FLT3/ITD (WBC count below 50 × 109/L or higher in 52% vs 38%, respectively; P = .17). There was no difference in sex distribution between FLT3/ITD-positive and -negative patients (P = .16).
FAB-type classifications of FLT3/ITD-positive patients are shown in Table 1. The number of FAB M1/M2 patients was 59% in the FLT3/ITD-positive group versus 39% in the FLT3/ITD-negative group (χ2 analysis, M1/M2 vs non-M1/M2; P = .04). FAB M5 represented only 7% of the FLT3/ITD-positive patients compared with 23% in the FLT3/ITD-negative group (M5 vs non-M5; P = .04). In addition, no FLT3/ITD occurred in the 21 FAB M4Eo patients (M4Eo vs non-M4Eo; P = .08).
The FLT3/ITD-positive group contained only 2 patients with “good risk” cytogenetics (of the 22 patients for whom cytogenetic data were available) compared with 40 of the 188 FLT3/ITD-negative patients (χ2 analysis; P = .37). Twelve (54.5%) FLT3/ITD-positive patients were found to have a normal karyotype compared with 42 (24.7%) FLT3/ITD-negative patients (P = .003). FLT3/ITD did not occur in the cytogenetic subgroups with 11q23 abnormalities (P = .02), inv(16) (P = .20), or abnormalities of chromosome 5 or 7 (P = .30).
No FLT3/ITDs were detected in the 13 AML patients with Down syndrome (not included in the study population). Of the 12 patients with secondary AML (also excluded from the study population), 2 were positive for FLT3/ITD.
Gene scan analysis of the mutant-wild-type ratio
From 21 of 27 patients with FLT3/ITD, we successfully determined the mutant/WT-FLT3 ratio. Failure was attributed to lack of cells or of DNA recovery. Median blast percentage of the cytospin slides (prepared after blast cell enrichment, as described earlier) used to determine the ratios was 92% (range, 84%-98%; 1 outlier had 70% blasts with a ratio of 0.3). The ratios varied from 0.14 to 3.90, with a median value of 0.69. There was no significant correlation between blast cell count and ratios (ρ = 0.2; P = .38). Patients with a ratio lower than or equal to the median had a lower WBC count (39.7 × 109/L) than patients with a ratio greater than the median (61.5 × 109/L), but this difference was not statistically significant (P = .23). In addition, there were no age differences between the 2 groups (P = .55).
Clinical outcome and prognostic significance of FLT3/ITD
The CR rate was 70% for FLT3/ITD-positive patients and 88% for patients without FLT3/ITD (P = .01). The Kaplan-Meier estimate for 5-year probability of EFS (pEFS) was 46% (SE, 4%) for FLT3/ITD-negative patients versus 29% (SE, 9%) for patients with FLT3/ITD (P = .0046; Figure 2A). Considering overall survival (OS), the estimated 5-year pOS of FLT3/ITD-negative patients was 58% (SE, 4%) compared with 32% (SE, 12%) for FLT3/ITD-positive patients (P = .037). The probability of DFS at 5 years (for patients in CR only) was 53% (SE, 5%) for FLT3/ITD-negative versus 41% (SE, 12%) for FLT3/ITD-positive patients (P = .09). Censoring SCT in first CR did not change the results considering DFS (FLT3/ITD-negative, 57% [SE, 5%]; FLT3/ITD-positive, 42% [SE, 15%]; P = .43). The 5-year cumulative incidence of relapses for patients in CR was 39% (SE, 5%) for FLT3/ITD-negative and 47% (SE, 15%) for FLT3/ITD-positive patients (P = .17). Median follow-up for patients still under observation was 3.3 years (range, 0.5-10.0 years) for FLT3/ITD-negative patients versus 2.2 years (range, 0.6-9.4 years) for FLT3/ITD-positive patients.
Probability of EFS. (A) Five-year probability of EFS in relation to FLT3/ITD. Children with FLT3/ITD-positive AML (n = 27) have significantly poorer 5-year pEFS (46%; [SE, 4%]) when compared with children without this particular mutation (n = 207; pEFS, 29% [SE, 9%]; P = .0046, log-rank test). (B) Two-year probability of EFS related to the mutant/WT-FLT3 ratio. Patients with FLT3/ITD mutations were divided into 2 groups according to the mutant/WT-FLT3 ratio. Children with ratios lower than or equal to the median (0.69) have outcomes similar to those of FLT3/ITD-negative patients (P = .26), whereas patients with ratios greater than the median have significantly worse outcomes than ITD-negative patients (P = .037). The difference between patients with ratios lower than or equal to the median versus those with high ratios was not significant (P = .41), but numbers were small.
Probability of EFS. (A) Five-year probability of EFS in relation to FLT3/ITD. Children with FLT3/ITD-positive AML (n = 27) have significantly poorer 5-year pEFS (46%; [SE, 4%]) when compared with children without this particular mutation (n = 207; pEFS, 29% [SE, 9%]; P = .0046, log-rank test). (B) Two-year probability of EFS related to the mutant/WT-FLT3 ratio. Patients with FLT3/ITD mutations were divided into 2 groups according to the mutant/WT-FLT3 ratio. Children with ratios lower than or equal to the median (0.69) have outcomes similar to those of FLT3/ITD-negative patients (P = .26), whereas patients with ratios greater than the median have significantly worse outcomes than ITD-negative patients (P = .037). The difference between patients with ratios lower than or equal to the median versus those with high ratios was not significant (P = .41), but numbers were small.
Subsequently, we divided the patients into SR and HR subgroups according to the BFM criteria. The difference in 5-year pEFS between FLT3/ITD-negative and -positive patients was significant in the subgroup of SR patients (pEFS = 61% [SE, 8%] vs 22% [SE, 14%], respectively; P = .0002) but not in the HR group (pEFS = 39% [SE, 5%] vs 34% [SE, 12%]; P = .22). The difference in 5-year pOS between FLT3/ITD-negative and -positive patients was significant in the subgroup of SR patients (pOS = 73% [SE, 7%] vs 22% [SE, 14%], respectively; P < .0001) but again not in the HR group (pOS = 50% [SE, 5%] vs 53% [SE, 12%], respectively; P = .78).
Within the subgroup with normal cytogenetics, the difference in 5-year pEFS was 59% (SE, 11%) for FLT3/ITD-negative (n = 42) versus 39% (SE, 15%) for FLT3/ITD-positive patients (n = 12; P = .06), with a difference in 5-year pOS of 77% (SE, 8%) for FLT3/ITD-negative versus 56% (SE, 15%) for FLT3/ITD-positive patients (P = .18).
We next studied the prognostic impact of the mutant/WT-FLT3 ratios. Patients with high ratios had relatively short follow-up (ie, the 2 patients with high ratios who did not yet experience an adverse event); therefore, 2-year EFS data were used for comparison instead of 5-year EFS data. There was no significant difference in CR rate (50% vs 73%; P = .28) or 2-year pEFS rate between patients with ratios greater than (n = 10) versus those with ratios less than or equal to (n = 11) the median (pEFS, 20% [SE, 13%] vs 44% [SE, 15%]; P = .41), but numbers were small (depicted in Figure 2B). When we compared the FLT3/ITD-negative patients with ITD-positive patients with a ratio less than or equal to the median (0.69), there was no significant difference in CR rate (88% vs 73%; P = .14) or 2-year pEFS (61% [SE, 3%] vs 44% [SE, 15%]; P = .26). However, the FLT3/ITD-positive patients with ratios greater than 0.69 fared significantly worse than the ITD-negative patients considering CR rate (50% vs 88%; P = .001) and 2-year EFS rate (pEFS, 20% [SE, 13%] vs 61% [SE, 3%]; P = .0037).
We evaluated several prognostic variables for pEFS in a multivariate Cox regression model. No significant prognostic relevance was found for treatment according to Dutch versus German protocols (P = .55), FLT3/ITD analysis performed in the USA or in Germany (P = .66), or interaction between location and FLT3/ITD (ie, different prognostic impact of FLT3/ITD in the 2 cohorts) (P = .27). To further determine which factors were independent prognostic factors for poor outcome (pEFS), we included well-known prognostic factors in BFM studies such as the BFM risk group classification (SR or HR), WBC count (less than 50 × 109/L or more than or equal to 50 × 109/L), FLT3/ITD status, and SCT in first CR as a time-dependent variable. FLT3/ITD strongly predicted for poor outcome (P = .01), with an increase in relative risk (RR) for events of 1.92 (95% confidence interval [CI], 1.16-3.17). In addition, the AML-BFM risk group classification strongly predicted for EFS (RR, 1.79 [95% CI, 1.17-2.72]; P = .007) and WBC count larger than or equal to 50 × 109/L (RR, 1.58 [95% CI, 1.09-2.30]; P = .016). When cytogenetic subgroups—with favorable cytogenetics defined as inv(16), t(8;21), and t(15;17)—were added to this model instead of the AML-BFM risk group classification, we again found FLT3/ITD to be the strongest independent predictor of outcome (RR, 1.86; P = .022). The mutant/WT-FLT3 ratio did not have independent prognostic significance, but when we added a ratio greater than 0.69 to the model we found this had an independent adverse prognostic significance (RR, 2.5; P = .016).
Within the group of FLT3/ITD-positive patients, we compared patients in continuous CR with those with events. However, there were no significant differences in WBC count (P = .87), age (P = .63), or sex distribution (P = .55) between these 2 groups. In addition, the FAB-type distribution was similar.
MTT assay
The MTT assay was successfully performed in 15 of 20 (75%) FLT3/ITD-positive and in 119 of 153 (78%) FLT3/ITD-negative patients (χ2 analysis, P = .78). In the FLT3/ITD-positive subgroup, failure was due to lack of cells before culture (n = 1), transport longer than 48 hours (n = 1), and low blast percentage after 4 days of culture (n = 3). In the FLT3/ITD-negative subgroup, MTT assay failure was due to lack of cells before culture (n = 14), low blast percentage after 4 days of culture (n = 14), low OD (n = 5), and infection of the culture (n = 1). There were no significant differences in OD/105 cells (P = .42) or CCS (P = .37) between FLT3/ITD-positive and -negative samples.
FLT3/ITD-positive samples appeared to be significantly more resistant to 2-chlorodeoxyadenosine than FLT3/ITD-negative samples (median, 2.0-fold; P = .006). Although FLT3/ITD-positive samples were 1.8-fold more resistant to etoposide than FLT3/ITD-negative samples, this difference did not achieve statistical significance (P = .27). For the other drugs tested, including cytarabine and the anthracyclines, no significant differences in cellular resistance were found between FLT3/ITD-positive and -negative samples. When we compared samples with a mutant/WT-FLT3 ratio greater than those with a ratio less than or equal to the median, no statistically significant differences in drug resistance were found. In addition, there were no significant differences when we compared these subgroups with the ITD-negative samples.
We previously reported on differences within childhood AML considering FAB-type and cytogenetic subgroups and cellular drug resistance.27,36 Given the differences in FAB-type distribution between FLT3/ITD-positive and -negative patients, we also analyzed whether there were differences in drug resistance within the FAB M1/M2 subgroup with regard to FLT3/ITD status. No significant differences were found. In addition, when we analyzed differences in cellular drug resistance within the subgroups with normal cytogenetics, again no significant differences between FLT3/ITD-positive and -negative cases were found.
Discussion
FLT3/ITD was found in 11.5% of the children with newly diagnosed AML in this study, which is the largest pediatric series to date. The occurrence of FLT3/ITD appeared to be age dependent—no cases were found in infant AML, 5.1% were found in children 1 to 10 years of age, but in older children (10-18 years of age) the frequency rose to 19.4%. FLT3/ITD was not equally distributed among the different FAB types, with a significantly higher frequency in patients with FAB M1/M2. Considering the cytogenetic subgroups, normal karyotype was found in 54.5% of the FLT3/ITD-positive patients; this was significantly higher than the 22.3% found in FLT3/ITD-negative patients. Moreover, no FLT3/ITDs were found in the subgroup of AML with 11q23 abnormalities (P = .02). Although FLT3/ITD has been described to occur frequently in acute promyelocytic leukemia (APL), we could not assess this because of the low number of patients with APL included in our study.16,23,25,38 Although not included for determination in the study population, we found no FLT3/ITD in the 13 AML patients with Down syndrome. When all the reported pediatric study results are taken together, the incidence of FLT3/ITD in newly diagnosed childhood AML is 12.8%, with 52% classified as having FAB M1/M2 and 45% classified in the normal cytogenetics subgroup (data not shown).19-23,38
The frequency of FLT3/ITD positivity is lower in children than the reported frequency of 20% to 35% in the adult series.15,16,18,25 It must be noted that for other cytogenetic abnormalities, differences in frequencies between adults and children have been reported. For instance, the normal cytogenetics subgroup comprises approximately 15% to 30% of children with AML and approximately 40% to 50% of adults with AML.3,18,39-41 Because FLT3/ITD occurs mainly in this subgroup, this may add to the lower frequency observed in children. There are no data yet about the frequency of point mutations of FLT3 in children with AML.25,42
FLT3/ITD-positive patients fared significantly worse in terms of CR rate, EFS, and OS than FLT3/ITD-negative patients. The poor clinical outcomes of FLT3/ITD-positive patients could be attributed mainly to induction failure. However, FLT3/ITD-positive patients who achieved CR seemed to have a lower DFS, though this did not reach statistical significance. The median follow-up time for the FLT3/ITD-positive patients at risk was shorter (2.1 years) than for FLT3/ITD-negative patients (3.3 years); hence, longer follow-up might have affected the relapse rates reported here. Meshinchi et al19 also reported a significant difference in CR rate, and only 1 of the 6 FLT3/ITD-positive patients achieving remission was a long-term survivor (4 had relapses; 1 died of toxicity). This differs from the results found in 2 adult series, both of which reported increased relapse rates and no significant differences in CR rate or resistant disease.16,25 In part, these differences may be explained by differences in definitions of CR (especially regarding hematologic recovery) and differences in the induction regimens.
We also analyzed the prognostic significance of the mutant versus WT FLT3 ratios. High ratios have been described as more predictive of poor clinical outcome than the presence of FLT3/ITD alone.23-25 Patients with ratios greater than the median did not do significantly worse than patients with ratios lower than or equal to the median in terms of CR rate or 2-year EFS, but the numbers were small. We subsequently compared FLT3/ITD-positive patients with higher ratios and those with lower ratios with the ITD-negative patients; results showed that patients with higher ratios had the worst clinical outcomes. This confirms the results of other studies.23-25 However, we feel this should not lead to the conclusion that patients with ratios lower than the median have prognoses similar to those without FLT3/ITD. The ratios described by Thiede et al25 for adult AML have a much wider range (0.03-32.56) than we found (0.14-3.9) in our patient cohort. Patients with a ratios greater than 2.0 fared worse in their series, but we observed this in only 1 patient. Larger series are needed to study whether the ratios in pediatric AML differ from those in adults.
FLT3/ITD was an independent factor for pEFS in our study and was a stronger predictor than AML-BFM risk group classification and high WBC count at diagnosis. This confirms the results of Meshinchi et al19 and Kondo et al22 for AML in children.
Meshinchi et al19 suggested that SCT might improve outcomes in children with FLT3/ITD-positive AML. In this study, multivariate analysis did not show any prognostic benefit of SCT in first CR. This is in line with earlier publications from the AML-BFM group in which no clear benefit of SCT in first CR in childhood AML is found.3,43,44 Larger prospective studies are needed to further evaluate the role of SCT for patients with FLT3/ITD.
FLT3/ITD-positive samples were significantly more resistant in vitro to 2-chlorodeoxyadenosine (2-CdA) than FLT3/ITD-negative samples. This might be related to the lower number of FAB M5 samples in the FLT3/ITD-positive subgroup. In earlier studies, FAB M5 samples show enhanced sensitivity to 2-CdA, both in vivo45 and in vitro (C.M.Z. et al, unpublished observations, 2001). However, the difference in prognosis between FLT3/ITD-positive and -negative patients reported here was not related to differences in sensitivity to 2-CdA because patients were not treated with this drug. Although FLT3/ITD-positive samples were more resistant to etoposide, this difference was not statistically significant. FLT3/ITD-positive samples did not show enhanced in vitro resistance to the other drugs, including those frequently used in AML treatment. This suggests that the bulk of AML cells can be killed just as effectively in FLT3/ITD-mutated versus nonmutated samples. We cannot exclude however, that a small but resistant subclone might be responsible for the poorer prognosis because this cannot be determined by the MTT assay. In addition, the MTT assay is a nonclonogenic assay, and in an earlier study we found no predictive value of cellular resistance to cytarabine and daunorubicin for long-term clinical outcome.36
Explanations for the poor prognosis of FLT3/ITD other than cellular resistance may exist, such as enhanced regrowth potential of residual disease, resulting in an enhanced relapse rate. This is further supported by the myeloproliferative disease that developed in mice after the introduction of FLT3/ITD in their stem cells.17 Obtaining control of this regrowth mechanism might be achieved by the novel FLT3/ITD inhibitors that have been developed recently, which await further testing in clinical trials.46
One important potential selection bias of our study should be mentioned. We studied only a relatively small subset (18%) of patients included in the AML-BFM study group clinical trials. However, with the exception of higher WBC counts in this study, further analysis did not reveal clinically relevant differences regarding initial patient data and clinical outcomes between the patients from the entire cohort included in the AML-BFM studies and the patients tested for FLT3/ITD presented here. This might have resulted in a slight overestimation of the frequency of FLT3/ITD in our patients because this is associated with a higher WBC count.
In conclusion, FLT3/ITD can be found in 10% to 15% of children with AML, with increasing frequency found with increasing age. Despite that, in older children the frequency is still lower than the 20% to 30% reported for adults with AML. FLT3/ITD is not equally distributed among FAB types or cytogenetic subgroups, and it occurs more frequently in the FAB M1/M2 and the normal cytogenetics subgroups. It is associated with significantly worse clinical outcome, and it was the strongest independent predictor for EFS. In addition, a high mutant/WT-FLT3 ratio further compromises prognosis. We have no evidence for differences in cellular drug resistance between samples with and without FLT3/ITD, but numbers in the FLT3/ITD-positive subgroup were small. Further clinical studies will have to demonstrate the effectiveness of FLT3/ITD inhibitors to improve the prognosis of this subgroup of children with poor prognosis AML.
Prepublished online as Blood First Edition Paper, June 19, 2003; DOI 10.1182/blood-2002-12-3627.
Supported by the Landelijke Vereniging van Crematoria, Eindhoven, the Netherlands.
C.M.Z. and S.M. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank all the hospital staff and clinicians participating in the AML-BFM Study Group and the DCLSG and their reference laboratory personnel, who provided us with the patient samples and clinical and cell biologic data. We also thank all the research technicians of the involved laboratories and Mrs A. Heus for her secretarial help. This study was performed in conjunction with the AML Committee of the International-BFM Study Group (chaired by G.J.L.K.).

![Figure 2. Probability of EFS. (A) Five-year probability of EFS in relation to FLT3/ITD. Children with FLT3/ITD-positive AML (n = 27) have significantly poorer 5-year pEFS (46%; [SE, 4%]) when compared with children without this particular mutation (n = 207; pEFS, 29% [SE, 9%]; P = .0046, log-rank test). (B) Two-year probability of EFS related to the mutant/WT-FLT3 ratio. Patients with FLT3/ITD mutations were divided into 2 groups according to the mutant/WT-FLT3 ratio. Children with ratios lower than or equal to the median (0.69) have outcomes similar to those of FLT3/ITD-negative patients (P = .26), whereas patients with ratios greater than the median have significantly worse outcomes than ITD-negative patients (P = .037). The difference between patients with ratios lower than or equal to the median versus those with high ratios was not significant (P = .41), but numbers were small.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/7/10.1182_blood-2002-12-3627/6/m_h81935008002.jpeg?Expires=1769097810&Signature=ZLwKeRe9cYzLRlEryf7Wu2kJyfXx~TDkixMPxM07QqD-1RXxw4Lf~F59l27Q~1ym5eG5awiJzmqzR5PUbZOrCgkZwemz~1DF7XnJ8jsfzvpfIuIrJNL1C1HdUoXKMxTHmhKNGIHFpwdA8U4lup9mAanW~BK3QEDhKMJ8kCTBKwiukkQCl06UTT~Y2wJsBD8vbW7-OWiohw3wwY2SO~~sG6IVU4CvlCBPE1qbLlQ3BHTApZP~VIPE0yD3fQlN83WR1~QUzCRY66yzz~eMZZEzp81LidH3aHsWjzhLIeb~Ce~ozb35-pkxnBX4goEOGoDYfyYYTJB5Wn12P1PtRtEIdg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 2. Probability of EFS. (A) Five-year probability of EFS in relation to FLT3/ITD. Children with FLT3/ITD-positive AML (n = 27) have significantly poorer 5-year pEFS (46%; [SE, 4%]) when compared with children without this particular mutation (n = 207; pEFS, 29% [SE, 9%]; P = .0046, log-rank test). (B) Two-year probability of EFS related to the mutant/WT-FLT3 ratio. Patients with FLT3/ITD mutations were divided into 2 groups according to the mutant/WT-FLT3 ratio. Children with ratios lower than or equal to the median (0.69) have outcomes similar to those of FLT3/ITD-negative patients (P = .26), whereas patients with ratios greater than the median have significantly worse outcomes than ITD-negative patients (P = .037). The difference between patients with ratios lower than or equal to the median versus those with high ratios was not significant (P = .41), but numbers were small.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/7/10.1182_blood-2002-12-3627/6/m_h81935008002.jpeg?Expires=1769097811&Signature=gIOAXl2SKo5kDkwAe-Qb8cMeyGucFwKwKYsQ6UOE6xKnfHYZ-3ZJJBe74JX9oJRFzfY1775KwLuus8KUqKQYRWhko6uBI2W0skDOluTCHX0ykryuoNkhIEHtL9Rk-z6lbk0ZT9LqQTrEWuRnLXr8iDctfwW3NKh3R7y6M8g5wdoAzqTuNnBO-5tiySbo046B1aai~STIori2mrHOPjfC40w5Q3sp1fX~PeI3uVgi7OywwQbzDaihA-D9FHdE6isiIUX00SkdU3Awgvu00EMPdonrO6M7d~kHULAcB5vw-VkYknc1C~AfJJhP1QClUQcv6c9MpRriARz-mDvsKDBMXQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)